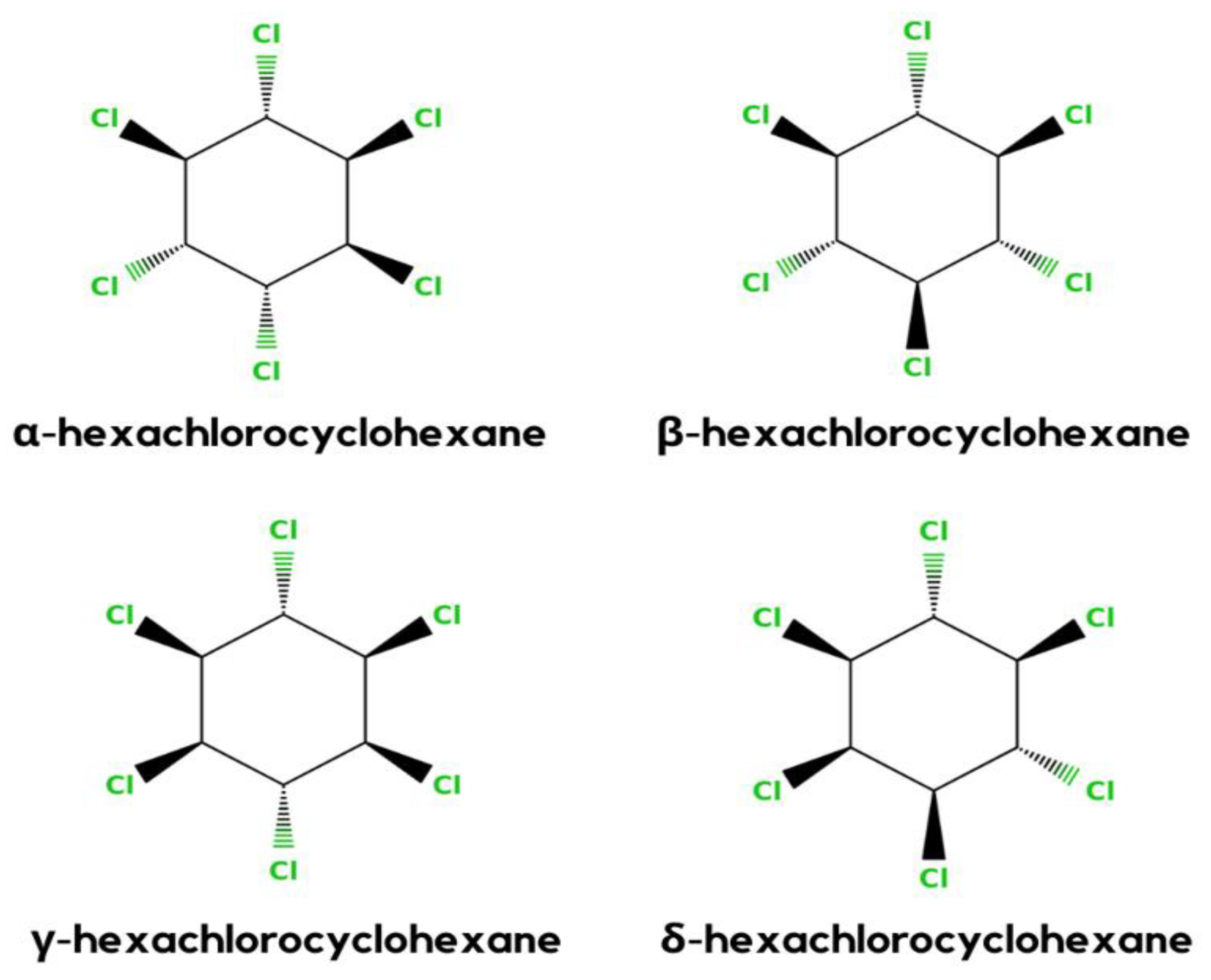
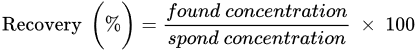Hexachlorocyclohexane (HCH) is an artificial organic pollutant also called hexachlorane. It has eight isomeric forms, but of these eight isomeric forms, four α, -β, -γ, and δ-HCHs are the most prevalent. From this compound, γ-HCH (also known as lindane) is the most constant and commonly used compound, and it is the supreme isomer. Lindane is a broad-spectrum chlorinated insecticide that has a mixture of several chemical forms of HCH and is written as γ-Hexachlorocyclohexane or γ-HCH. Organic pollutants are normally pesticides, insecticides, or fertilizer, but HCH is an insecticide that is used on fruits, plants, and animals. Lindane is one of the earliest generations of chlorinated organic insecticides, appearing shortly after the end of World War II. All of the pollutants have the same physical and chemical properties. Therefore, it has also had PBT (Persistent, Bioaccumulative, and Toxic) properties.
1. Classification of Lindane Based on the Isomeric Form
HCH is a synthetic organochlorinated pollutant. There are eight isomeric versions of HCH. α-, β-, γ-, and δ-HCH are the four most universal isomers shown in
Figure 1 [1]. γ-HCH (also known as lindane) is the most frequent isomer, and it is general. As the global usage of HCH decreases, so does the frequency of detection and the amounts identified in the environment
[2].
Figure 1. Different stereochemical structures of lindane (α, β, γ, and δ).
In general, HCH formulation is a mixture of β- (5–12%), δ- (6–10%), γ (10–12%), and α- (60–70%) isomers. However, only the γ-HCH isomer shows the maximum insecticidal activity. Therefore, it is used more in the agriculture sector than the other three isomers. γ-HCH is purified to at least 99% and the other isomers are discarded or not sent to the market
[3].
HCH isomers are widely dispersed in the environment. They accumulate in the food chain and cause toxicity in biological systems due to their lipophilic characteristics. They are volatilized in the environment and transferred to far-flung locations. As a result, HCH isomers are among nature’s most persistent and commonly encountered pollutants, with polluted sites documented all over the world. The half-life of lindane in soil and water was estimated to be 708 and 2292 days, respectively
[4]. Lindane and other HCHs leftovers survive in the environment for a long time and have lately been found in water, soil, sediments, plants, and animals all over the world owing to their persistence
[5].
2. Voltammetric Detection of Lindane Using Glassy Carbon Electrode
O.E. Fayemi et al.
[6] prepared the environmentally friendly modified working electrodes by altering the surface of a glassy carbon electrode (GCE) using polyaniline nanofibers (PANI), nanoparticles (ZnO and Fe
3O
4), and multiwalled carbon nanotubes (MWCNTs). GCE is used as a material for electrodes in electrochemistry, as well as for high-temperature crucibles due to its thermostability, chemical resistance, and impermeability to gases and liquids. Researchers prepared two types of composite electrodes (PANI/Zn, Fe(III) and Nylon 6,6/MWCNT/Zn, Fe(III)). Once preparing the electrodes, the researchers used cyclic voltammetry (CV) to observe the reduction behavior of lindane. Both composite electrodes and bare GCE displayed irreversible (reduction) CV peaks. Both the composite electrodes showed increased reduction peak currents for lindane electron-transfer reactions. GCE modified from Nylon 6,6/MWCNT/Fe
3O showed the maximum sensing current because of improved diffusion of lindane molecules through the micropores of Nylon 6,6/MWCNT/MO nanofibers. They also performed the analysis of lindane in tap-water samples and obtained a recovery range performance.
A. Kumaravel et al.
[7] prepared stable and reproducible cellulose acetate (CA)-modified GCE to sense lindane in the aqueous-alcoholic medium. The researchers prepared the modified GCE using a drop-dry method (deposited CA solution on pretreated GCE). They have also used CV to test the electrode performance for sensing lindane. The researchers observed the reduction peak of lindane at −1.5 V at both bare GCE and CA-modified GCE. However, at the CA-modified GCE, the reduction peak currents are quite high due to the CA-modified electron-transfer mechanism. They tested the analytical utility of the electrode in drinking water samples. They have used CV, differential pulse voltammetry (DPV), and amperometry to measure lindane in drinking-water samples. The recovery of lindane is higher at DPV.
Anu Prathap et al.
[8] detected lindane using NiCo
2O
4 modified glassy carbon electrode using differential pulse voltammetry (DPV) and cyclic voltammetry in the aqueous-methanol medium. At −1.5 V of the reduction peak, it shows a single well-defined irreversible lindane reduction. After the modification of the electrode, the reduction of lindane electron transfer can be calculated by the Randles–Sevcik equation. This modified GCE shows that lindane is irreversibly reduced, as there is no longer a reverse peak. They used four different metal oxides as an electrode, but all of these were found inactive to detect the reduction of lindane. Only NiCo2O4-modified GCEs are found to show a higher current response compared to other metal oxide electrodes. Researchers have used CV, DPV, and amperometry techniques for the detection of lindane in tap water with good recovery in differential pulse voltammetry (DPV). The GCE–AONP–PANI–SWCNT modified electrode was used in this experiment, and square wave voltammograms were obtained with varied lindane concentrations. After the modification of GCE with NiCo
2O
4, it showed excellent electrolytic activity enhanced the electron transfer rate, and gave a detection limit of 37,000 nM. It was discovered that when the concentration of lindane increased, the lindane reduction peak current increased as well, and the potential gradually switched to the negative side
[9]. The linear graph’s standard deviation error value was found to be 0.21. The detection limit (LOD) for lindane at the GCE–AONP–PANI–SWCNT modified electrode was calculated using the relationship 3.3/m, where m is the slope of the same line and r is the relative standard deviation of the intercept of the y-coordinates. The GCE–AONP–PANI–SWCNT electrode’s limit of detection (LOD), the limit of quantification (LOQ), and sensitivity to lindane were determined to be 2.01 nM, 6.09 nM, and 202.5 µA/µM, respectively
[6]. This shows that the AONP–PANI–SWCNT-modified GCE has a superior electrocatalytic activity for lindane electrooxidation. The results showed that the GCE–AONP–PANI–SWCNT-modified electrode demonstrated anti-interference behavior when detecting lindane in the presence of interfering species (benzene, cyclohexane, phenol, Ca
2+, Fe
2+, K
+, and Mg
2+), with an average current drop of only 12.1 percent on the lindane reduction signal. To determine the practical application of the GCE–AONP–PANI–SWCNT-modified electrode for lindane detection in both river-water and tap-water samples, a real sample analysis was performed. Lindane/water samples with concentrations ranging from 0.5 to 100 M were generated using a 60:40 methanol/river-water mixture containing 0.05 M TBAB, and CV tests were conducted to measure the quantity of lindane in the samples. The recovery value of lindane at lower concentrations was found to be higher than the presence of lindane in river-water samples. The sensor’s kinetic range could be responsible for this result.
3. Voltammetric Detection of Lindane Using Modified Pencil Carbon Electrode
Abdul Rahim Mohd Yusoff
[9] proposed Nylon 6,6 modified graphite HB pencil as a working electrode to detect lindane using differential pulse cathodic stripping voltammetry (DPCSV). Researchers replaced the traditional mercury electrode to avoid the toxicity of mercury with economical HB pencil electrodes with Nylon 6,6 graphite HB pencil electrodes. Pencil graphite electrodes have received a lot of attention because of their low background currents, exceptional sensitivity, repeatability, implementable electroactive surface area, cost-effectiveness, and convenience of discharge. To modify the pencil electrode researchers, use alumina paste, ethanol, and Nylon 6,6 solution. This modified electrode is proved that it has good stability, a good recovery percentage, and a low concentration could be determined. The recovery percentage of pesticides for water sample analysis was calculated using the following Equation.
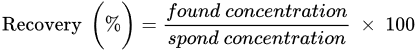
Some parameters are also used to detect lindane such as Ei (effect of initial potential), Each (effect of accumulation potential), and T
acc (time accumulation) using the DPCSV technique. These parameters are best for the current peak of lindane using bare HBPE. The increasing and decreasing value of E
acc (effect of initial accumulation) affects the peak value of lindane. Additionally, the same trend can be observed by using Nyl-MHBPE. Therefore, the redox reaction of lindane is easier and it is more effective to detect the metals which are also present in the sample (Cu
2+, Zn
2+, Pb
2+, Cd
2+, and Fe
3+)
[9].
At pH 10, the peak was shifted towards positive potential, but in the case of Nyl-MHBPE, it shifted towards the negative potential by increasing the pH value. The stripping signal at Nyl-MHBPE was greater as compared to bare HBPE.
4. Electrochemical Reduction of Lindane
There are a number of ways to reduce lindane, but if researchers focus on the electrochemical reduction, it has the property to pick out good oxidizing and reducing agents, so it is very useful to reduce lindane because it is a good reducing agent. The sequential electrochemical reduction of lindane takes place with one electron by expulsing chloride ions.
STEP: 1
In this process weakness, the C-Cl bond cleaves and there will be the formation of an intermediate.
STEP: 2
Free radical ions will be formed in step 2 as a result of the remotion of the chlorine atom.
STEP: 3
The axial C-Cl bond is weakly held and completely broken by two hydrogen bonds. The chloride ion gives a conjugated intermediate.
STEP: 4
After the conjugated intermediate, the C- Cl bond cleaves and forms a radical ion compound.
STEP: 5
The radical ion forms a double bond that leads to the formation of a radical anion.
STEP: 6
Cl atom weakly binds to the benzene ring, the unusual structure forms, and due to the stability of the aromatic compound, the formations of benzene is the major product.
STEP: 7
The minor isomer can be produced from the same conformational isomer after the major product has been formed. The rupture of the C-Cl bond, which is held in a hydrogen-bonded structure, and removal of the chloride ion is given by comparing the whole six electron reduction of one to afford three. This method for the synthesis of 4 is less, so a base is required to approach and abstract an equatorial proton form. Chlorobenzene is a minor result of the reaction
[10].