Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Spinella | -- | 1479 | 2022-07-15 17:10:28 | | | |
| 2 | Jessie Wu | -31 word(s) | 1448 | 2022-07-18 04:25:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Erdogan, Z.M.; Irudayaraj, J.; Spinella, M.J. Potential Mechanisms of Poly- and Perfluoroalkylated Substances Carcinogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/25186 (accessed on 07 February 2026).
Boyd RI, Ahmad S, Singh R, Fazal Z, Prins GS, Erdogan ZM, et al. Potential Mechanisms of Poly- and Perfluoroalkylated Substances Carcinogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/25186. Accessed February 07, 2026.
Boyd, Raya I., Saeed Ahmad, Ratnakar Singh, Zeeshan Fazal, Gail S. Prins, Zeynep Madak Erdogan, Joseph Irudayaraj, Michael J. Spinella. "Potential Mechanisms of Poly- and Perfluoroalkylated Substances Carcinogenesis" Encyclopedia, https://encyclopedia.pub/entry/25186 (accessed February 07, 2026).
Boyd, R.I., Ahmad, S., Singh, R., Fazal, Z., Prins, G.S., Erdogan, Z.M., Irudayaraj, J., & Spinella, M.J. (2022, July 15). Potential Mechanisms of Poly- and Perfluoroalkylated Substances Carcinogenesis. In Encyclopedia. https://encyclopedia.pub/entry/25186
Boyd, Raya I., et al. "Potential Mechanisms of Poly- and Perfluoroalkylated Substances Carcinogenesis." Encyclopedia. Web. 15 July, 2022.
Copy Citation
Poly- and perfluoroalkylated substances (PFAS) are chemicals that persist and bioaccumulate in the environment and are found in nearly all human populations through several routes of exposure. Human occupational and community exposure to PFAS has been associated with several cancers, including cancers of the kidney, testis, prostate, and liver.
epigenetics
PFOA
testicular cancer
1. Introducition
Unlike known carcinogens such as benzo(a)pyrene and UV light that are genotoxic due to direct damage to DNA, there is little evidence that poly- and perfluoroalkylated substances (PFAS) are direct mutagens or deregulators of DNA repair or genomic stability [1][2][3]. However, at high concentrations, PFAS have been demonstrated to damage DNA via reactive oxygen species generation [4][5]. It is unclear if this mechanism is relevant for typical levels of human PFAS exposure. In contrast, most of the evidence for PFAS-mediated effects has focused on epigenetics, transcription, cellular metabolism, and endocrine effects [1][6][7][8][9][10].
2. Metabolism
Metabolic plasticity is one of the hallmarks of cancer [11]. PFAS exposure causes numerous metabolic alterations, through both PPAR-dependent and -independent mechanisms in the liver and other tissues [6][8]. Structurally, PFAS resemble fatty acids (FAs) and there is evidence that PFAS can act as ligands for peroxisome proliferator-activated receptors (PPARs) [12][13]. PPARs are transcription factors with many biological effects beyond their canonical role in controlling lipid and glucose metabolism [14]. Hence, activation of PPARs is an attractive mechanism to explain many of the biological effects of PFAS. The activation of PPARα has been extensively studied as a mechanism of PFAS-mediated liver toxicities, including fibrosis, cirrhosis, steatosis, non-alcoholic fatty acid liver disease, and liver cancer [15][16][17][18]. Similarly, the PFAS activation of PPARs has also been proposed to mediate dyslipidemia (especially high cholesterol), insulin resistance, adipogenesis, and several cancers, including colon, breast, and prostate cancer [6][8][19][20][21][22][23]. Likely related again to a structural similarity with FAs, PFAS are known to accumulate in the liver and have been proposed as altering FA metabolism by binding to FA transporters and metabolic enzymes [6][8]. In contrast to PFAS activation of PPARs, there is less evidence for direct activation by PFAS of other metabolic and xenobiotic nuclear receptors that respond to FAs, including liver X (LXR), farnesoid X (FXR), constitutive androstane (CAR), and pregnane X (PXR). Since altered metabolism is a key feature of the cancer phenotype, the alteration of metabolic regulators such as PPARs offers an attractive mechanism for the proposed pro-carcinogenetic actions of PFAS [11]. Another mechanism related to FA mimicry is the proposed direct effect of PFAS on regulating cell membrane fluidity [24][25]. Published studies demonstrate a central role for PPARα signaling in PFOA/PFOS-induced liver and kidney carcinogenesis [26][27]. In addition, an important role for fatty acid metabolism has been proposed for other cancers including breast, prostate, and colon cancer [28][29][30].
PFOA has been proposed to increase the risk of metabolic syndrome in humans [23]. PFAS alter the hepatic metabolism, with alterations in amino acid biogenesis and the Krebs cycle [31]. In addition, the upregulation of enzymes involved in β-oxidation has been reported upon PFOS exposure [32]. PFOS also induced high peroxisome, endoplasmic reticulum, mitochondria, and membrane protein levels, and deregulated lipid and amino acid metabolism [33][34]. Prenatal exposure to PFAS can contribute to pediatric liver toxicity [35]. A study of 1105 mother-child pairs that assessed multiple PFAS in maternal blood found higher liver enzyme levels of alanine aminotransferase, aspartate aminotransferase and gamma-glutamyl transferase [35]. Furthermore, PFAS levels were associated with alterations in serum amino acid levels in children [36]. In a study of male Chinese subjects, six PFAS were associated with metabolic serum changes associated with oxidative stress [37]. Metabolic stress, as evidenced by metabolites of oxidative DNA damage and lipid peroxidation, has also been documented for both animal and cell line studies for a number of PFAS compounds [20][37]. An additional study of targeted metabolomics found perturbations in branched-chain and aromatic amino acid biosynthesis and glycerophospholipid metabolism and a link between PFAS and increased risk of non-alcoholic steatohepatitis in children [35]. Rodent experiments have shown that early and prenatal PFAS is associated with liver injury in offspring [38][39].
In summary, the activation of PPARs and associated metabolic perturbations, especially in the liver, is one of the most studied mechanisms of PFAS actions. The recent appreciation that many cancers are driven and sustained by metabolic reprogramming underscores the potential importance of this pathway in studying the proposed pro-carcinogenic effects of PFAS. How metabolic reprogramming at the hepatic and cancer cell/cancer progenitor cell level cross-talks with epigenetic and endocrine reprogramming is a key area of future research for understanding the potential carcinogenicity of PFAS (Figure 1).
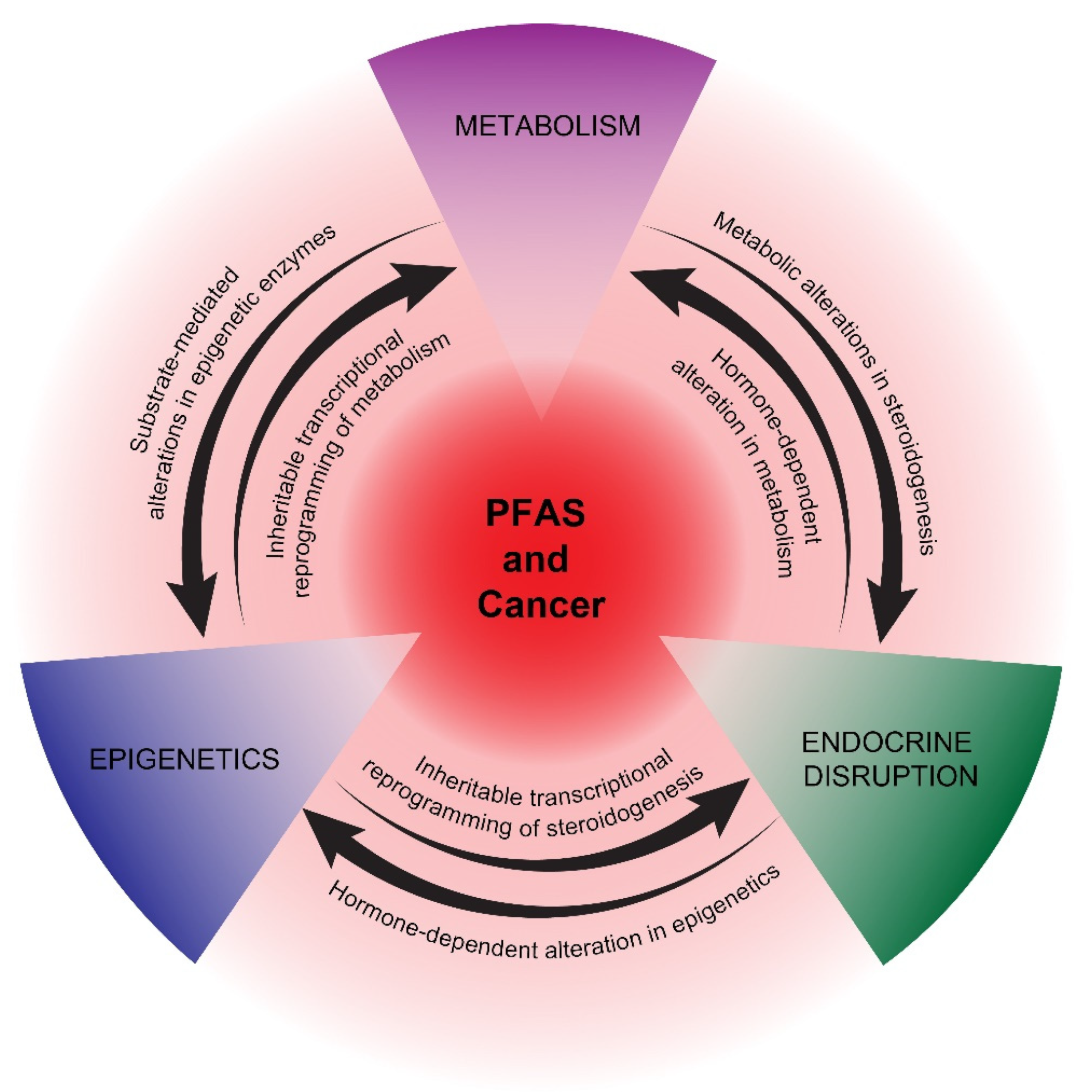
Figure 1. Proposed mechanisms of potential PFAS cancer promotion. PPAR-dependent and -independent reprogramming of metabolism, epigenetics, and endocrine disruption are represented as interconnecting, mutually reinforcing pathways of potential PFAS tumor promotion. The precise details of how PFAS influences these pathways are still uncertain, as is the impact of other proposed PFAS mechanisms, including immunosuppression and oxidative stress.
3. Endocrine Disruption
PFAS cross the placenta and concentrate in breast milk; thus, exposure to the developing fetus and infant occurs [40][41]. PFAS are known to have endocrine-disrupting properties [42][43]. There are reports of adverse reproductive health and decreased fecundity linked to PFAS exposure [44][45]. Human semen quality has decreased over the last several decades. This time period coincides with the rise in production of endocrine-disrupting chemicals (EDCs), and PFAS have been associated with infertility in male mice and subfertility in female mice [46][47]. In several studies, estrogenic and anti-androgen activities were observed for a number of PFAS compounds [48][49][50][51]. There is evidence that PFAS exposure is associated with decreased testosterone and poor sperm quality and numbers in humans [45][52]. For example, in a Japanese study, in utero PFOA and PFOS exposure was associated with decreased testosterone in male neonates [53]. In addition to in human studies, in rodents, PFAS have been observed to alter testosterone and estrogen levels, and were associated with impaired spermatogenesis and steroidogenesis and reduced sperm quality [48][49][50][51], although some inconsistent findings also exist [54]. In female rodents, PFOA alters mammary development [55][56]. PFOA has been associated with changes in the uterus and the reproductive health of female mice [57].
Several cancers associated with PFAS are hormone-dependent, including prostate and breast cancer, or have an etiology closely associated with endocrine disruption, as in testicular cancer [58][59][60][61][62][63][64]. In addition, endometrial cancer has been associated with endocrine disruption [65]. There is evidence that PFAS can alter endocrine hormone levels, potentially leading to disrupted reproductive health, especially with neonatal or pubertal exposure [66][67][68]. A major proposed mechanism of EDCs, in general, is their binding to nuclear receptors [69]. While there is strong evidence supporting the direct activation of PPARs, there is less evidence that PFAS directly activate endocrine receptors, including estrogen (ER) and androgen receptors (AR). Hence, the mechanism of endocrine disruption mediated by PFAS remains unclear, suggesting that indirect mechanisms, including epigenetic and/or metabolic reprogramming, may play roles in disrupting the production and secretion of endocrine hormones during critical windows of exposure [10][70] (Figure 1). In turn, early-life exposure to EDCs has been associated with epigenetic reprogramming that manifests later in life [71].
4. Epigenetics
Despite the likelihood that non-mutagenic, epigenetic pathways play a major role in PFAS biological effects, studies have been sparse, and these have mainly focused on DNA methylation. PFAS have been shown to be associated with both hypomethylation and hypermethylation in genome-wide and gene-specific molecular epidemiology studies [72][73][74][75][76][77][78][79]. PFAS levels have also been linked to decreased and differential DNA methylation in infants [76][77][78]. For example, reduced insulin-like growth factor methylation in cord blood was observed with prenatal PFOA exposure [78]. Another study reported that PFAS exposure was associated with increased long interspersed nuclear element-1 methylation [73]. Associations between PFAS exposures and altered methylation, either genome-wide or at specific loci, have been described in limited in vitro and animal studies, including early life exposures in rodents [80][81][82][83][84][85][86][87][88][89][90]. One study revealed PFOA-mediated hypomethylation of the glutathione-S-transferase Pi gene in liver cells [82]. Significant alterations in DNA methylation have been reported in vitro in HepG2 cells and in vivo in mouse kidney and liver tissues [85][86][87]. Globally, DNA methylation is altered during PFOS-induced fat cell differentiation [83]. Additionally, recent studies have reported PFAS-mediated alterations of epigenetic regulators, such as DNA methyltransferases, ten-eleven translocation methylcytosine dioxygenases, and histone deacetylase enzymes in different mouse organs and human cell lines [80][81][84][91][92][93]. PFAS-mediated effects on histone modifications and microRNAs have also been described [15][80][81][92][94][95][96].
In summary, epigenetics may play a key role in initiating and maintaining potential pro-cancerous states mediated by non-mutagenic PFAS chemicals. Despite this, very few mechanistic studies have been reported. Researchers speculate that epigenetic reprogramming by PFAS may be driven, in part, by metabolomic alterations in substrates and cofactors of epigenetic enzymes and, reciprocally, that epigenetic-mediated, transcriptional reprogramming plays a key role in establishing and stabilizing the metabolic and hormonal states required for continued tumorigenesis [97][98][99][100][101] (Figure 1). This hypothesis is motivated by the above-mentioned association between PFAS and metabolic, epigenetic, and endocrine disruptions and the recent appreciation of mechanistic relationships between these three pathways.
References
- Temkin, A.M.; Hocevar, B.A.; Andrews, D.Q.; Naidenko, O.V.; Kamendulis, L.M. Application of the key characteristics of carcinogens to per and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 1668.
- Crebelli, R.; Caiola, S.; Conti, L.; Cordelli, E.; De Luca, G.; Dellatte, E.; Eleuteri, P.; Iacovella, N.; Leopardi, P.; Marcon, F.; et al. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul. Toxicol. Pharmacol. 2019, 106, 169–177.
- Emerce, E.; Çetin, Ö. Genotoxicity assessment of perfluoroalkyl substances on human sperm. Toxicol. Ind. Health 2018, 34, 884–890.
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245.
- Panaretakis, T.; Shabalina, I.G.; Grandér, D.; Shoshan, M.C.; DePierre, J.W. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol. Appl. Pharmacol. 2001, 173, 56–64.
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630.
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147.
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and emerging per- and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects. Int. J. Mol. Sci. 2021, 22, 995.
- Klaunig, J.E.; Hocevar, B.A.; Kamendulis, L.M. Mode of action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and human relevance. Reprod. Toxicol. 2012, 33, 410–418.
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Vanden Heuvel, J.P.; Thompson, J.T.; Frame, S.R.; Gillies, P.J. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol. Sci. 2006, 92, 476–489.
- Wolf, C.J.; Takacs, M.L.; Schmid, J.E.; Lau, C.; Abbott, B.D. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol. Sci. 2008, 106, 162–171.
- Dixit, G.; Prabhu, A. The pleiotropic peroxisome proliferator activated receptors: Regulation and therapeutics. Exp. Mol. Pathol. 2022, 124, 104723.
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 18866–18875.
- Sheng, N.; Pan, Y.; Guo, Y.; Sun, Y.; Dai, J. Hepatotoxic effects of hexafluoropropylene oxide trimer acid (HFPO-TA), A novel perfluorooctanoic acid (PFOA) alternative, on mice. Environ. Sci. Technol. 2018, 52, 8005–8015.
- Das, K.P.; Wood, C.R.; Lin, M.T.; Starkov, A.A.; Lau, C.; Wallace, K.B.; Corton, J.C.; Abbott, B.D. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 2017, 378, 37–52.
- Chappell, G.A.; Thompson, C.M.; Wolf, J.C.; Cullen, J.M.; Klaunig, J.E.; Haws, L.C. Assessment of the mode of action underlying the effects of GenX in mouse liver and implications for assessing human health risks. Toxicol. Pathol. 2020, 48, 494–508.
- Schlezinger, J.J.; Hyötyläinen, T.; Sinioja, T.; Boston, C.; Puckett, H.; Oliver, J.; Heiger-Bernays, W.; Webster, T.F. Perfluorooctanoic acid induces liver and serum dyslipidemia in humanized PPARα mice fed an American diet. Toxicol. Appl. Pharmacol. 2021, 426, 115644.
- Fragki, S.; Dirven, H.; Fletcher, T.; Grasl-Kraupp, B.; Bjerve Gützkow, K.; Hoogenboom, R.; Kersten, S.; Lindeman, B.; Louisse, J.; Peijnenburg, A.; et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? Crit. Rev. Toxicol. 2021, 51, 141–164.
- Frisbee, S.J.; Brooks, A.P., Jr.; Maher, A.; Flensborg, P.; Arnold, S.; Fletcher, T.; Steenland, K.; Shankar, A.; Knox, S.S.; Pollard, C.; et al. The C8 health project: Design, methods, and participants. Environ. Health Perspect. 2009, 117, 1873–1882.
- Eriksen, K.T.; Raaschou-Nielsen, O.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE 2013, 8, e56969.
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014. Environ. Pollut. 2018, 232, 73–79.
- Hu, W.; Jones, P.D.; DeCoen, W.; King, L.; Fraker, P.; Newsted, J.; Giesy, J.P. Alterations in cell membrane properties caused by perfluorinated compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 77–88.
- Fitzgerald, N.J.M.; Wargenau, A.; Sorenson, C.; Pedersen, J.; Tufenkji, N.; Novak, P.J.; Simcik, M.F. Partitioning and accumulation of perfluoroalkyl substances in model lipid bilayers and bacteria. Environ. Sci. Technol. 2018, 52, 10433–10440.
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. perfluorinated chemicals as emerging environmental threats to kidney health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492.
- Wolf, D.C.; Moore, T.; Abbott, B.D.; Rosen, M.B.; Das, K.P.; Zehr, R.D.; Lindstrom, A.B.; Strynar, M.J.; Lau, C. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-alpha knockout and wild-type mice. Toxicol. Pathol. 2008, 36, 632–639.
- Attané, C.; Milhas, D.; Hoy, A.J.; Muller, C. Metabolic remodeling induced by adipocytes: A new achilles’ heel in invasive breast cancer? Curr. Med. Chem. 2020, 27, 3984–4001.
- Xu, H.; Chen, Y.; Gu, M.; Liu, C.; Chen, Q.; Zhan, M.; Wang, Z. Fatty acid metabolism reprogramming in advanced prostate cancer. Metabolites 2021, 11, 765.
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420.
- Yu, N.; Wei, S.; Li, M.; Yang, J.; Li, K.; Jin, L.; Xie, Y.; Giesy, J.P.; Zhang, X.; Yu, H. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci. Rep. 2016, 6, 23963.
- Tan, F.; Jin, Y.; Liu, W.; Quan, X.; Chen, J.; Liang, Z. Global liver proteome analysis using iTRAQ labeling quantitative proteomic technology to reveal biomarkers in mice exposed to perfluorooctane sulfonate (PFOS). Environ. Sci. Technol. 2012, 46, 12170–12177.
- Domazet, S.L.; Grøntved, A.; Timmermann, A.G.; Nielsen, F.; Jensen, T.K. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: The European Youth Heart Study. Diabetes Care 2016, 39, 1745–1751.
- Alderete, T.L.; Jin, R.; Walker, D.I.; Valvi, D.; Chen, Z.; Jones, D.P.; Peng, C.; Gilliland, F.D.; Berhane, K.; Conti, D.V.; et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ. Int. 2019, 126, 445–453.
- Stratakis, N.; Conti, D.V.; Jin, R.; Margetaki, K.; Valvi, D.; Siskos, A.P.; Maitre, L.; Garcia, E.; Varo, N.; Zhao, Y.; et al. Prenatal exposure to perfluoroalkyl substances associated with increased susceptibility to liver injury in children. Hepatology 2020, 72, 1758–1770.
- Kingsley, S.L.; Walker, D.I.; Calafat, A.M.; Chen, A.; Papandonatos, G.D.; Xu, Y.; Jones, D.P.; Lanphear, B.P.; Pennell, K.D.; Braun, J.M. Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics 2019, 15, 95.
- Wang, X.; Liu, L.; Zhang, W.; Zhang, J.; Du, X.; Huang, Q.; Tian, M.; Shen, H. Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environ. Pollut. 2017, 229, 168–176.
- Lv, Z.; Li, G.; Li, Y.; Ying, C.; Chen, J.; Chen, T.; Wei, J.; Lin, Y.; Jiang, Y.; Wang, Y.; et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ. Toxicol. 2013, 28, 532–542.
- Quist, E.M.; Filgo, A.J.; Cummings, C.A.; Kissling, G.E.; Hoenerhoff, M.J.; Fenton, S.E. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol. Pathol. 2015, 43, 546–557.
- Varsi, K.; Huber, S.; Averina, M.; Brox, J.; Bjørke-Monsen, A.L. Quantitation of linear and branched perfluoroalkane sulfonic acids (PFSAs) in women and infants during pregnancy and lactation. Environ. Int. 2021, 160, 107065.
- Appel, M.; Forsthuber, M.; Ramos, R.; Widhalm, R.; Granitzer, S.; Uhl, M.; Hengstschläger, M.; Stamm, T.; Gundacker, C. The transplacental transfer efficiency of per- and polyfluoroalkyl substances (PFAS): A first meta-analysis. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 23–42.
- Ješeta, M.; Navrátilová, J.; Franzová, K.; Fialková, S.; Kempisty, B.; Ventruba, P.; Žáková, J.; Crha, I. Overview of the mechanisms of action of selected bisphenols and perfluoroalkyl chemicals on the male reproductive axes. Front. Genet. 2021, 12, 692897.
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694.
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031.
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794.
- Wan, H.T.; Zhao, Y.G.; Wong, M.H.; Lee, K.F.; Yeung, W.S.; Giesy, J.P.; Wong, C.K. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol. Reprod. 2011, 84, 1016–1023.
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the star promoter leading to deficits in follicular development and ovulation. Toxicol. Sci. 2015, 148, 368–379.
- Zhao, B.; Li, L.; Liu, J.; Li, H.; Zhang, C.; Han, P.; Zhang, Y.; Yuan, X.; Ge, R.S.; Chu, Y. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE 2014, 9, e78888.
- Jensen, A.A.; Leffers, H. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 2008, 31, 161–169.
- Zhang, H.; Lu, Y.; Luo, B.; Yan, S.; Guo, X.; Dai, J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J. Proteome Res. 2014, 13, 3370–3385.
- López-Doval, S.; Salgado, R.; Pereiro, N.; Moyano, R.; Lafuente, A. Perfluorooctane sulfonate effects on the reproductive axis in adult male rats. Environ. Res. 2014, 134, 158–168.
- Di Nisio, A.; Sabovic, I.; Valente, U.; Tescari, S.; Rocca, M.S.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; Pozzi, N.; Plebani, M.; et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: Clinical and experimental evidence. J. Clin. Endocrinol. Metab 2019, 104, 1259–1271.
- Itoh, S.; Araki, A.; Mitsui, T.; Miyashita, C.; Goudarzi, H.; Sasaki, S.; Cho, K.; Nakazawa, H.; Iwasaki, Y.; Shinohara, N.; et al. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ. Int. 2016, 94, 51–59.
- Song, P.; Li, D.; Wang, X.; Zhong, X. Effects of perfluorooctanoic acid exposure during pregnancy on the reproduction and development of male offspring mice. Andrologia 2018, 50, e13059.
- White, S.S.; Stanko, J.P.; Kato, K.; Calafat, A.M.; Hines, E.P.; Fenton, S.E. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ. Health Perspect. 2011, 119, 1070–1076.
- White, S.S.; Calafat, A.M.; Kuklenyik, Z.; Villanueva, L.; Zehr, R.D.; Helfant, L.; Strynar, M.J.; Lindstrom, A.B.; Thibodeaux, J.R.; Wood, C.; et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007, 96, 133–144.
- Zhang, Y.; Zhang, L.; Bao, J.; Liu, L.; Wang, X. Perfluorooctanoic acid exposure in early pregnancy induces oxidative stress in mice uterus and liver. Environ. Sci. Pollut. Res. Int. 2021, 28, 66355–66365.
- Gilliland, F.D.; Mandel, J.S. Mortality among employees of a perfluorooctanoic acid production plant. J. Occup. Med. 1993, 35, 950–954.
- Lundin, J.I.; Alexander, B.H.; Olsen, G.W.; Church, T.R. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 2009, 20, 921–928.
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318.
- Vieira, V.M.; Hoffman, K.; Shin, H.M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 2013, 121, 318–323.
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst. 2009, 101, 605–609.
- Bonefeld-Jorgensen, E.C.; Long, M.; Bossi, R.; Ayotte, P.; Asmund, G.; Krüger, T.; Ghisari, M.; Mulvad, G.; Kern, P.; Nzulumiki, P.; et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: A case control study. Environ. Health 2011, 10, 88.
- Bonefeld-Jørgensen, E.C.; Long, M.; Fredslund, S.O.; Bossi, R.; Olsen, J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: A case-control study nested in the Danish National Birth Cohort. Cancer Causes Control. 2014, 25, 1439–1448.
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine disrupting chemicals and endometrial cancer: An overview of recent laboratory evidence and epidemiological studies. Int. J. Environ. Res. Public Health 2017, 14, 334.
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Kristensen, S.L.; Halldorsson, T.I.; Becher, G.; Haug, L.S.; Ernst, E.H.; Toft, G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Perspect. 2013, 121, 453–458.
- Lopez-Espinosa, M.J.; Mondal, D.; Armstrong, B.G.; Eskenazi, B.; Fletcher, T. perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6-9 years of age: A cross-sectional analysis within the C8 Health Project. Environ. Health Perspect. 2016, 124, 1269–1275.
- Tsai, M.S.; Lin, C.Y.; Lin, C.C.; Chen, M.H.; Hsu, S.H.; Chien, K.L.; Sung, F.C.; Chen, P.C.; Su, T.C. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int. J. Hyg. Environ. Health 2015, 218, 437–443.
- Toporova, L.; Balaguer, P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol. Cell Endocrinol. 2020, 502, 110665.
- Margolis, R.; Sant, K.E. Associations between Exposures to Perfluoroalkyl Substances and Diabetes, Hyperglycemia, or Insulin Resistance: A Scoping Review. J. Xenobiot. 2021, 11, 115–129.
- Walker, C.L. Minireview: Epigenomic plasticity and vulnerability to EDC exposures. Mol. Endocrinol. 2016, 30, 848–855.
- van den Dungen, M.W.; Murk, A.J.; Kampman, E.; Steegenga, W.T.; Kok, D.E. Association between DNA methylation profiles in leukocytes and serum levels of persistent organic pollutants in Dutch men. Environ. Epigenet. 2017, 3, dvx001.
- Watkins, D.J.; Wellenius, G.A.; Butler, R.A.; Bartell, S.M.; Fletcher, T.; Kelsey, K.T. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ. Int. 2014, 63, 71–76.
- Guerrero-Preston, R.; Goldman, L.R.; Brebi-Mieville, P.; Ili-Gangas, C.; Lebron, C.; Witter, F.R.; Apelberg, B.J.; Hernández-Roystacher, M.; Jaffe, A.; Halden, R.U.; et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics 2010, 5, 539–546.
- Kingsley, S.L.; Kelsey, K.T.; Butler, R.; Chen, A.; Eliot, M.N.; Romano, M.E.; Houseman, A.; Koestler, D.C.; Lanphear, B.P.; Yolton, K.; et al. Maternal serum PFOA concentration and DNA methylation in cord blood: A pilot study. Environ. Res. 2017, 158, 174–178.
- Leung, Y.K.; Ouyang, B.; Niu, L.; Xie, C.; Ying, J.; Medvedovic, M.; Chen, A.; Weihe, P.; Valvi, D.; Grandjean, P.; et al. Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 2018, 13, 290–300.
- Miura, R.; Araki, A.; Miyashita, C.; Kobayashi, S.; Kobayashi, S.; Wang, S.L.; Chen, C.H.; Miyake, K.; Ishizuka, M.; Iwasaki, Y.; et al. An epigenome-wide study of cord blood DNA methylations in relation to prenatal perfluoroalkyl substance exposure: The Hokkaido study. Environ. Int. 2018, 115, 21–28.
- Kobayashi, S.; Azumi, K.; Goudarzi, H.; Araki, A.; Miyashita, C.; Kobayashi, S.; Itoh, S.; Sasaki, S.; Ishizuka, M.; Nakazawa, H.; et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido Study. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 251–259.
- Liu, C.Y.; Chen, P.C.; Lien, P.C.; Liao, Y.P. Prenatal perfluorooctyl sulfonate exposure and Alu DNA hypomethylation in cord blood. Int. J. Environ. Res Public Health 2018, 15, 1066.
- Guo, X.X.; He, Q.Z.; Li, W.; Long, D.X.; Pan, X.Y.; Chen, C.; Zeng, H.C. Brain-derived neurotrophic factor mediated perfluorooctane sulfonate induced-neurotoxicity via epigenetics regulation in SK-N-SH cells. Int. J. Mol. Sci. 2017, 18, 893.
- Sonkar, R.; Kay, M.K.; Choudhury, M. PFOS modulates interactive epigenetic regulation in first-trimester human trophoblast cell line HTR-8/SV(neo). Chem. Res. Toxicol. 2019, 32, 2016–2027.
- Tian, M.; Peng, S.; Martin, F.L.; Zhang, J.; Liu, L.; Wang, Z.; Dong, S.; Shen, H. Perfluorooctanoic acid induces gene promoter hypermethylation of glutathione-S-transferase Pi in human liver L02 cells. Toxicology 2012, 296, 48–55.
- van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol. Vitr. 2017, 40, 79–87.
- Ma, Y.; Yang, J.; Wan, Y.; Peng, Y.; Ding, S.; Li, Y.; Xu, B.; Chen, X.; Xia, W.; Ke, Y.; et al. Low-level perfluorooctanoic acid enhances 3 T3-L1 preadipocyte differentiation via altering peroxisome proliferator activated receptor gamma expression and its promoter DNA methylation. J. Appl. Toxicol. 2018, 38, 398–407.
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol. Rep. 2020, 7, 125–132.
- Wen, Y.; Chen, J.; Li, J.; Arif, W.; Kalsotra, A.; Irudayaraj, J. Effect of PFOA on DNA methylation and alternative splicing in mouse liver. Toxicol. Lett. 2020, 329, 38–46.
- Wen, Y.; Mirji, N.; Irudayaraj, J. Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol. Vitr. 2020, 65, 104797.
- Liu, W.; Irudayaraj, J. Perfluorooctanoic acid (PFOA) exposure inhibits DNA methyltransferase activities and alters constitutive heterochromatin organization. Food Chem. Toxicol. 2020, 141, 111358.
- Ahmad, S.; Wen, Y.; Irudayaraj, J.M.K. PFOA induces alteration in DNA methylation regulators and SARS-CoV-2 targets Ace2 and Tmprss2 in mouse lung tissues. Toxicol. Rep. 2021, 8, 1892–1898.
- Wan, Y.J.; Li, Y.Y.; Xia, W.; Chen, J.; Lv, Z.Q.; Zeng, H.C.; Zhang, L.; Yang, W.J.; Chen, T.; Lin, Y.; et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology 2010, 274, 57–64.
- Tian, J.; Xu, H.; Zhang, Y.; Shi, X.; Wang, W.; Gao, H.; Bi, Y. SAM targeting methylation by the methyl donor, a novel therapeutic strategy for antagonize PFOS transgenerational fertility toxicity. Ecotoxicol. Environ. Saf 2019, 184, 109579.
- Rashid, F.; Ahmad, S.; Irudayaraj, J.M.K. Effect of Perfluorooctanoic acid on the epigenetic and tight junction genes of the mouse intestine. Toxics 2020, 8, 64.
- Jabeen, M.; Fayyaz, M.; Irudayaraj, J. Epigenetic modifications, and alterations in cell cycle and apoptosis pathway in A549 lung carcinoma cell line upon exposure to perfluoroalkyl substances. Toxics 2020, 8, 112.
- Dong, H.; Curran, I.; Williams, A.; Bondy, G.; Yauk, C.L.; Wade, M.G. Hepatic miRNA profiles and thyroid hormone homeostasis in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). Environ. Toxicol. Pharmacol. 2016, 41, 201–210.
- Wang, J.; Zhang, Y.; Zhang, W.; Jin, Y.; Dai, J. Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents. Environ. Sci. Technol. 2012, 46, 9274–9281.
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.Y.; Liu, Y.J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.Y.; Prins, G.S.; Madak Erdogan, Z. Per- and polyfluoroalkyl substance exposure combined with high-fat diet supports prostate cancer progression. Nutrients 2021, 13, 3902.
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, 5473.
- Chandra, V.; Hong, K.M. Effects of deranged metabolism on epigenetic changes in cancer. Arch. Pharm. Res. 2015, 38, 321–337.
- Boukouris, A.E.; Zervopoulos, S.D.; Michelakis, E.D. metabolic enzymes moonlighting in the nucleus: Metabolic regulation of gene transcription. Trends Biochem. Sci. 2016, 41, 712–730.
- Fleisch, A.F.; Wright, R.O.; Baccarelli, A.A. Environmental epigenetics: A role in endocrine disease? J. Mol. Endocrinol. 2012, 49, R61–R67.
- Feroe, A.; Broene, R.; Albuquerque, D.; Ruiz, P. Endocrine disrupting chemicals, transgenerational epigenetics and metabolic diseases. EC Endocrinol. Metab. Res. 2017, 21, 31–51.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
18 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




