
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samir Mouhssine | -- | 3871 | 2022-07-12 16:41:50 | | | |
| 2 | Lindsay Dong | Meta information modification | 3871 | 2022-07-20 03:17:58 | | |
Video Upload Options
Acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) represent an unmet clinical need whose prognosis is still dismal. Alterations of immune response play a prominent role in AML/MDS pathogenesis, revealing novel options for immunotherapy. Among immune system regulators, CD47, immune checkpoints, and toll-like receptor 2 (TLR2) are major targets. Magrolimab antagonizes CD47, which is overexpressed by AML and MDS cells, thus inducing macrophage phagocytosis with clinical activity in AML/MDS. Sabatolimab, an inhibitor of T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), which disrupts its binding to galectin-9, has shown promising results in AML/MDS, enhancing the effector functions of lymphocytes and triggering tumor cell death. Several other surface molecules, namely CD33, CD123, CD45, and CD70, can be targeted with monoclonal antibodies (mAbs) that exert different mechanisms of action and include naked and conjugated antibodies, bispecific T-cell engagers, trispecific killer engagers, and fusion proteins linked to toxins.
1. Immune System Dysfunction in AML/MDS
| NCT Code | Trial | Target | Study Population | Efficacy Results | Ref. |
|---|---|---|---|---|---|
| NCT03248479 | Ongoing phase Ib, magrolimab + AZA | CD47 | untreated AML unfit for induction chemotherapy. | ORR 69%: 50% CR or CRi, 13% PR and 31% SD | [8] |
| NCT02678338 | Phase I, magrolimab | CD47 | R/R AML | N/A | [9] |
| NCT04755244 | Ongoing phase I/II, evorpacept + venetoclax + AZA | CD47 | R/R AML ineligible for standard induction chemotherapy | N/A | N/A |
| NCT01822509 | Phase I/Ib, ipilimumab | CTLA-4 | R/R AML after allogeneic HSCT | Durable response (>1 year): 4/22 | [10] |
| NCT02397720 | Ongoing phase II, nivolumab + AZA | PD-1 | R/R AML | ORR: 33% mOS: 10.6 months |
[11] |
| NCT02530463 | Ongoing phase II, ipilimumab + nivolumab + AZA vs. nivolumab + AZA vs. AZA | PD-1 | R/R AML | Ipilimumab + nivolumab + AZA arm: mOS 7.6 months; Nivolumab + AZA arm: mOS 5.9 months; AZA control arm: mOS 4.4 months |
[12] |
| NCT03066648 | Phase Ib, sabatolimab +/− PDR001 + HMA | TIM-3 | AML | ND AML unsuitable for induction chemotherapy: ORR 41.2%, CR 8%, CRi 3%, PR 3% | [13] |
| NCT02785900 | Phase III, vadastuximab talirine + AZA/decitabine vs. placebo | CD33 | Older ND AML | Terminated (due to poor safety) | [14][15] |
| NCT02575963 | Phase II, 225 Ac-lintuzumab | CD33 | AML | 69% remission | [16] |
| NCT02520427 | Ongoing phase I, AMG330 | CD33 | R/R AML | CR/CRi 11.4% | [17] |
| NCT03647800 | Phase IB, APVO436 | CD123 | R/R AML | N/A | [18] |
| NCT02730312 | Ongoing phase I, vibecotamab | CD123 | R/R AML | CR/CRi: 23% | [19] |
| NCT03386513 | Ongoing phase I/II, IMGN632 | CD123 | R/R AML | CR: 1/12, CRi: 3/12 | [20] |
| NCT03113643 | Ongoing phase I, tagraxofusp + AZA vs. AZA/venetoclax | CD123 | AML | N/A | [21] |
| NCT02152956 | Ongoing phase I/II, flotetuzumab | CD123 | R/R AML | ORR 13.6%, CR 11.7% | [22] |
| NCT00008177 | Phase I, iomab-B + FLU + 2 Gy TBI | CD45 | Over 50 years AML | N/A | [23] |
| NCT02665065 | Ongoing phase III, iomab-B + FLU + low-dose TBI | CD45 | R/R AML | N/A | [23] |
| NCT01300572 | Phase I, 90Y-BC8 + FLU/TBI | CD45 | AML ineligible for allogeneic HSCT | OS at 1.8 years: 53% | [24] |
| NCT03030612 | Phase I/II, cusatuzumab monotherapy followed by cusatuzumab + AZA | CD70 | Untreated older AML | CR/CRi: 83% | [25] |
| NCT Code | Trial | Target | Study Population | Efficacy Results | Ref. |
|---|---|---|---|---|---|
| NCT03248479 | Ongoing phase Ib, magrolimab + AZA | CD47 | treatment-naïve MDS from intermediate to very high | ORR 91%: CR 42%, mCR 24%; PR 3% |
[26] |
| NCT04313881 | Ongoing phase III, magrolimab + AZA vs. AZA + placebo | CD47 | Treatment-naïve HR-MDS | NA | N/A |
| NCT04417517 | Ongoing phase I/II, evorpacept + AZA | CD47 | R/R or ND HR-MDS | mCR: 3/10; cytogenic response: 2/10 SD: 2/10 |
[27] |
| NCT02530463 | Ongoing phase II, ipilimumab and/or nivolumab +/− AZA | CTLA-4 | HMA-failure MDS or untreated MDS | HMA-failure arm: ORR 36%, CR 9%, CRi 9%, mOS 11.4 months; frontline arm: ORR 67%, CR 33%, mOS 12% |
[28] |
| NCT03094637 | Ongoing phase II, pembrolizumab + AZA | PD-1 | HMA-failure or untreated INT1 or HR-MDS | HMA-failure arm: ORR 25%; frontline arm: ORR 76%, CR 18%, mCR 29% |
[29] |
| NCT03066648 | Ongoing phase Ib, sabatolimab + HMA | TIM-3 | High risk and very high risk MDS | ORR 56.9%, mDOR: 16.1 months |
[30] |
| NCT02363491 | Ongoing phase I/II, tomaralimab | TLR-2 | HMA-failure and transfusion-dependent LR-MDS patients | ORR: 50% | [31] |
| NCT03337451 | Ongoing phase I/II, tomaralimab | TLR-2 | HMA-failure and transfusion-dependent LR-MDS patients | ORR: 50% | [32] |
| NCT03214666 | Phase I/II, GTB-3550 | CD33 | HR-MDS | N/A | [33] |
| NCT03647800 | Ongoing Ib, APVO436 | CD123 | R/R MDS after HMA-failure | mCR: 50% | [18] |
| NCT03113643 | Ongoing phase Ib, tagraxofusp + AZA | CD123 | MDS | CR 50%, mCR: 25% | [34] |
2. Immune System Regulators
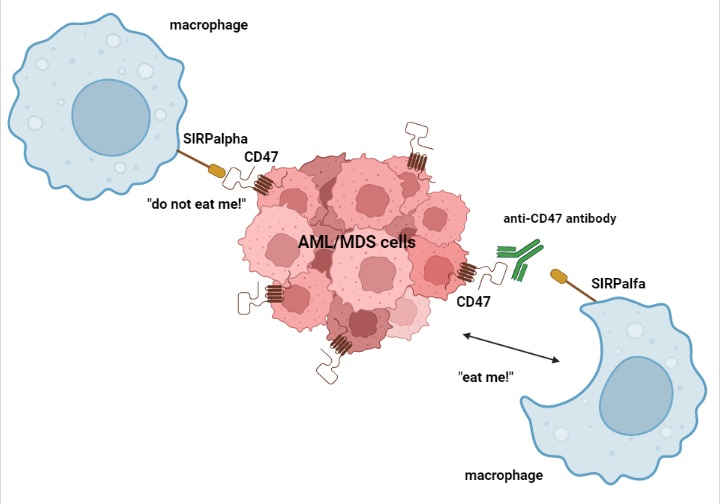
2.1. CD47
2.2. Immune Checkpoint Regulators
2.3. TLR-2
TLR2 is a member of the Toll-like receptor family and is expressed on the surface of various cells, including hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HSPCs), and plays a fundamental role in pathogen recognition and in the activation of innate immunity [43]. Overexpression of TLR2 leads to upregulation of the IL-8 molecular pathway, which is often dysregulated in MDS patients [44][45]]. Antagonizing TLR2 with a mAb that interacts with its ligand-binding site may prevent heterodimerization of the receptor with TLR1 or TLR6, resulting in TLR2 pathway blockade [46].
3. Other Molecular Targets on the AML/MDS Cell Membrane
3.1. CD33
3.2. CD123
3.3. CD45
3.4. CD70
4. Target Immunotherapies in AML
Several clinical trials with mAbs (naked and conjugated) are currently ongoing in the frontline, relapsed/refractory (R/R), post allogeneic HSCT, and minimal residual disease (MRD)/maintenance setting, with the aim of improving the outcomes of AML patients. These mAbs may target immune regulatory molecules (CD47 and immune checkpoints) and other membrane antigens (CD 33, CD123, CD45, and CD70) (Table 1).
4.1. Targeting CD47
4.2. Immune Checkpoint Inhibitors
4.3. Targeting CD33
4.4. Targeting CD123
4.5. Targeting CD45
4.6. Targeting CD70
5. Target Immunotherapies in MDS
5.1. Targeting CD47
5.2. Immune Checkpoint Inhibitors
5.3. Targeting TLR-2
5.4. Targeting CD33
Recent studies have analyzed anti-CD33 BiTEs and trispecific killer engagers (TriKEs) in MDS. AMV564, a CD33XCD3 BiTE, was evaluated in a preclinical study, which showed its in vitro ability to reduce theMDSCcount and to increase the anti-PD1 antibody activity [67]. GTB-3550 is a CD33/CD16/IL15 TriKE, consisting of a fusion of two scFv, one against CD33 and one against CD16, bridged by an IL15 linker that promotes NK activation, with no significant reported toxicities [33]. A second-generation TriKE, GTB-3650, is under development, although clinical trials have not started yet. Overall, this evidence indicates that CD33 could be a possible target for MDS treatment, but further investigations are needed.
5.5. Targeting CD123
Preliminary results concerning the CD123XCD3 BiTE APVO436 are coming from an ongoing phase Ib trial (NCT03647800) [18]. This study reported an acceptable safety profile for the few enrolled MDS patients, all with R/R MDS after HMA failure. No severe AEs were reported and 50% achieved mCR [18]. Another strategy of CD123 targeting is represented by challenging CD123 with its ligand (IL3) fused to a toxin. In this setting, an ongoing phase Ib study (NCT03113643) has demonstrated the safety of tagraxofusp + AZA, reporting anemia, thrombocytopenia, and neutropenia as the most common grade ≥3 AEs [34].
6. Conclusions and Perspectives
References
- Winter, S.; Shoaie, S.; Kordasti, S.; Platzbecker, U. Integrating the “Immunome” in the Stratification of Myelodysplastic Syndromes and Future Clinical Trial Design. J. Clin. Oncol. 2020, 38, 1723–1735.
- Kapoor, S.; Champion, G.; Basu, A.; Mariampillai, A.; Olnes, M.J. Immune Therapies for Myelodysplastic Syndromes and Acute Myeloid Leukemia. Cancers 2021, 13, 5026.
- Zeng, W.; Miyazato, A.; Chen, G.; Kajigaya, S.; Young, N.S.; Maciejewski, J.P. Interferon-gamma-induced gene expression in CD34 cells: Identification of pathologic cytokine-specific signature profiles. Blood 2006, 107, 167–175.
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years on. Cell 2018, 174, 1054–1066.
- Lordo, M.R.; Scoville, S.D.; Goel, A.; Yu, J.; Freud, A.G.; Caligiuri, M.A.; Mundy-Bosse, B.L. Unraveling the Role of Innate Lymphoid Cells in AcuteMyeloid Leukemia. Cancers 2021, 13, 320.
- Sendker, S.; Reinhardt, D.; Niktoreh, N. Redirecting the Immune Microenvironment in Acute Myeloid Leukemia. Cancers 2021, 13, 1423.
- Mahalleh, M.; Shabani, M.; Rayzan, E.; Rezaei, N. Reinforcing the primary immunotherapy modulators against acute leukemia; monoclonal antibodies in AML. Immunotherapy 2019, 11, 1583–1600.
- Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569.
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254.
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153.
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383.
- Daver, N.; Basu, S.; Garcia-Manero, G.; Abbas, H.A.; Konopleva, M.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Alotaibi, A.S.; Pemmaraju, N.; et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory (R/R) Acute Myeloid Leukemia: Clinical and Immune Biomarkers of Response. Blood 2020, 136, 43–45.
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Vey, N.; Scholl, S.; Garcia-Manero, G.; Wermke, M.; Janssen, J.; Traer, E.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients with Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (HR-MDS): Updated Results from a Phase 1b Study. Blood 2020, 136, 1–2.
- Fathi, A.T.; Erba, H.P.; Lancet, J.E.; Stein, E.M.; Ravandi, F.; Faderl, S.; Walter, R.B.; Advani, A.S.; DeAngelo, D.J.; Kovacsovics, T.J.; et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood 2018, 132, 1125–1133.
- Wang, E.S.; Adés, L.; Fathi, A.T.; Kreuzer, K.A.; O’Meara, M.M.; Liang, S.-Y.; Ravandi, F. CASCADE: A phase 3, randomized, double-blind study of vadastuximab talirine (33A) versus placebo in combination with azacitidine or decitabine in the treatment of older patients with newly diagnosed acute myeloid leukemia (AML). J. Clin. Oncol. 2017, 35, TPS7066.
- Jurcic, J.G. Targeted Alpha-Particle Therapy for Hematologic Malignancies. J. Med. Imaging Radiat. Sci. 2019, 50, S53–S57.
- Ravandi, F.; Walter, R.B.; Subklewe, M.; Buecklein, V.; Jongen-Lavrencic, M.; Paschka, P.; Ossenkoppele, G.J.; Kantarjian, H.M.; Hindoyan, A.; Agarwal, S.K.; et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J. Clin. Oncol. 2020, 38, 7508.
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113.
- Ravandi, F.; Bashey, A.; Foran, J.M.; Stock, W.; Mawad, R.; Blum, W.; Saville, M.W.; Johnson, C.M.; Vanasse, K.G.J.; Ly, T.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of XmAb14045, a CD123 x CD3 T Cell-Engaging Bispecific Antibody: Initial Results of a Phase 1 Study. Blood 2018, 132, 763.
- Daver, N.G.; Erba, H.P.; Papadantonakis, N.; DeAngelo, D.J.; Wang, E.S.; Konopleva, M.Y.; Sloss, C.M.; Culm-Merdek, K.; Zweidler-McKay, P.A.; Kantarjian, H.M. A Phase I, First-in-Human Study Evaluating the Safety and Preliminary Antileukemia Activity of IMGN632, a Novel CD123-Targeting Antibody-Drug Conjugate, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Other CD123-Positive Hematologic Malignancies. Blood 2018, 132, 27.
- Wang, S.Y.; Thomassen, K.; Kurch, L.; Opitz, S.; Franke, G.N.; Bach, E.; Platzbecker, U.; Kayser, S. Combination of Tagraxofusp and Azacitidine Is an Effective Option for Relapsed Blastic Plasmacytoid Dendritic Cell Neoplasm After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2021, 21, e579–e582.
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762.
- Pagel, J.M.; Gooley, T.A.; Rajendran, J.; Fisher, D.R.; Wilson, W.A.; Sandmaier, B.M.; Matthews, D.C.; Deeg, H.J.; Gopal, A.K.; Martin, P.J.; et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009, 114, 5444–5453.
- Vo, P.; Gooley, T.A.; Rajendran, J.G.; Fisher, D.R.; Orozco, J.J.; Green, D.J.; Gopal, A.K.; Haaf, R.; Nartea, M.; Storb, R.; et al. Yttrium-90-labeled anti-CD45 antibody followed by a reduced-intensity hematopoietic cell transplantation for patients with relapsed/refractory leukemia or myelodysplasia. Haematologica 2020, 105, 1731–1737.
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467.
- Sallman, D.A.; Al Malki, M.; Asch, A.S.; Lee, D.J.; Kambhampati, S.; Donnellan, W.B.; Bradley, T.J.; Vyas, P.; Jeyakumar, D.; Marcucci, G.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results. J. Clin. Oncol. 2020, 38, 7507.
- Garcia-Manero, G.; Erba, H.P.; Sanikommu, S.R.; Altman, J.K.; Sayar, H.; Scott, B.L.; Fong, A.P.; Guan, S.; Jin, F.; Forgie, A.J.; et al. Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine: A Phase 1/2 Study in Patients with Myelodysplastic Syndrome (ASPEN-02). Blood 2021, 138, 2601.
- Morita, K.; Kantarjian, H.M.; Montalban Bravo, G.; Sasaki, K.; Daver, N.; Jabbour, E.; Alvarado, Y.; Chien, K.S.; DiNardo, C.D.; Ravandi, F.; et al. A Phase II Study of Double Immune Checkpoint Inhibitor Blockade with Nivolumab and Ipilimumab with or without Azacitidine in Patients with Myelodysplastic Syndrome (MDS). Blood 2020, 136, 7–9.
- Chien, K.S.; Kim, K.; Nogueras-Gonzalez, G.M.; Borthakur, G.; Naqvi, K.; Daver, N.G.; Montalban-Bravo, G.; Cortes, J.E.; DiNardo, C.D.; Jabbour, E.; et al. Phase II study of azacitidine with pembrolizumab in patients with intermediate-1 or higher-risk myelodysplastic syndrome. Br. J. Haematol. 2021, 195, 378–387.
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients (Pts) with Very High/High-Risk Myelodysplastic Syndrome (vHR/HR-MDS) and Acute Myeloid Leukemia (AML): Final Analysis from a Phase Ib Study. Blood 2021, 138, 244.
- Garcia-Manero, G.; Montalban-Bravo, G.; Yang, H.; Wei, Y.; Alvarado, Y.; DiNardo, C.D.; Daver, N.G.; Konopleva, M.; Hearn, K.P.; Miller, R.; et al. A Clinical Study of OPN-305, a Toll-like Receptor 2 (TLR-2) Antibody, in Patients with Lower Risk Myelodysplastic Syndromes (MDS) That Have Received Prior Hypomethylating Agent (HMA) Therapy. Blood 2016, 128, 227.
- Garcia-Manero, G.; Jabbour, E.J.; Konopleva, M.Y.; Daver, N.G.; Borthakur, G.; DiNardo, C.D.; Bose, P.; Patel, P.; Komrokji, R.S.; Shastri, A.; et al. A Clinical Study of Tomaralimab (OPN-305), a Toll-like Receptor 2 (TLR-2) Antibody, in Heavily Pre-Treated Transfusion Dependent Patients with Lower Risk Myelodysplastic Syndromes (MDS) That Have Received and Failed on Prior Hypomethylating Agent (HMA) Therapy. Blood 2018, 132, 798.
- Warlick, E.D.; Weisdorf, D.J.; Vallera, D.A.; Wangen, R.; Lewis, D.; Knox, J.; Schroeder, M.; Felices, M.; Miller, J.S. GTB-3550 TriKE™ for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (AML) Safely Drives Natural Killer (NK) Cell Proliferation At Initial Dose Cohorts. Blood 2020, 136, 7–8.
- Lane, A.A.; Stein, A.S.; Garcia, J.S.; Garzon, J.L.; Galinsky, I.; Luskin, M.R.; Stone, R.M.; Winer, E.S.; Leonard, R.; Mughal, T.I.; et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood 2021, 138, 2346.
- Xu, Z.; Gao, J.; Yao, J.; Yang, T.; Wang, D.; Dai, C.; Ding, Y. Preclinical efficacy and toxicity studies of a highly specific chimeric anti-CD47 antibody. FEBS Open Bio 2021, 11, 813–825.
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108.
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779.
- Daver, N. Immune checkpoint inhibitors in acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2021, 34, 101247.
- Yang, H.; Bueso-Ramos, C.; DiNardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014, 28, 1280–1288.
- Shrikant, P.; Khoruts, A.; Mescher, M.F. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999, 11, 483–493.
- Alfayez, M.; Borthakur, G. Checkpoint inhibitors and acute myelogenous leukemia: Promises and challenges. Expert Rev. Hematol. 2018, 11, 373–389.
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148.
- Darlene A. Monlish; Zev J. Greenberg; Sima T. Bhatt; Kathryn M. Leonard; Molly P. Romine; Qian Dong; Lauren Bendesky; Eric J. Duncavage; Jeffrey A. Magee; Laura G. Schuettpelz; et al. TLR2/6 signaling promotes the expansion of premalignant hematopoietic stem and progenitor cells in the NUP98–HOXD13 mouse model of MDS. Experimental Hematology 2020, 88, 42-55.
- Wei, Y.; Dimicoli, S.; Bueso-Ramos, C.; Chen, R.; Yang, H.; Neuberg, D.; Pierce, S.; Jia, Y.; Zheng, H.; Wang, H.; et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013, 27, 1832–1840.
- Carolina Schinke; Orsolya Giricz; Schinke Carolina; Aditi Shastri; Shanisha Gordon; Laura Barreyro; Tushar Bhagat; Sanchari Bhattacharyya; Nandini Ramachandra; Matthias Bartenstein; et al.Andrea PellagattiJacqueline BoultwoodAmittha WickremaYiting YuBritta WillLi WeijuanUlrich SteidlAmit Verma IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood 2015, 125, 3144-3152.
- Reilly, M.; Miller, R.M.; Thomson, M.H.; Patris, V.; Ryle, P.; McLoughlin, L.; Mutch, P.; Gilboy, P.; Miller, C.; Broekema, M.; et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin. Pharmacol. Ther. 2013, 94, 593–600.
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119.
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers 2019, 11, 1358.
- Hercus, T.R.; Dhagat, U.; Kan, W.L.T.; Broughton, S.E.; Nero, T.L.; Perugini, M.; Sandow, J.J.; D’Andrea, R.J.; Ekert, P.G.; Hughes, T.; et al. Signalling by the βc family of cytokines. Cytokine Growth Factor Rev. 2013, 24, 189–201.
- Allen, C.; Zeidan, A.M.; Bewersdorf, J.P. BiTEs, DARTS, BiKEs and TriKEs-Are Antibody Based Therapies Changing the Future Treatment of AML? Life 2021, 11, 465.
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32.
- Riether, C.; Schürch, C.M.; Bührer, E.D.; Hinterbrandner, M.; Huguenin, A.L.; Hoepner, S.; Zlobec, I.; Pabst, T.; Radpour, R.; Ochsenbein, A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 2017, 214, 359–380.
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345.
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232.
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299.
- Liu, Y.; Bewersdorf, J.P.; Stahl, M.; Zeidan, A.M. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: The dawn of a new era? Blood Rev. 2019, 34, 67–83.
- Steven E. Kauder; Tracy C. Kuo; Ons Harrabi; Amy Chen; Emma Sangalang; Laura Doyle; Sony S. Rocha; Sangeetha Bollini; Bora Han; Janet Sim; et al.Jaume PonsHong I. Wan ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLOS ONE 2018, 13, e0201832.
- Berger, R.; Rotem-Yehudar, R.; Slama, G.; Landes, S.; Kneller, A.; Leiba, M.; Koren-Michowitz, M.; Shimoni, A.; Nagler, A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008, 14, 3044–3051.
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496.
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559.
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516.
- Muresan, B.; Mamolo, C.; Cappelleri, J.C.; Mokgokong, R.; Palaka, A.; Soikkeli, F.; Heeg, B. Comparing cure rates for gemtuzumab ozogamicin plus standard chemotherapy vs standard chemotherapy alone in acute myeloid leukemia patients. Future Oncol. 2021, 17, 2883–2892.
- Arthur E. Frankel; Jung H. Woo; Chul Ahn; Naveen Pemmaraju; Bruno C. Medeiros; Hetty E. Carraway; Olga Frankfurt; Stephen J. Forman; Xuezhong A. Yang; Marina Konopleva; et al.Francine Garnache-OttouFanny Angelot-DelettreChristopher BrooksMichael SzarekEric Rowinsky Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014, 124, 385-392.
- A. E. Frankel; M. A. Weir; P. D. Hall; M. Holguin; C. Cable; D. A. Rizzieri; D. E. Hogge; Induction of remission in patients with acute myeloid leukemia without prolonged myelosuppression using diphtheria toxin-interleukin 3 fusion protein. Journal of Clinical Oncology 2007, 25, 7068-7068.
- Abaza, Y.; Fathi, A.T. Monoclonal Antibodies in Acute Myeloid Leukemia-Are We There Yet? Cancer J. 2022, 28, 37–42.
- Zeidan, A.M.; Knaus, H.A.; Robinson, T.M.; Towlerton, A.M.H.; Warren, E.H.; Zeidner, J.F.; Blackford, A.L.; Duffield, A.S.; Rizzieri, D.; Frattini, M.G.; et al. A Multi-center Phase I Trial of Ipilimumab in Patients with Myelodysplastic Syndromes following Hypomethylating Agent Failure. Clin. Cancer Res. 2018, 24, 3519–3527.
- Cheng, P.; Chen, X.; Dalton, R.; Calescibetta, A.; So, T.; Gilvary, D.; Ward, G.; Smith, V.; Eckard, S.; Fox, J.A.; et al. Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol. Ther. 2022, 30, 2315–2326.
- Brauchle, B.; Goldstein, R.L.; Karbowski, C.M.; Henn, A.; Li, C.M.; Bücklein, V.L.; Krupka, C.; Boyle, M.C.; Koppikar, P.; Haubner, S.; et al. Characterization of a Novel FLT3 BiTE Molecule for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2020, 19, 1875–1888.
- Wu, M.; Li, C.; Zhu, X. FLT3 inhibitors in acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 133.




