Acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) represent an unmet clinical need whose prognosis is still dismal. Alterations of immune response play a prominent role in AML/MDS pathogenesis, revealing novel options for immunotherapy. Among immune system regulators, CD47, immune checkpoints, and toll-like receptor 2 (TLR2) are major targets. Magrolimab antagonizes CD47, which is overexpressed by AML and MDS cells, thus inducing macrophage phagocytosis with clinical activity in AML/MDS. Sabatolimab, an inhibitor of T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), which disrupts its binding to galectin-9, has shown promising results in AML/MDS, enhancing the effector functions of lymphocytes and triggering tumor cell death. Several other surface molecules, namely CD33, CD123, CD45, and CD70, can be targeted with monoclonal antibodies (mAbs) that exert different mechanisms of action and include naked and conjugated antibodies, bispecific T-cell engagers, trispecific killer engagers, and fusion proteins linked to toxins.
- acute myeloid leukemia
- myelodysplastic syndromes
- molecular targets
- monoclonal antibodies
- therapy
1. Immune System Dysfunction in AML/MDS
| NCT Code | Trial | Target | Study Population | Efficacy Results | Ref. |
|---|---|---|---|---|---|
| NCT03248479 | Ongoing phase Ib, magrolimab + AZA | CD47 | untreated AML unfit for induction chemotherapy. | ORR 69%: 50% CR or CRi, 13% PR and 31% SD | [8] |
| NCT02678338 | Phase I, magrolimab | CD47 | |||
| NCT02665065 | |||||
| Ongoing phase III, iomab-B + FLU + low-dose TBI | |||||
| CD45 | |||||
| CR/CRi: 83% | |||||
| [ | |||||
| 25 | |||||
| ] | |||||
| NCT Code | Trial | Target | Study Population | Efficacy Results | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03248479 | Ongoing phase Ib, magrolimab + AZA | CD47 | treatment-naïve MDS from intermediate to very high | ORR 91%: CR 42%, mCR 24%; PR 3% |
[26] | ||||||
| R/R AML | |||||||||||
| NCT04313881 | Ongoing phase III, magrolimab + AZA vs. AZA + placebo | CD47 | N/A | Treatment-naïve HR-MDS | [9] | ||||||
| NA | N/A | NCT04755244 | Ongoing phase I/II, evorpacept + venetoclax + AZA | CD47 | R/R AML ineligible for standard induction chemotherapy | N/A | N/A | ||||
| NCT04417517 | Ongoing phase I/II, evorpacept + AZA | CD47 | R/R or ND HR-MDS | mCR: 3/10; cytogenic response: 2/10 SD: 2/10 |
[27] | NCT01822509 | Phase I/Ib, ipilimumab | CTLA-4 | R/R AML after allogeneic HSCT | Durable response (>1 year): 4/22 | [10] |
| [ | 29 | ] | |||||||||
| R/R AML | |||||||||||
| N/A | |||||||||||
| [ | |||||||||||
| NCT02530463 | Ongoing phase II, ipilimumab and/or nivolumab +/− AZA | CTLA-4 | HMA-failure MDS or untreated MDS | HMA-failure arm: ORR 36%, CR 9%, CRi 9%, mOS 11.4 months; frontline arm: ORR 67%, CR 33%, mOS 12% |
[28 | NCT02397720 | Ongoing phase II, nivolumab + AZA | PD-1 | R/R AML | ORR: 33% mOS: 10.6 months |
[11 |
| 23 | |||||||||||
| ] | |||||||||||
| ] | ] | ||||||||||
| NCT03094637 | Ongoing phase II, pembrolizumab + AZA | PD-1 | HMA-failure or untreated INT1 or HR-MDS | HMA-failure arm: ORR 25%; frontline arm: ORR 76%, CR 18%, mCR 29% |
NCT02530463 | Ongoing phase II, ipilimumab + nivolumab + AZA vs. nivolumab + AZA vs. AZA | PD-1 | R/R AML | Ipilimumab + nivolumab + AZA arm: mOS 7.6 months; Nivolumab + AZA arm: mOS 5.9 months; AZA control arm: mOS 4.4 months |
[12] | |
| NCT03066648 | Ongoing phase Ib, sabatolimab + HMA | TIM-3 | High risk and very high risk MDS | ORR 56.9%, mDOR: 16.1 months |
[30] | NCT03066648 | Phase Ib, sabatolimab +/− PDR001 + HMA | TIM-3 | AML | ND AML unsuitable for induction chemotherapy: ORR 41.2%, CR 8%, CRi 3%, PR 3% | [13] |
| NCT01300572 | |||||||||||
| NCT02363491 | Ongoing phase I/II, tomaralimab | TLR-2 | NCT02785900 | Phase III, vadastuximab talirine + AZA/decitabine vs. placebo | CD33 | Older ND AML | Terminated (due to poor safety) | [14][15] | |||
| HMA-failure and transfusion-dependent LR-MDS patients | ORR: 50% | [ | 31 | ] | |||||||
| NCT03337451 | Ongoing phase I/II, tomaralimab | TLR-2 | HMA-failure and transfusion-dependent LR-MDS patients | ORR: 50% | [32] | NCT02575963 | Phase II, 225 Ac-lintuzumab | CD33 | AML | ||
| NCT03214666 | Phase I/II, GTB-3550 | CD33 | 69% remission | HR-MDS | [16] | ||||||
| N/A | [ | 33 | ] | NCT02520427 | Ongoing phase I, AMG330 | CD33 | R/R AML | CR/CRi 11.4% | [17] | ||
| NCT03647800 | Ongoing Ib, APVO436 | CD123 | R/R MDS after HMA-failure | mCR: 50% | [18] | NCT03647800 | Phase IB, APVO436 | CD123 | R/R AML | N/A | [18] |
| NCT03113643 | Ongoing phase Ib, tagraxofusp + AZA | CD123 | MDS | CR 50%, mCR: 25% | [34] | NCT02730312 | Ongoing phase I, vibecotamab | CD123 | R/R AML | CR/CRi: 23% | [19] |
| NCT03386513 | Ongoing phase I/II, IMGN632 | CD123 | R/R AML | CR: 1/12, CRi: 3/12 | [20] | ||||||
| NCT03113643 | Ongoing phase I, tagraxofusp + AZA vs. AZA/venetoclax | CD123 | AML | N/A | [21] | ||||||
| NCT02152956 | Ongoing phase I/II, flotetuzumab | CD123 | R/R AML | ORR 13.6%, CR 11.7% | [22] | ||||||
| NCT00008177 | Phase I, iomab-B + FLU + 2 Gy TBI | CD45 | Over 50 years AML | N/A | [23] | ||||||
| Phase I, 90Y-BC8 + FLU/TBI | CD45 | AML ineligible for allogeneic HSCT | OS at 1.8 years: 53% | [ | 24] | ||||||
| NCT03030612 | Phase I/II, cusatuzumab monotherapy followed by cusatuzumab + AZA | CD70 | Untreated older AML |
2. Immune System Regulators
2.1. CD47
CD-47 is a transmembrane protein whose interaction with signal regulatory protein α (SIRPα), a regulatory membrane glycoprotein expressed mainly by macrophages, determines an inhibitory regulation against macrophage-mediated phagocytosis. This, in turn, allows CD47 overexpressing cells to escape immune surveillance and destruction. Anti-CD47 mAbs block this interaction, thus facilitating the killing of tumor cells by macrophages and the cross-priming of tumor-specific cytotoxic T cells, which, in turn, activate the adaptive immune response (Figure 21) [35][36]. As adverse events of anti-CD47 mAbs, many CD47+ cells, such as erythrocytes and platelets, may be less protected against phagocytosis, leading to hemagglutination, acute anemia, and thrombocytopenia [8].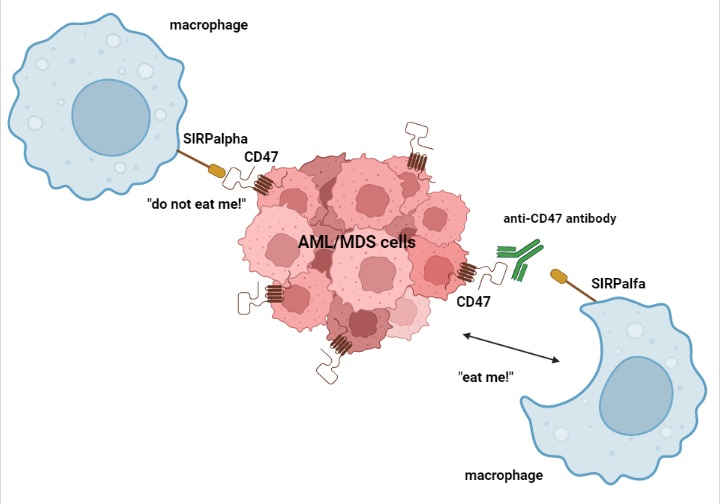
2.2. Immune Checkpoint Regulators
2.3. TLR-2
TLR2 is a member of the Toll-like receptor family and is expressed on the surface of various cells, including hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HSPCs), and plays a fundamental role in pathogen recognition and in the activation of innate immunity [43]. Overexpression of TLR2 leads to upregulation of the IL-8 molecular pathway, which is often dysregulated in MDS patients [44][45]]. Antagonizing TLR2 with a mAb that interacts with its ligand-binding site may prevent heterodimerization of the receptor with TLR1 or TLR6, resulting in TLR2 pathway blockade [46].
3. Other Molecular Targets on the AML/MDS Cell Membrane
3.1. CD33
CD33 is a sialic acid-binding Ig-like lectin (Siglec) expressed as a transmembrane protein on the surface of malignant AML blasts and MDSCs of MDS, but not on HSCs. These features render CD33 an ideal target for immunotherapy by different modalities [2]. The binding of anti-CD33 immunoconjugates to CD33 on the tumor cell surface results in the internalization of the antibody drug conjugates (ADCs)-CD33 complex into the cytoplasm and in delivery of the cytotoxic payload [47].3.2. CD123
CD123 represents the alpha-chain of the IL-3 receptor (IL-3Rα) expressed on myeloid pluripotent progenitor cells [48]. Its interaction with IL-3 induces intracellular tyrosine transphosphorylation by JAK-2, promoting the proliferation and differentiation of myeloid cells [49]. IL-3Rα is frequently expressed on AML blasts and is overexpressed in leukemic cells compared with normal HSCs, making it a promising therapeutic target. Novel anti-CD123 mAbs are CD123XCD3 BiTEs and antibody drug conjugates (ADCs) [2]. The anti-CD123 flotetuzumab mAb belongs to a novel category of bispecific mAbs, represented by dual affinity retargeting antibodies (DARTs) [22][50].3.3. CD45
Protein tyrosine phosphatase receptor type C, also known as CD45, is a transmembrane protein present in various isoforms on almost all differentiated hematopoietic cells [51]. CD45 is a signaling molecule that regulates a variety of cellular processes, including cell growth, mitotic cell cycle, and cell differentiation. CD45 is widely expressed on AML blasts and has emerged as a target for radio-immunotherapy (anti-CD45 ADCs) as part of the conditioning regimen prior to allogeneic hematopoietic stem cell transplantation (HSCT), exerting its action by delivering a cytotoxic payload to leukemic cells [23].3.4. CD70
Although CD70 is mainly a lymphoid lineage marker, it is also expressed on myeloid leukemic blasts, with an absent or low-level expression in normal BM cells [52]. The interaction between CD70 and its ligand CD27 in AML stem cells induces the activation of molecular pathways, including Wnt, JAK/STAT, Hedgehog, and TGF-β signaling, and promotes cell division [52]. Blocking CD70/CD27 signaling with mAbs can result in increased killing of leukemic cells by antibody-dependent cellular cytotoxicity (ADCC) [25].4. Target Immunotherapies in AML
Several clinical trials with mAbs (naked and conjugated) are currently ongoing in the frontline, relapsed/refractory (R/R), post allogeneic HSCT, and minimal residual disease (MRD)/maintenance setting, with the aim of improving the outcomes of AML patients. These mAbs may target immune regulatory molecules (CD47 and immune checkpoints) and other membrane antigens (CD 33, CD123, CD45, and CD70) (Table 1).
4.1. Targeting CD47
Targeting CD47 in AML is currently being explored using mAbs or fusion proteins. The rationale for using magrolimab (Hu5F9-G4), a humanized anti-CD47 IgG4, stems from the overexpression of CD47 on AML cells and its association with an adverse prognosis [53][54][55][56]. In the ongoing phase Ib trial (NCT03248479), including 25 untreated AML patients unfit for high-dose induction chemotherapy, the combination of magrolimab and HMA azacytidine (AZA) led to an overall response rate (ORR) of 69%, of which 50% was complete response (CR) or CR with incomplete hematologic recovery (CRi) [8]. Treatment-related adverse events were anemia (37%), neutropenia (26%), and thrombocytopenia (26%). Sixty-nine percent of patients became red blood cell (RBC) transfusion independent. Importantly, 88% of the evaluable TP53 mutant patients achieved an objective response, suggesting the efficacy of magrolimab plus AZA in poor prognosis and refractory patients [8]. Evorpacept (ALX148) is a fusion protein consisting of a modified SIRPα D1 domain targeting CD47, bound to an inactive human IgG1 fragment (Fc) [57]. This molecule is currently being explored in an ongoing phase I/II clinical trial (NCT04755244) in combination with the BCL2 inhibitor venetoclax and AZA for untreated or R/R AML ineligible for standard induction chemotherapy.4.2. Immune Checkpoint Inhibitors
Several immune checkpoint inhibitors are currently under investigation in AML, alone or in combination with standard therapies. Data from phase I studies suggest a limited efficacy of these mAbs when used as monotherapy and a potential synergistic effect when combined with HMAs [38]. A phase I/IB study (NCT01822509) tested ipilimumab (a CTLA-4 inhibitor) in patients with R/R AML after allogeneic HSCT. Durable responses (>1 year) were observed in 4/22 patients. Notably, 21% of patients had immune-mediated toxic effects [10]. PD-1 inhibitors are safe, but do not seem to provide any beneficial impact on disease outcome if used alone [58]. The observation that AZA upregulates PD-1 signaling provides the rationale for combining PD-1 inhibition with HMAs in R/R AML [11][58]. In an ongoing phase II study (NCT02397720), AZA combined with nivolumab in 70 R/R AML patients induced an ORR of 33% with a median OS of 10.6 months [11]. An additional study cohort (NCT02530463) of R/R AML patients treated with AZA+ nivolumab + ipilimumab demonstrated a median OS of 7.6 months, in contrast with 5.9 months and 4.4 months in the AZA + nivolumab cohort and HMAs control arm, respectively [12]. Sabatolimab (MBG453), a novel antibody directed against TIM-3, is under investigation in a phase Ib trial (NCT03066648) with or without PDR001 (anti PD-1) in combination with HMAs in AML patients [13]. Among the 34 evaluable patients with newly diagnosed AML unsuitable for standard induction chemotherapy or HSCT, the ORR was 41.2%: 8 CR, 3 CRi, and 3 PR [13].4.3. Targeting CD33
In 2000, the FDA approved gemtuzumab ozogamicin (GO), an immunoconjugate drug targeting CD33, for elderly (≥60 years) CD33+ relapsed AML unfit for chemotherapy [2][59]. In 2010, however, GO was withdrawn because of unacceptable toxicities, including major bleeding events, infection, and/or acute respiratory distress syndrome. Subsequently, the ALFA-0701 phase III multicentric randomized trial demonstrated adequate tolerability if GO was administered in a fractionated dose [2]. Therefore, in 2017, the FDA approved a GO fractionated dose for AML treatment [60][61][62]. A novel strategy for AML treatment is represented by the conjugation of anti-CD33 mAbs with radionuclides. Lintuzumab (SGN-33) is an anti-CD33 mAb that can be linked to α-emitters bismuth-213 (213Bi) or actinium-225 (225Ac). Initial studies have demonstrated that 213Bi-lintuzumab and 225Ac-lintuzumab may have an antileukemic effect, being able to induce remissions after low-dose cytarabine cytoreduction in untreated AML patients [16]. Finally, CD33 targeting is also being investigated with BiTEs. Early evidence of an antitumor activity has been shown with AMG 330, a CD33XCD3 BiTE, in R/R AML. However, only 11.4% of patients achieved CR/CRi in a phase I study (NCT02520427) evaluating the safety and tolerability of AMG 330, with reported serious AEs including cytokine release syndrome (CRS) and severe cytopenias [17].4.4. Targeting CD123
Challenging CD123 with bispecific mAbs is currently under investigation. APVO436, a CD123XCD3 BiTE, was evaluated in a phase Ib study (NCT03647800) that demonstrated adequate safety in R/R AML patients [18]. Vibecotamab (XmAb14045), a CD123XCD3 BiTE, is being tested in an ongoing phase I study (NCT02730312) to evaluate the safety and tolerability in R/R AML patients with CD123+ blasts. The study demonstrated evidence of an anti-leukemic activity, with a 23% CR/CRi rate. Grade ≥3 CRS was the most common AE (11% of patients), but no CRS-related deaths were recorded. The study is ongoing, with further optimization of the dose, schedule, and premedication regimens for CRS [19]. A different strategy of targeting CD123 is represented by tagraxofusp (SL-401), a fusion protein consisting of IL3 (CD123 ligand) linked to a truncated diphtheria toxin, which inactivates protein synthesis [63]. This compound was studied in a phase I trial enrolling R/R AML patients, with one patient achieving a durable CR of 8 months, two patients a PR lasting one and three months, and three patients presenting a minimal response [64]. Flotetuzumab (MGD006), a DART engineered for binding CD3 and CD123 on AML cells, is under investigation in a phase I/II trial (NCT02152956) for R/R AML. Among the 88 patients enrolled, the ORR was 13.6%, with 11.7% CR, and the most common treatment-emergent AE was CRS, which led to a dose interruption in 60% of patients [22].4.5. Targeting CD45
Iomab-B, an anti-CD45 antibody conjugated to 131I, was studied in combination with a reduced-intensity conditioning (RIC) regimen of fludarabine (FLU) plus total body irradiation (TBI) in R/R AML patients over 50 years in a phase I clinical trial (NCT00008177) in order to estimate the maximum tolerated dose [23]. Among the AEs, infusion toxicities, chills, nausea, vomiting, respiratory symptoms, and hypotension were reported. It showed that Iomab-B can be safely combined with a RIC regimen to achieve complete remission for older, HR patients with AML, and it is currently being tested in the phase III SIERRA trial (NCT02665065) [23]. In contrast with 131I, 90Y does not require isolation of the patient, providing a potential advantage in the management and quality of life for AML patients; 90Y-BC8, an anti-CD45 monoclonal antibody conjugated with 90Y, was proven to be well tolerated in a phase I trial (NCT01300572) in combination with FLU/TBI in R/R AML ineligible for myeloablative HSCT [24].4.6. Targeting CD70
Based on preclinical results, a phase I/II trial (NCT03030612) evaluated a single dose of cusatuzumab (ARGX-110), an anti-CD70 mAb, monotherapy followed by AZA in untreated AML older patients [25]. AZA induces CD70 expression on LSCs and therefore favors in vitro killing when combined with cusatuzumab [52]. Ten patients (83%) achieved CR/Cri, with four patients achieving MRD negativity by flow cytometry. No dose-limiting toxicities were reported [25][65].5. Target Immunotherapies in MDS
5.1. Targeting CD47
Magrolimab is one of the most innovative drugs for MDS treatment [53]. An ongoing phase Ib study (NCT03248479) has reported initial encouraging results for magrolimab in combination with AZA in treatment-naïve MDS patients at intermediate, high, or very high IPSS-R risk [26]. The ORR was 91% with a high rate of deep responses: 42% CR, 24% marrow CR (mCR, half of them also with hematological improvement, HI), 21% HI only, and 3% PR. Among the patients who reached CR or mCR, 22% were MRD negative by flow cytometry [26]. Moreover, 58% of RBC transfusion-dependent patients achieved transfusion independence. The most relevant AEs were myelosuppression (particularly anemia) and fatigue; the median duration of response (mDOR) was not reached, with 91% of responding patients maintaining a response at 6 months; OS was 100% at 6 months [26]. Remarkably, patients who also had the TP53 mutation achieved an objective response [8]. A novel treatment approach involves Evorpacept, studied in the phase I of the ASPEN02 multicentric phase I/II trial (NCT04417517), evaluating the safety and tolerability of its association with AZA in patients with untreated or R/R HR MDS. The initial results demonstrated a safety profile similar to AZA monotherapy: dose limiting toxicities were not observed and the maximum tolerated dose was not reached [27].5.2. Immune Checkpoint Inhibitors
Although studies involving ipilimumab monotherapy have demonstrated a limited efficacy, more encouraging results are emerging from an ongoing phase II study (NCT02530463) analyzing treatment with ipilimumab and/or nivolumab with or without AZA in MDS [28][66]. The most relevant results are from two cohorts of this trial: the HMA-failure cohort treated with ipilimumab + nivolumab and the frontline cohort treated with ipilimumab + nivolumab + AZA. For the HMA-failure cohort, the ORR was 36% (9% CR, 9% CR with incomplete count recovery or Cri, and 18% HI), with a median OS and progression-free survival (PFS) of 11.4 and 7.1 months, respectively. For the frontline cohort, the ORR was 67% (33% CR and 33% HI), with a median OS and PFS of 12 and 10 months, respectively. Over the median follow-up duration of 25 months, 38% of patients experienced disease progression. Grade ≥3 AEs included infection in 55% of patients, febrile neutropenia in 46%, rash in 24%, and transaminitis in 24% [28]. Additional positive results have been obtained in an ongoing phase II study (NCT03094637) evaluating the safety and efficacy of pembrolizumab, a humanized mAb targeting PD-1, combined with AZA in intermediate-1 or HR MDS. For the HMA-failure cohort (n = 20), the ORR of 25% was modest (but not irrelevant), with no significant survival benefit. The frontline cohort (n = 17) reached better outcomes: the ORR was 76% (18% CR, 29% mCR only, 24% mCR with HI, 6% HI only), and the median OS was not reached, with a median follow up of 12.8 months [29]. TIM-3 is a recently investigated immune checkpoint, which is being studied together with its pathway inhibitor sabatolimab [13]. An ongoing phase Ib clinical trial (NCT03066648) has shown promising results in high/very high risk MDS patients treated with a combination of sabatolimab and HMA. The safety was similar to HMA monotherapy and, notably, all of the patients with immune-mediated AEs achieved remission, probably due to the increased immune activity promoted by sabatolimab [30].5.3. Targeting TLR-2
Early results of two phase I/II trials (NCT02363491 and NCT03337451) with tomaralimab (OPN-305), a fully humanized IgG4 monoclonal antibody against TLR2, suggest its safety and efficacy profile in HMA-failure and transfusion-dependent LR MDS patients [31][32][44][46]. No significant toxicities were reported, and the ORR was 50%, with 27% of patients reaching transfusion independence [31][32]. These favorable outcomes may be confirmed and expanded once the complete results are finalized.5.4. Targeting CD33
Recent studies have analyzed anti-CD33 BiTEs and trispecific killer engagers (TriKEs) in MDS. AMV564, a CD33XCD3 BiTE, was evaluated in a preclinical study, which showed its in vitro ability to reduce theMDSCcount and to increase the anti-PD1 antibody activity [67]. GTB-3550 is a CD33/CD16/IL15 TriKE, consisting of a fusion of two scFv, one against CD33 and one against CD16, bridged by an IL15 linker that promotes NK activation, with no significant reported toxicities [33]. A second-generation TriKE, GTB-3650, is under development, although clinical trials have not started yet. Overall, this evidence indicates that CD33 could be a possible target for MDS treatment, but further investigations are needed.
5.5. Targeting CD123
Preliminary results concerning the CD123XCD3 BiTE APVO436 are coming from an ongoing phase Ib trial (NCT03647800) [18]. This study reported an acceptable safety profile for the few enrolled MDS patients, all with R/R MDS after HMA failure. No severe AEs were reported and 50% achieved mCR [18]. Another strategy of CD123 targeting is represented by challenging CD123 with its ligand (IL3) fused to a toxin. In this setting, an ongoing phase Ib study (NCT03113643) has demonstrated the safety of tagraxofusp + AZA, reporting anemia, thrombocytopenia, and neutropenia as the most common grade ≥3 AEs [34].
6. Conclusions and Perspectives
Only a minority of AML patients become long-term survivors using the standard treatments that are approved. The identification of several genetic targets has allowed for the design of small molecule inhibitors, best exemplified by FLT3 inhibitors, that have improved the outcome in patients carrying these molecular predictors [68][69]. However, the advantage in outcome is restricted to the fraction of patients carrying these genetic alterations. The outcome of AML is particularly dismal in elderly patients that are not candidates of allogeneic HSCT, for whom HMA plus venetoclax is the only available molecular treatment. In this context, the development of novel strategies of immunotherapy with mAbs targeting diverse surface molecules, alone or in combination with HMA and possibly BCL2 inhibitors, may provide a substantial clinical benefit to patients. In the context of MDS, HMA represents the only approved treatment strategy for HR patients. However, a response is limited to a fraction of cases and survival is still inadequate. In addition, the effect of HMA is treatment dependent and patients who discontinue treatment eventually lose their response, progress, and die. The availability of innovative immunotherapy therapies will allow for designing combinations that may possibly target MDS stem cells, thus increasing the response and prolonging survival. Finally, and perhaps most importantly, in view of a precision medicine approach for AML and MDS immunotherapy, the studies that are ongoing should also aim at the identification of molecular predictors of treatment response to a given mAb. This, in turn, will allow for a biologically rational choice of a specific immunotherapy strategy for the individual patient with AML or MDS.References
- Winter, S.; Shoaie, S.; Kordasti, S.; Platzbecker, U. Integrating the “Immunome” in the Stratification of Myelodysplastic Syndromes and Future Clinical Trial Design. J. Clin. Oncol. 2020, 38, 1723–1735.
- Kapoor, S.; Champion, G.; Basu, A.; Mariampillai, A.; Olnes, M.J. Immune Therapies for Myelodysplastic Syndromes and Acute Myeloid Leukemia. Cancers 2021, 13, 5026.
- Zeng, W.; Miyazato, A.; Chen, G.; Kajigaya, S.; Young, N.S.; Maciejewski, J.P. Interferon-gamma-induced gene expression in CD34 cells: Identification of pathologic cytokine-specific signature profiles. Blood 2006, 107, 167–175.
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years on. Cell 2018, 174, 1054–1066.
- Lordo, M.R.; Scoville, S.D.; Goel, A.; Yu, J.; Freud, A.G.; Caligiuri, M.A.; Mundy-Bosse, B.L. Unraveling the Role of Innate Lymphoid Cells in AcuteMyeloid Leukemia. Cancers 2021, 13, 320.
- Sendker, S.; Reinhardt, D.; Niktoreh, N. Redirecting the Immune Microenvironment in Acute Myeloid Leukemia. Cancers 2021, 13, 1423.
- Mahalleh, M.; Shabani, M.; Rayzan, E.; Rezaei, N. Reinforcing the primary immunotherapy modulators against acute leukemia; monoclonal antibodies in AML. Immunotherapy 2019, 11, 1583–1600.
- Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569.
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254.
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153.
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383.
- Daver, N.; Basu, S.; Garcia-Manero, G.; Abbas, H.A.; Konopleva, M.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Alotaibi, A.S.; Pemmaraju, N.; et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory (R/R) Acute Myeloid Leukemia: Clinical and Immune Biomarkers of Response. Blood 2020, 136, 43–45.
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Vey, N.; Scholl, S.; Garcia-Manero, G.; Wermke, M.; Janssen, J.; Traer, E.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients with Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (HR-MDS): Updated Results from a Phase 1b Study. Blood 2020, 136, 1–2.
- Fathi, A.T.; Erba, H.P.; Lancet, J.E.; Stein, E.M.; Ravandi, F.; Faderl, S.; Walter, R.B.; Advani, A.S.; DeAngelo, D.J.; Kovacsovics, T.J.; et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood 2018, 132, 1125–1133.
- Wang, E.S.; Adés, L.; Fathi, A.T.; Kreuzer, K.A.; O’Meara, M.M.; Liang, S.-Y.; Ravandi, F. CASCADE: A phase 3, randomized, double-blind study of vadastuximab talirine (33A) versus placebo in combination with azacitidine or decitabine in the treatment of older patients with newly diagnosed acute myeloid leukemia (AML). J. Clin. Oncol. 2017, 35, TPS7066.
- Jurcic, J.G. Targeted Alpha-Particle Therapy for Hematologic Malignancies. J. Med. Imaging Radiat. Sci. 2019, 50, S53–S57.
- Ravandi, F.; Walter, R.B.; Subklewe, M.; Buecklein, V.; Jongen-Lavrencic, M.; Paschka, P.; Ossenkoppele, G.J.; Kantarjian, H.M.; Hindoyan, A.; Agarwal, S.K.; et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J. Clin. Oncol. 2020, 38, 7508.
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113.
- Ravandi, F.; Bashey, A.; Foran, J.M.; Stock, W.; Mawad, R.; Blum, W.; Saville, M.W.; Johnson, C.M.; Vanasse, K.G.J.; Ly, T.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of XmAb14045, a CD123 x CD3 T Cell-Engaging Bispecific Antibody: Initial Results of a Phase 1 Study. Blood 2018, 132, 763.
- Daver, N.G.; Erba, H.P.; Papadantonakis, N.; DeAngelo, D.J.; Wang, E.S.; Konopleva, M.Y.; Sloss, C.M.; Culm-Merdek, K.; Zweidler-McKay, P.A.; Kantarjian, H.M. A Phase I, First-in-Human Study Evaluating the Safety and Preliminary Antileukemia Activity of IMGN632, a Novel CD123-Targeting Antibody-Drug Conjugate, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Other CD123-Positive Hematologic Malignancies. Blood 2018, 132, 27.
- Wang, S.Y.; Thomassen, K.; Kurch, L.; Opitz, S.; Franke, G.N.; Bach, E.; Platzbecker, U.; Kayser, S. Combination of Tagraxofusp and Azacitidine Is an Effective Option for Relapsed Blastic Plasmacytoid Dendritic Cell Neoplasm After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2021, 21, e579–e582.
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762.
- Pagel, J.M.; Gooley, T.A.; Rajendran, J.; Fisher, D.R.; Wilson, W.A.; Sandmaier, B.M.; Matthews, D.C.; Deeg, H.J.; Gopal, A.K.; Martin, P.J.; et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009, 114, 5444–5453.
- Vo, P.; Gooley, T.A.; Rajendran, J.G.; Fisher, D.R.; Orozco, J.J.; Green, D.J.; Gopal, A.K.; Haaf, R.; Nartea, M.; Storb, R.; et al. Yttrium-90-labeled anti-CD45 antibody followed by a reduced-intensity hematopoietic cell transplantation for patients with relapsed/refractory leukemia or myelodysplasia. Haematologica 2020, 105, 1731–1737.
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467.
- Sallman, D.A.; Al Malki, M.; Asch, A.S.; Lee, D.J.; Kambhampati, S.; Donnellan, W.B.; Bradley, T.J.; Vyas, P.; Jeyakumar, D.; Marcucci, G.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results. J. Clin. Oncol. 2020, 38, 7507.
- Garcia-Manero, G.; Erba, H.P.; Sanikommu, S.R.; Altman, J.K.; Sayar, H.; Scott, B.L.; Fong, A.P.; Guan, S.; Jin, F.; Forgie, A.J.; et al. Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine: A Phase 1/2 Study in Patients with Myelodysplastic Syndrome (ASPEN-02). Blood 2021, 138, 2601.
- Morita, K.; Kantarjian, H.M.; Montalban Bravo, G.; Sasaki, K.; Daver, N.; Jabbour, E.; Alvarado, Y.; Chien, K.S.; DiNardo, C.D.; Ravandi, F.; et al. A Phase II Study of Double Immune Checkpoint Inhibitor Blockade with Nivolumab and Ipilimumab with or without Azacitidine in Patients with Myelodysplastic Syndrome (MDS). Blood 2020, 136, 7–9.
- Chien, K.S.; Kim, K.; Nogueras-Gonzalez, G.M.; Borthakur, G.; Naqvi, K.; Daver, N.G.; Montalban-Bravo, G.; Cortes, J.E.; DiNardo, C.D.; Jabbour, E.; et al. Phase II study of azacitidine with pembrolizumab in patients with intermediate-1 or higher-risk myelodysplastic syndrome. Br. J. Haematol. 2021, 195, 378–387.
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients (Pts) with Very High/High-Risk Myelodysplastic Syndrome (vHR/HR-MDS) and Acute Myeloid Leukemia (AML): Final Analysis from a Phase Ib Study. Blood 2021, 138, 244.
- Garcia-Manero, G.; Montalban-Bravo, G.; Yang, H.; Wei, Y.; Alvarado, Y.; DiNardo, C.D.; Daver, N.G.; Konopleva, M.; Hearn, K.P.; Miller, R.; et al. A Clinical Study of OPN-305, a Toll-like Receptor 2 (TLR-2) Antibody, in Patients with Lower Risk Myelodysplastic Syndromes (MDS) That Have Received Prior Hypomethylating Agent (HMA) Therapy. Blood 2016, 128, 227.
- Garcia-Manero, G.; Jabbour, E.J.; Konopleva, M.Y.; Daver, N.G.; Borthakur, G.; DiNardo, C.D.; Bose, P.; Patel, P.; Komrokji, R.S.; Shastri, A.; et al. A Clinical Study of Tomaralimab (OPN-305), a Toll-like Receptor 2 (TLR-2) Antibody, in Heavily Pre-Treated Transfusion Dependent Patients with Lower Risk Myelodysplastic Syndromes (MDS) That Have Received and Failed on Prior Hypomethylating Agent (HMA) Therapy. Blood 2018, 132, 798.
- Warlick, E.D.; Weisdorf, D.J.; Vallera, D.A.; Wangen, R.; Lewis, D.; Knox, J.; Schroeder, M.; Felices, M.; Miller, J.S. GTB-3550 TriKE™ for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (AML) Safely Drives Natural Killer (NK) Cell Proliferation At Initial Dose Cohorts. Blood 2020, 136, 7–8.
- Lane, A.A.; Stein, A.S.; Garcia, J.S.; Garzon, J.L.; Galinsky, I.; Luskin, M.R.; Stone, R.M.; Winer, E.S.; Leonard, R.; Mughal, T.I.; et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood 2021, 138, 2346.
- Xu, Z.; Gao, J.; Yao, J.; Yang, T.; Wang, D.; Dai, C.; Ding, Y. Preclinical efficacy and toxicity studies of a highly specific chimeric anti-CD47 antibody. FEBS Open Bio 2021, 11, 813–825.
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108.
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779.
- Daver, N. Immune checkpoint inhibitors in acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2021, 34, 101247.
- Yang, H.; Bueso-Ramos, C.; DiNardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014, 28, 1280–1288.
- Shrikant, P.; Khoruts, A.; Mescher, M.F. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999, 11, 483–493.
- Alfayez, M.; Borthakur, G. Checkpoint inhibitors and acute myelogenous leukemia: Promises and challenges. Expert Rev. Hematol. 2018, 11, 373–389.
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148.
- Darlene A. Monlish; Zev J. Greenberg; Sima T. Bhatt; Kathryn M. Leonard; Molly P. Romine; Qian Dong; Lauren Bendesky; Eric J. Duncavage; Jeffrey A. Magee; Laura G. Schuettpelz; et al. TLR2/6 signaling promotes the expansion of premalignant hematopoietic stem and progenitor cells in the NUP98–HOXD13 mouse model of MDS. Experimental Hematology 2020, 88, 42-55.
- Wei, Y.; Dimicoli, S.; Bueso-Ramos, C.; Chen, R.; Yang, H.; Neuberg, D.; Pierce, S.; Jia, Y.; Zheng, H.; Wang, H.; et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013, 27, 1832–1840.
- Carolina Schinke; Orsolya Giricz; Schinke Carolina; Aditi Shastri; Shanisha Gordon; Laura Barreyro; Tushar Bhagat; Sanchari Bhattacharyya; Nandini Ramachandra; Matthias Bartenstein; et al.Andrea PellagattiJacqueline BoultwoodAmittha WickremaYiting YuBritta WillLi WeijuanUlrich SteidlAmit Verma IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood 2015, 125, 3144-3152.
- Reilly, M.; Miller, R.M.; Thomson, M.H.; Patris, V.; Ryle, P.; McLoughlin, L.; Mutch, P.; Gilboy, P.; Miller, C.; Broekema, M.; et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin. Pharmacol. Ther. 2013, 94, 593–600.
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119.
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers 2019, 11, 1358.
- Hercus, T.R.; Dhagat, U.; Kan, W.L.T.; Broughton, S.E.; Nero, T.L.; Perugini, M.; Sandow, J.J.; D’Andrea, R.J.; Ekert, P.G.; Hughes, T.; et al. Signalling by the βc family of cytokines. Cytokine Growth Factor Rev. 2013, 24, 189–201.
- Allen, C.; Zeidan, A.M.; Bewersdorf, J.P. BiTEs, DARTS, BiKEs and TriKEs-Are Antibody Based Therapies Changing the Future Treatment of AML? Life 2021, 11, 465.
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32.
- Riether, C.; Schürch, C.M.; Bührer, E.D.; Hinterbrandner, M.; Huguenin, A.L.; Hoepner, S.; Zlobec, I.; Pabst, T.; Radpour, R.; Ochsenbein, A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 2017, 214, 359–380.
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345.
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232.
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299.
- Liu, Y.; Bewersdorf, J.P.; Stahl, M.; Zeidan, A.M. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: The dawn of a new era? Blood Rev. 2019, 34, 67–83.
- Steven E. Kauder; Tracy C. Kuo; Ons Harrabi; Amy Chen; Emma Sangalang; Laura Doyle; Sony S. Rocha; Sangeetha Bollini; Bora Han; Janet Sim; et al.Jaume PonsHong I. Wan ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLOS ONE 2018, 13, e0201832.
- Berger, R.; Rotem-Yehudar, R.; Slama, G.; Landes, S.; Kneller, A.; Leiba, M.; Koren-Michowitz, M.; Shimoni, A.; Nagler, A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008, 14, 3044–3051.
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496.
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559.
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516.
- Muresan, B.; Mamolo, C.; Cappelleri, J.C.; Mokgokong, R.; Palaka, A.; Soikkeli, F.; Heeg, B. Comparing cure rates for gemtuzumab ozogamicin plus standard chemotherapy vs standard chemotherapy alone in acute myeloid leukemia patients. Future Oncol. 2021, 17, 2883–2892.
- Arthur E. Frankel; Jung H. Woo; Chul Ahn; Naveen Pemmaraju; Bruno C. Medeiros; Hetty E. Carraway; Olga Frankfurt; Stephen J. Forman; Xuezhong A. Yang; Marina Konopleva; et al.Francine Garnache-OttouFanny Angelot-DelettreChristopher BrooksMichael SzarekEric Rowinsky Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014, 124, 385-392.
- A. E. Frankel; M. A. Weir; P. D. Hall; M. Holguin; C. Cable; D. A. Rizzieri; D. E. Hogge; Induction of remission in patients with acute myeloid leukemia without prolonged myelosuppression using diphtheria toxin-interleukin 3 fusion protein. Journal of Clinical Oncology 2007, 25, 7068-7068.
- Abaza, Y.; Fathi, A.T. Monoclonal Antibodies in Acute Myeloid Leukemia-Are We There Yet? Cancer J. 2022, 28, 37–42.
- Zeidan, A.M.; Knaus, H.A.; Robinson, T.M.; Towlerton, A.M.H.; Warren, E.H.; Zeidner, J.F.; Blackford, A.L.; Duffield, A.S.; Rizzieri, D.; Frattini, M.G.; et al. A Multi-center Phase I Trial of Ipilimumab in Patients with Myelodysplastic Syndromes following Hypomethylating Agent Failure. Clin. Cancer Res. 2018, 24, 3519–3527.
- Cheng, P.; Chen, X.; Dalton, R.; Calescibetta, A.; So, T.; Gilvary, D.; Ward, G.; Smith, V.; Eckard, S.; Fox, J.A.; et al. Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol. Ther. 2022, 30, 2315–2326.
- Brauchle, B.; Goldstein, R.L.; Karbowski, C.M.; Henn, A.; Li, C.M.; Bücklein, V.L.; Krupka, C.; Boyle, M.C.; Koppikar, P.; Haubner, S.; et al. Characterization of a Novel FLT3 BiTE Molecule for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2020, 19, 1875–1888.
- Wu, M.; Li, C.; Zhu, X. FLT3 inhibitors in acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 133.
