
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Margherita Sisto | -- | 1668 | 2022-07-18 09:59:20 | | | |
| 2 | Sirius Huang | Meta information modification | 1668 | 2022-07-18 11:02:37 | | | | |
| 3 | Sirius Huang | + 7 word(s) | 1675 | 2022-07-20 08:28:30 | | |
Video Upload Options
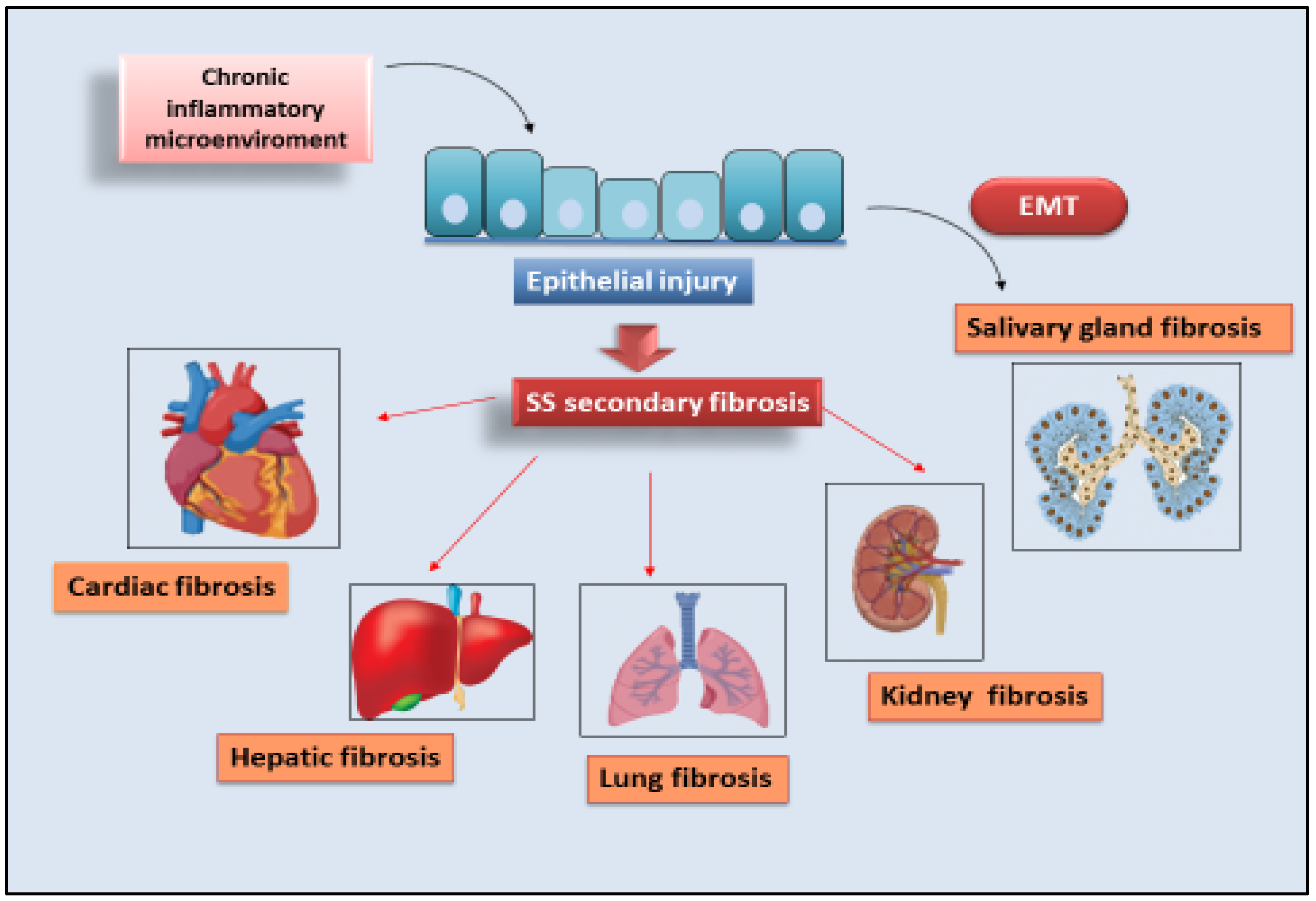
Sjögren’s syndrome (SS) is a systemic chronic autoimmune disorder characterized by lymphoplasmacytic infiltration of salivary glands (SGs) and lacrimal glands, causing glandular damage. The disease shows a combination of dryness symptoms found in the oral cavity, pharynx, larynx, and vagina, representing a systemic disease. The data in the literature relating to organ fibrosis correlated with SS are presented. The phenomenon has been extensively studied in SGs, where the molecular mechanisms that could trigger fibrosis are known and have been correlated with epithelial to mesenchymal transition (EMT). Cases of secondary fibrosis have also been observed, which could be correlated with the state of chronic inflammation that characterizes SS.
1. EMT-Dependent Salivary Gland Fibrosis

2. Cardiac Fibrosis
3. Liver Fibrosis
4. Lung Fibrosis
References
- Koski, H.; Janin, A.; Humphreys-Beher, M.G.; Sorsa, T.; Malmström, M.; Konttinen, Y.T. Tumor necrosis factor-alpha and receptors for it in labial salivary glands in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2001, 19, 131–137.
- Skopouli, F.; Li, L.; Boumba, D.; Stefanaki, S.; Hanel, K.; Moutsopoulos, H.M.; Krilis, S.A. Association of mast cells with fibrosis and fatty infiltration in the minor salivary glands of patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 1998, 16, 63–65.
- Leehan, K.M.; Pezant, N.P.; Rasmussen, A.; Grundahl, K.; Moore, J.S.; Radfar, L.; Lewis, D.M.; Stone, D.U.; Lessard, C.J.; Rhodus, N.L.; et al. Minor salivary gland fibrosis in Sjögren’s syndrome is elevated, associated with focus score and not solely a consequence of aging. Clin. Exp. Rheumatol. 2018, 112, 80–88.
- Zavadil, J.; Bottinger, E.P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774.
- Hall, B.E.; Zheng, C.; Swaim, W.D.; Cho, A.; Nagineni, C.N.; Eckhaus, M.A.; Flanders, K.C.; Ambudkar, I.S.; Baum, B.J.; Kulkarni, A.B. Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Investig. 2010, 90, 543–555.
- Woods, L.T.; Camden, J.M.; El-Sayed, F.G.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Weisman, G.A. Increased expression of TGF-β signaling components in a mouse model of fibrosis induced by submandibular gland duct ligation. PLoS ONE 2015, 10, e0123641.
- González, C.R.; Amer, M.A.; Vitullo, A.D.; González-Calvar, S.I.; Vacas, M.I. Immunolocalization of the TGFB1 system in submandibular gland fibrosis after experimental periodontitis in rats. Acta Odont. Latinoam. 2016, 29, 138–143.
- Mason, G.I.; Hamburger, J.; Bowman, S.; Matthews, J.B. Salivary gland expression of transforming growth factor beta isoforms in Sjogren’s syndrome and benign lymphoepithelial lesions. Mol. Pathol. 2003, 56, 52–59.
- Sisto, M.; Lisi, S.; Ribatti, D. The role of the epithelial-to-mesenchymal transition (EMT) in diseases of the salivary glands. Histochem. Cell Biol. 2018, 150, 133–147.
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Ribatti, D.; Lisi, S. TGFβ1-Smad canonical and -Erk non-canonical pathways participate in interleukin-17-induced epithelial–mesenchymal transition in Sjögren’s syndrome. Lab. Investig. 2020, 100, 824–836.
- Sisto, M.; Lorusso, L.; Tamma, R.; Ingravallo, G.; Ribatti, D.; Lisi, S. Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjögren’s syndrome. Clin. Exp. Immunol. 2019, 198, 261–272.
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Overall and cause-specific mortality in systemic lupus erythematosus: An updated meta-analysis. Lupus 2016, 25, 727–734.
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003, 107, 1303–1307.
- Wu, X.F.; Huang, J.Y.; Chiou, J.Y.; Chen, H.H.; Wei, J.C.; Dong, L.L. Increased risk of coronary heart disease among patients with primary Sjögren’s syndrome: A nationwide population-based cohort study. Sci. Rep. 2018, 8, 2209.
- Beltai, A.; Barnetche, T.; Daien, C.; Lukas, C.; Gaujoux-Viala, C.; Combe, B.; Morel, J. Cardiovascular morbidity and mortality in primary Sjögren’s syndrome: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 131–139.
- Nishiwaki, A.; Kobayashi, H.; Ikumi, N.; Kobayashi, Y.; Yokoe, I.; Sugiyama, K.; Matsukawa, Y.; Takei, M.; Kitamura, N. Salivary Gland Focus Score Is Associated with Myocardial Fibrosis in Primary Sjögren’s Syndrome Assessed by a Cardiac Magnetic Resonance Approach. J. Rheumatol. 2021, 48, 627.
- Yokoe, I.; Kobayashi, H.; Nishiwaki, A.; Nagasawa, Y.; Kitamura, N.; Haraoka, M.; Kobayashi, Y.; Takei, M.; Nakamura, H. Asymptomatic myocardial dysfunction was revealed by feature tracking cardiac magnetic resonance imaging in patients with primary Sjögren’s syndrome. Int. J. Rheum. Dis. 2021, 24, 1482–1490.
- Voulgarelis, M.; Tzioufas, A. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat. Rev. Rheumatol. 2010, 6, 529–537.
- Kaplan, M.J.; Ike, R.W. The liver is a common non-exocrine target in primary Sjögren’s syndrome: A retrospective review. BMC Gastroenterol. 2002, 2, 21.
- Lee, S.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Song, J.; Park, Y.B.; Lee, S.K.; Han, K.H.; Kim, S.U. Clinical predictors of silent but substantial liver fibrosis in primary Sjogren’s syndrome. Mod. Rheumatol. 2016, 26, 576–582.
- Zeron, P.B.; Retamozo, S.; Bové, A.; Kostov, B.A.; Sisó, A.; Ramos-Casals, M. Diagnosis of Liver Involvement in Primary Sjögren Syndrome. J. Clin. Transl. Hepatol. 2013, 1, 94–102.
- Liedtke, C.; Nevzorova, Y.A.; Luedde, T.; Zimmermann, H.; Kroy, D.; Strnad, P.; Berres, M.L.; Bernhagen, J.; Tacke, F.; Nattermann, J.; et al. Liver Fibrosis-From Mechanisms of Injury to Modulation of Disease. Front. Med. 2022, 8, 814496.
- Jiao, J.; Friedman, S.L.; Aloman, C. Hepatic fibrosis. Curr. Opin. Gastroenterol. 2009, 25, 223–229.
- Fairfax, A.J.; Haslam, P.L.; Pavia, D.; Sheahan, N.F.; Bateman, J.R.; Agnew, J.E.; Clarke, S.W.; Turner-Warwick, M. Pulmonary disorders associated with sjogren’s syndrome. Q. J. Med. 1981, 50, 279–295.
- Dong, X.; Zhou, J.; Guo, X.; Li, Y.; Xu, Y.; Fu, Q.; Lu, Y.; Zheng, Y. A retrospective analysis of distinguishing features of chest HRCT and clinical manifestation in primary sjogren’s syndrome-related interstitial lung disease in a Chinese population. Clin. Rheumatol. 2018, 37, 2981–2988.
- Kamiya, Y.; Fujisawa, T.; Kono, M.; Nakamura, H.; Yokomura, K.; Koshimizu, N.; Toyoshima, M.; Imokawa, S.; Sumikawa, H.; Johkoh, T.; et al. Prognostic factors for primary Sjögren’s syndrome-associated interstitial lung diseases. Respir. Med. 2019, 159, 105811.
- Roca, F.; Dominique, S.; Schmidt, J.; Smail, A.; Duhaut, P.; Lévesque, H.; Marie, I. Interstitial lung disease in primary Sjögren’s syndrome. Autoimmun. Rev. 2017, 16, 48–54.
- Sogkas, G.; Hirsch, S.; Olsson, K.M.; Hinrichs, J.B.; Thiele, T.; Seeliger, T.; Skripuletz, T.; Schmidt, R.E.; Witte, T.; Jablonka, A.; et al. Lung Involvement in Primary Sjögren’s Syndrome-An Under-Diagnosed Entity. Front. Med. 2020, 7, 332.
- Palm, O.; Garen, T.; Berge Enger, T.; Jensen, J.L.; Lund, M.B.; Aalokken, T.M.; Gran, J.T. Clinical pulmonary involvement in primary sjogren’s syndrome: Prevalence, quality of life and mortality–a retrospective study based on registry data. Rheumatology 2013, 52, 173–179.
- Belenguer, R.; Ramos-Casals, M.; Brito-Zeron, P.; del Pino, J.; Sentis, J.; Aguilo, S.; Font, J. Influence of clinical and immunological parameters on the health-related quality of life of patients with primary sjogren’s syndrome. Clin. Exp. Rheumatol. 2005, 23, 351–356.
- Gao, H.; Sun, Y.; Zhang, X.Y.; Xie, L.; Zhang, X.W.; Zhong, Y.C.; Zhang, J.; Hou, Y.K.; Li, Z.G. Characteristics and mortality in primary Sjögren syndrome-related interstitial lung disease. Medicine 2021, 100, e26777.
- Kakugawa, T.; Sakamoto, N.; Ishimoto, H.; Shimizu, T.; Nakamura, H.; Nawata, A.; Ito, C.; Sato, S.; Hanaka, T.; Oda, K.; et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjögren’s syndrome. Respir. Med. 2018, 137, 95–102.
- Lin, W.; Xin, Z.; Zhang, J.; Liu, N.; Ren, X.; Liu, M.; Su, Y.; Liu, Y.; Yang, L.; Guo, S.; et al. Interstitial lung disease in Primary Sjögren’s syndrome. BMC Pulm. Med. 2022, 22, 73.




