| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Noriyuki Miyoshi | -- | 2412 | 2022-07-15 01:17:45 | | | |
| 2 | Jessie Wu | Meta information modification | 2412 | 2022-07-15 04:23:34 | | | | |
| 3 | Jessie Wu | Meta information modification | 2412 | 2022-07-15 04:32:41 | | | | |
| 4 | Jessie Wu | + 10 word(s) | 2422 | 2022-07-15 04:34:53 | | |
Video Upload Options
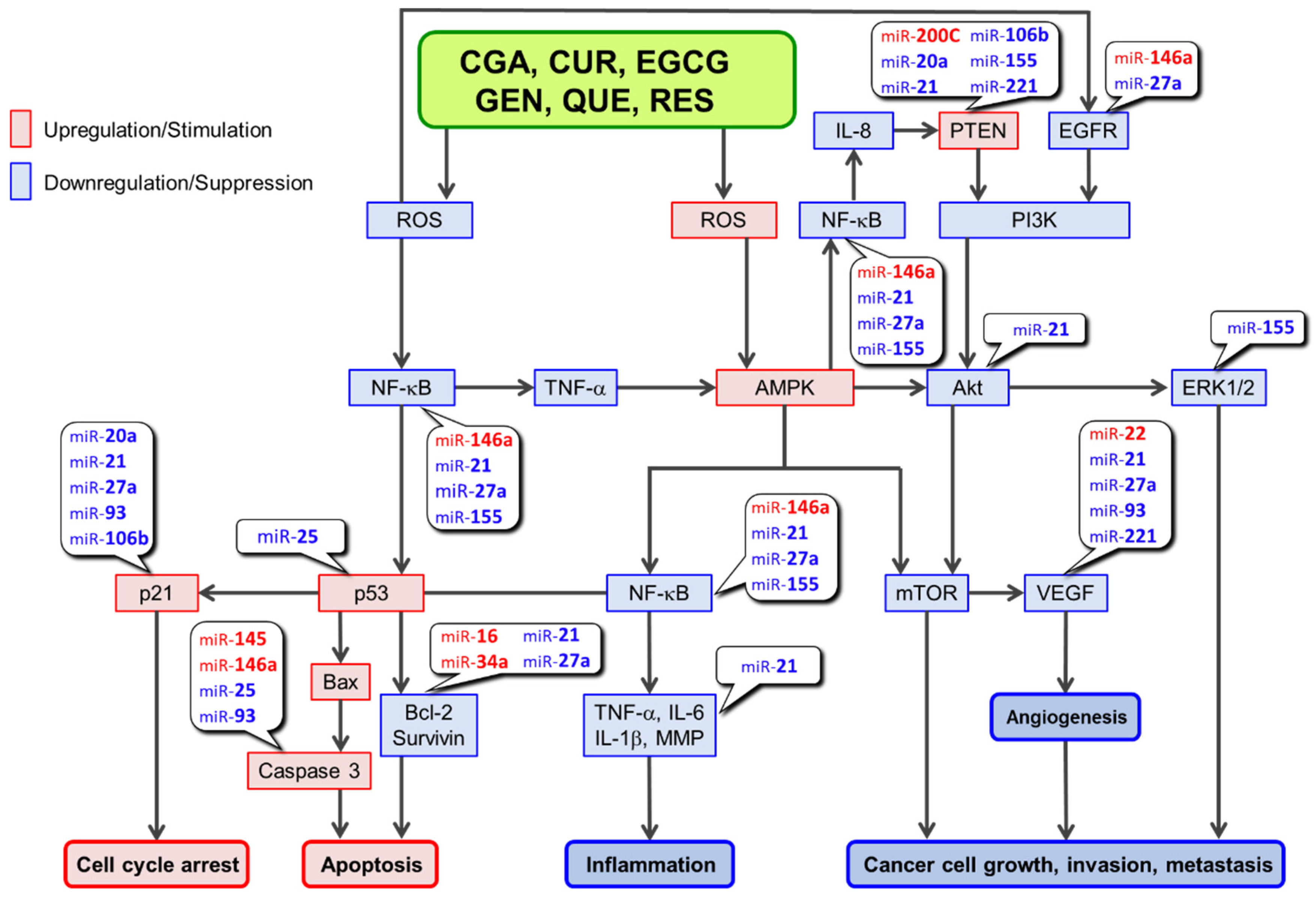
Consumption of coffee, tea, wine, curry, and soybeans has been linked to a lower risk of cancer in epidemiological studies. Several cell-based and animal studies have shown that dietary polyphenols like chlorogenic acid, curcumin, epigallocatechin-3-O-gallate, genistein, quercetin and resveratrol play a major role in these anticancer effects. Several mechanisms have been proposed to explain the anticancer effects of polyphenols. Depending on the cellular microenvironment, these polyphenols can exert double-faced actions as either an antioxidant or a prooxidant, and one of the representative anticancer mechanisms is a reactive oxygen species (ROS)-mediated mechanism. These polyphenols can also influence microRNA (miR) expression. In general, they can modulate the expression/activity of the constituent molecules in ROS-mediated anticancer pathways by increasing the expression of tumor-suppressive miRs and decreasing the expression of oncogenic miRs.
1. Introduction
| Polyphenol | Major Food Source |
|---|---|
| Chlorogenic acid (CGA) | Coffee bean |
| (−)-Epigallocatechin gallate (EGCG) | Green tea |
| Resveratrol (RES) | Red wine |
| Curcumin (CUR) | Curry |
| Quercetin (QUE) | Onion |
| Genistein (GEN) | Soy |
2. Anticancer Mechanism of Tumor Suppressor miRs Upregulated by Polyphenols
| miR | CUR | EGCG | GEN | QUE | RES | Effects of miRs Upregulated by Polyphenols on Molecules in the ROS-Mediated Pathway: ↑, Upregulation; ↓ Downregulation |
|---|---|---|---|---|---|---|
| miR-16 | MCF-7 (breast cancer) (Yang, et al.) [17] |
HepG2 (liver cancer) (Tsang, et al.) [18] |
A549 (lung cancer) (Sonoki, et al.) [19] HSC-6 SCC-9 (oral cancer) (Zhao, et al.) [20] |
MCF7-ADR MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [21] CCRF-CEM (acute lymphoblastic leukemia) (Azimi, et al.) [22] |
↓Bcl-2 [17][18] | |
| miR-22 | BxPC-3 (pancreatic carcinoma) (Sun, et al.) [23] Y79 (retinoblastoma) (Sreenivasan, et al.) [24] Downregulated * MyLa2059, SeAx (malignant cutaneous lymphoma) (Sibbesen, et al.) [25] |
CNE2 (nasopharyngeal carcinoma) (Li, et al.) [26] |
Tca8113 SAS (oral squamous cell carcinoma) (Zhang, et al.) [27] |
↓VEGF via↓Sp1 [23] | ||
| miR-34a | MDA-MB-231 MDA-MB-435 (breast cancer) (Guo, et al.) [28] SGC-7901 (gastric cancer) (Sun, et al.) [29] HCT116 (colorectal cancer) (Toden, et al.) [30] BxPC-3 (pancreatic cancer) (Sun, et al.) [23] Downregulated * TE-7 (esophageal adenocarcinoma) (Subramaniam, et al.) [31] |
SK-N-BE2 IMR-32 (malignant neuroblastoma) (Chakrabarti, et al.) [32] SH-SY5Y SK-N-DZ (malignant neuroblastoma) (Chakrabarti, et al.) [33] HCT116 HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [34] CNE2 (nasopharyngeal carcinoma) (Li, et al.) [26] HepG2 (hepatocellular carcinoma) (Mostafa, et al.) [35] |
HNC-TICs (tumor-initiating cells of head and neck cancer) (Hsieh, et al.) [36] DU145 (prostate cancer) (Chiyomaru, et al.) [37] AsPC-1 MiaPaCa-2 (pancreatic cancer) (Xia, et al.) [38] |
MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [21] DLD-1 (colon cancer) (Kumazaki, et al.) [39] MCF-7 (breast cancer) (Otsuka, et al.) [40] SKOV-3 OV-90 (ovarian cancer) (Yao, et al.) [41] |
↓Bcl-2 [28][29][30][41] ↓NF-κB via Notch-1 [38] |
|
| miR-141 | HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [42] |
Downregulated * MM1.s (multiple myeloma) (Gordon, et al.) [43] |
786-O ACHN (renal carcinoma) (Chiyomaru, et al.) [44] |
MCF7-ADR MCF-7 MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [21] |
||
| miR-145 | U-87 MG (glioblastoma) Mirgani, et al.) [45] DU145 22RV1 (prostate cancer) (Liu, et al.) [46] |
HCT116 HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [34] |
Y79 (retinoblastoma) (Wei, et al.) [47] |
SKOV-3 A2780 (ovarian cancer) (Zhou, et al.) [48] |
BT-549 MDA-MB-231 MCF-7 (breast cancer) (Sachdeva, et al.) [49] |
↑Caspase-3 [48] |
| miR-146a | U-87 MG (glioblastoma) (Wu, et al.) [50] AsPC-1 (pancreatic cancer) CDF (analog) (Bao, et al.) [51] |
Colo357 Panc-1 (pancreatic cancer) G2535 (mixture of genistein and other isoflavones) (Li, et al.) [52] |
MCF-7 MDA-MB-231 (breast cancer) (Tao, et al.) [53] |
↓NF-κB [50] ↑Caspase-3 [53] ↓EGFR [53] |
||
| miR-200c | HCT116-5FUR SW480-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [42] MiaPaCa-2 MiaPaCa-2-GR BxPC-3 (pancreatic cancer) CDF (analog) (Soubani, et al.) [54] |
HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [34] |
Cancer stem cells of nasopharyngeal carcinoma (Shen, et al.) [55] MCF7-ADR MCF-7 MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [21] HCT116 (colorectal cancer) (Dermani, et al.) [56] |
↑PTEN [54] |
2.1. miR-16
2.2. miR-22
2.3. miR-34a
2.4. miR-141
2.5. miR-145
2.6. miR-146a
2.7. miR-200c
References
- Wahle, K.W.J.; Brown, I.; Rotondo, D.; Heys, S.D. Plant Phenolics in the Prevention and Treatment of Cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51.
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370.
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of MicroRNA Modifications in Anticancer Effects of Major Polyphenols from Green Tea, Coffee, Wine, and Curry. Crit. Rev. Food Sci. Nutr. 2022, 1–32.
- Tanabe, H.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Plant Polyphenols on Obesity. Obes. Control Ther. 2017, 4, 1–16.
- Lam, T.K.; Rotunno, M.; Lubin, J.H.; Wacholder, S.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Chanock, S.J.; Burdette, L.; Goldstein, A.M.; et al. Dietary Quercetin, Quercetin-Gene Interaction, Metabolic Gene Expression in Lung Tissue and Lung Cancer Risk. Carcinogenesis 2010, 31, 634–642.
- Bandera, E.V.; Williams, M.G.; Sima, C.; Bayuga, S.; Pulick, K.; Wilcox, H.; Soslow, R.; Zauber, A.G.; Olson, S.H. Phytoestrogen Consumption and Endometrial Cancer Risk: A Population-Based Case-Control Study in New Jersey. Cancer Causes Control 2009, 20, 1117–1127.
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary Quercetin Intake and Risk of Gastric Cancer: Results from a Population-Based Study in Sweden. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 438–443.
- Woo, H.D.; Kim, J. Dietary Flavonoid Intake and Smoking-Related Cancer Risk: A Meta-Analysis. PLoS ONE 2013, 8, e75604.
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy Food Consumption and Risk of Prostate Cancer: A Meta-Analysis of Observational Studies. Nutr. Cancer 2009, 61, 598–606.
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419.
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863.
- Wang, Q.; Huang, H.; Zhao, N.; Ni, X.; Udelsman, R.; Zhang, Y. Phytoestrogens and Thyroid Cancer Risk: A Population-Based Case-Control Study in Connecticut. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 500–508.
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 40.
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439.
- Shahinfar, H.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Coffee Consumption and Cardiovascular Diseases and Mortality in Patients with Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2526–2538.
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442.
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin Reduces the Expression of Bcl-2 by Upregulating MiR-15a and MiR-16 in MCF-7 Cells. Med. Oncol. 2010, 27, 1114–1118.
- Tsang, W.P.; Kwok, T.T. Epigallocatechin Gallate Up-Regulation of MiR-16 and Induction of Apoptosis in Human Cancer Cells. J. Nutr. Biochem. 2010, 21, 140–146.
- Sonoki, H.; Sato, T.; Endo, S.; Matsunaga, T.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of MicroRNA MiR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 2015, 7, 4578–4592.
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin Inhibits Cell Viability, Migration and Invasion by Regulating MiR-16/HOXA10 Axis in Oral Cancer. Eur. J. Pharmacol. 2019, 847, 11–18.
- Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.-U.; Takeshita, F.; Ochiya, T. Stilbene Derivatives Promote Ago2-Dependent Tumour-Suppressive MicroRNA Activity. Sci. Rep. 2012, 2, 314.
- Azimi, A.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Hossein pour feizi, A.A.; Baradaran, B.; Movassaghpour, A.A.; Shamsasenjan, K.; Khanzedeh, T.; Ghaderi, A.H.; et al. Time-and Concentration-Dependent Effects of Resveratrol on MiR 15a and MiR16-1 Expression and Apoptosis in the CCRF-CEM Acute Lymphoblastic Leukemia Cell Line. Asian Pac. J. Cancer Prev. 2015, 16, 6463–6468.
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (Diferuloylmethane) Alters the Expression Profiles of MicroRNAs in Human Pancreatic Cancer Cells. Mol. Cancer Ther. 2008, 7, 464–473.
- Sreenivasan, S.; Thirumalai, K.; Danda, R.; Krishnakumar, S. Effect of Curcumin on MiRNA Expression in Human Y79 Retinoblastoma Cells. Curr. Eye Res. 2012, 37, 421–428.
- Sibbesen, N.A.; Kopp, K.L.; Litvinov, I.V.; Jønson, L.; Willerslev-Olsen, A.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Krejsgaard, T.; Lindahl, L.M.; et al. Jak3, STAT3, and STAT5 Inhibit Expression of MiR-22, a Novel Tumor Suppressor MicroRNA, in Cutaneous T-Cell Lymphoma. Oncotarget 2015, 6, 20555–20569.
- Li, B.-B.; Huang, G.-L.; Li, H.-H.; Kong, X.; He, Z.-W. Epigallocatechin-3-Gallate Modulates MicroRNA Expression Profiles in Human Nasopharyngeal Carcinoma CNE2 Cells. Chin. Med. J. 2017, 130, 93–99.
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin Suppresses the Tumorigenesis of Oral Squamous Cell Carcinoma by Regulating MicroRNA-22/WNT1/β-Catenin Axis. J. Pharmacol. Sci. 2019, 140, 128–136.
- Guo, J.; Li, W.; Shi, H.; Xie, X.; Li, L.; Tang, H.; Wu, M.; Kong, Y.; Yang, L.; Gao, J.; et al. Synergistic Effects of Curcumin with Emodin against the Proliferation and Invasion of Breast Cancer Cells through Upregulation of MiR-34a. Mol. Cell. Biochem. 2013, 382, 103–111.
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted MiR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641.
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of MiR-34a and MiR-27a in Colorectal Cancer. Cancer Prev. Res. 2015, 8, 431–443.
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin Induces Cell Death in Esophageal Cancer Cells through Modulating Notch Signaling. PLoS ONE 2012, 7, e30590.
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in Expression of Specific MicroRNAs by Combination of 4-HPR and EGCG Inhibited Growth of Human Malignant Neuroblastoma Cells. Brain Res. 2012, 1454, 1–13.
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of MiR-7-1 Increases Efficacy of Green Tea Polyphenols for Induction of Apoptosis in Human Malignant Neuroblastoma SH-SY5Y and SK-N-DZ Cells. Neurochem. Res. 2013, 38, 420–432.
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget 2016, 7, 16158–16171.
- Mostafa, S.M.; Gamal-Eldeen, A.M.; Maksoud, N.A.E.; Fahmi, A.A. Epigallocatechin Gallate-Capped Gold Nanoparticles Enhanced the Tumor Suppressors Let-7a and MiR-34a in Hepatocellular Carcinoma Cells. An. Acad. Bras. Cienc. 2020, 92, e20200574.
- Hsieh, P.-L.; Liao, Y.-W.; Hsieh, C.-W.; Chen, P.-N.; Yu, C.-C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating MiR-34a/RTCB Axis. Nutrients 2020, 12, 1924.
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting MiR-34a and Oncogenic HOTAIR. PLoS ONE 2013, 8, e70372.
- Xia, J.; Duan, Q.; Ahmad, A.; Bao, B.; Banerjee, S.; Shi, Y.; Ma, J.; Geng, J.; Chen, Z.; Rahman, K.M.W.; et al. Genistein Inhibits Cell Growth and Induces Apoptosis through Up-Regulation of MiR-34a in Pancreatic Cancer Cells. Curr. Drug Targets 2012, 13, 1750–1756.
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-Cancer Effects of Naturally Occurring Compounds through Modulation of Signal Transduction and MiRNA Expression in Human Colon Cancer Cells. J. Nutr. Biochem. 2013, 24, 1849–1858.
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory Role of Resveratrol, a MicroRNA-Controlling Compound, in HNRNPA1 Expression, Which Is Associated with Poor Prognosis in Breast Cancer. Oncotarget 2018, 9, 24718–24730.
- Yao, S.; Gao, M.; Wang, Z.; Wang, W.; Zhan, L.; Wei, B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med. J. 2021, 62, 691–701.
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin Mediates Chemosensitization to 5-Fluorouracil through MiRNA-Induced Suppression of Epithelial-to-Mesenchymal Transition in Chemoresistant Colorectal Cancer. Carcinogenesis 2015, 36, 355–367.
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of P53-Targeting MicroRNAs by Polycyclic Aromatic Hydrocarbons: Implications in the Etiology of Multiple Myeloma. Mol. Carcinog. 2015, 54, 1060–1069.
- Chiyomaru, T.; Fukuhara, S.; Saini, S.; Majid, S.; Deng, G.; Shahryari, V.; Chang, I.; Tanaka, Y.; Enokida, H.; Nakagawa, M.; et al. Long Non-Coding RNA HOTAIR Is Targeted and Regulated by MiR-141 in Human Cancer Cells. J. Biol. Chem. 2014, 289, 12550–12565.
- Tahmasebi Mirgani, M.; Isacchi, B.; Sadeghizadeh, M.; Marra, F.; Bilia, A.R.; Mowla, S.J.; Najafi, F.; Babaei, E. Dendrosomal Curcumin Nanoformulation Downregulates Pluripotency Genes via MiR-145 Activation in U87MG Glioblastoma Cells. Int. J. Nanomedicine 2014, 9, 403–417.
- Liu, T.; Chi, H.; Chen, J.; Chen, C.; Huang, Y.; Xi, H.; Xue, J.; Si, Y. Curcumin Suppresses Proliferation and in Vitro Invasion of Human Prostate Cancer Stem Cells by CeRNA Effect of MiR-145 and LncRNA-ROR. Gene 2017, 631, 29–38.
- Wei, D.; Yang, L.; Lv, B.; Chen, L. Genistein Suppresses Retinoblastoma Cell Viability and Growth and Induces Apoptosis by Upregulating MiR-145 and Inhibiting Its Target ABCE1. Mol. Vis. 2017, 23, 385–394.
- Zhou, J.; Gong, J.; Ding, C.; Chen, G. Quercetin Induces the Apoptosis of Human Ovarian Carcinoma Cells by Upregulating the Expression of MicroRNA-145. Mol. Med. Rep. 2015, 12, 3127–3131.
- Sachdeva, M.; Liu, Q.; Cao, J.; Lu, Z.; Mo, Y.-Y. Negative Regulation of MiR-145 by C/EBP-β through the Akt Pathway in Cancer Cells. Nucleic Acids Res. 2012, 40, 6683–6692.
- Wu, H.; Liu, Q.; Cai, T.; Chen, Y.-D.; Wang, Z.-F. Induction of MicroRNA-146a Is Involved in Curcumin-Mediated Enhancement of Temozolomide Cytotoxicity against Human Glioblastoma. Mol. Med. Rep. 2015, 12, 5461–5466.
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin Analogue CDF Inhibits Pancreatic Tumor Growth by Switching on Suppressor MicroRNAs and Attenuating EZH2 Expression. Cancer Res. 2012, 72, 335–345.
- Li, Y.; Vandenboom, T.G.; Wang, Z.; Kong, D.; Ali, S.; Philip, P.A.; Sarkar, F.H. MiR-146a Suppresses Invasion of Pancreatic Cancer Cells. Cancer Res. 2010, 70, 1486–1495.
- Tao, S.-F.; He, H.-F.; Chen, Q. Quercetin Inhibits Proliferation and Invasion Acts by Up-Regulating MiR-146a in Human Breast Cancer Cells. Mol. Cell. Biochem. 2015, 402, 93–100.
- Soubani, O.; Ali, A.S.; Logna, F.; Ali, S.; Philip, P.A.; Sarkar, F.H. Re-Expression of MiR-200 by Novel Approaches Regulates the Expression of PTEN and MT1-MMP in Pancreatic Cancer. Carcinogenesis 2012, 33, 1563–1571.
- Shen, Y.-A.; Lin, C.-H.; Chi, W.-H.; Wang, C.-Y.; Hsieh, Y.-T.; Wei, Y.-H.; Chen, Y.-J. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through P53 Activation. Evid. Based Complement. Alternat. Med. 2013, 2013, 590393.
- Karimi Dermani, F.; Saidijam, M.; Amini, R.; Mahdavinezhad, A.; Heydari, K.; Najafi, R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing MiR-200c Expression in HCT-116 Colorectal Cancer Cells. J. Cell Biochem. 2017, 118, 1547–1555.
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553.
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529.
- Vellingiri, B.; Iyer, M.; Devi Subramaniam, M.; Jayaramayya, K.; Siama, Z.; Giridharan, B.; Narayanasamy, A.; Abdal Dayem, A.; Cho, S.-G. Understanding the Role of the Transcription Factor Sp1 in Ovarian Cancer: From Theory to Practice. Int. J. Mol. Sci. 2020, 21, 1153.
- Huang, C.; Xie, K. Crosstalk of Sp1 and Stat3 Signaling in Pancreatic Cancer Pathogenesis. Cytokine Growth Factor Rev. 2012, 23, 25–35.
- Su, F.; Geng, J.; Li, X.; Qiao, C.; Luo, L.; Feng, J.; Dong, X.; Lv, M. SP1 Promotes Tumor Angiogenesis and Invasion by Activating VEGF Expression in an Acquired Trastuzumab-resistant Ovarian Cancer Model. Oncol. Rep. 2017, 38, 2677–2684.
- Wang, Q.; Wang, J.; Xiang, H.; Ding, P.; Wu, T.; Ji, G. The Biochemical and Clinical Implications of Phosphatase and Tensin Homolog Deleted on Chromosome Ten in Different Cancers. Am. J. Cancer Res. 2021, 11, 5833–5855.
- Lee, Y.-S.; Lim, K.-H.; Guo, X.; Kawaguchi, Y.; Gao, Y.; Barrientos, T.; Ordentlich, P.; Wang, X.-F.; Counter, C.M.; Yao, T.-P. The Cytoplasmic Deacetylase HDAC6 Is Required for Efficient Oncogenic Tumorigenesis. Cancer Res. 2008, 68, 7561–7569.
- Qin, L.; Wu, Y.-L.; Toneff, M.J.; Li, D.; Liao, L.; Gao, X.; Bane, F.T.; Tien, J.C.-Y.; Xu, Y.; Feng, Z.; et al. NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Res. 2014, 74, 3477–3488.
- Jiang, J.; Wang, S.; Wang, Z.; Cai, J.; Han, L.; Xie, L.; Han, Q.; Wang, W.; Zhang, Y.; He, X.; et al. HOTAIR Promotes Paclitaxel Resistance by Regulating CHEK1 in Ovarian Cancer. Cancer Chemother. Pharmacol. 2020, 86, 295–305.
- Yu, L.; Li, W. Abnormal Activation of Notch 1 Signaling Causes Apoptosis Resistance in Cervical Cancer. Int. J. Clin. Exp. Pathol. 2022, 15, 11–19.
- Cui, L.; Dong, Y.; Wang, X.; Zhao, X.; Kong, C.; Liu, Y.; Jiang, X.; Zhang, X. Downregulation of Long Noncoding RNA SNHG1 Inhibits Cell Proliferation, Metastasis, and Invasion by Suppressing the Notch-1 Signaling Pathway in Pancreatic Cancer. J. Cell Biochem. 2019, 120, 6106–6112.
- Kiesel, V.A.; Stan, S.D. Modulation of Notch Signaling Pathway by Bioactive Dietary Agents. Int. J. Mol. Sci. 2022, 23, 3532.
- Alemohammad, H.; Asadzadeh, Z.; Motafakker Azad, R.; Hemmat, N.; Najafzadeh, B.; Vasefifar, P.; Najafi, S.; Baradaran, B. Signaling Pathways and MicroRNAs, the Orchestrators of NANOG Activity during Cancer Induction. Life Sci. 2020, 260, 118337.