Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuji Mizumoto | -- | 1677 | 2022-07-14 08:34:15 | | | |
| 2 | Amina Yu | -98 word(s) | 1579 | 2022-07-14 08:48:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mizumoto, S.; Yamada, S. Dermatan Sulfate in Tissue Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/25134 (accessed on 05 March 2026).
Mizumoto S, Yamada S. Dermatan Sulfate in Tissue Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/25134. Accessed March 05, 2026.
Mizumoto, Shuji, Shuhei Yamada. "Dermatan Sulfate in Tissue Development" Encyclopedia, https://encyclopedia.pub/entry/25134 (accessed March 05, 2026).
Mizumoto, S., & Yamada, S. (2022, July 14). Dermatan Sulfate in Tissue Development. In Encyclopedia. https://encyclopedia.pub/entry/25134
Mizumoto, Shuji and Shuhei Yamada. "Dermatan Sulfate in Tissue Development." Encyclopedia. Web. 14 July, 2022.
Copy Citation
The crucial roles of dermatan sulfate (DS) have been demonstrated in tissue development of the cutis, blood vessels, and bone through construction of the extracellular matrix and cell signaling. DS classically exerts physiological functions via interaction with collagens, growth factors, and heparin cofactor-II.
biglycan
carbohydrate sulfotransferase 14
decorin
dermatan sulfate
1. Introduction
Dermatan sulfate (DS) was initially isolated from skin by Karl Myer in 1941 [1]. Although dermatan sulfate-proteoglycans (DS-PGs) ubiquitously exist in various tissues, they distribute abundantly throughout skin, cartilage, and the aorta [2]. DS-PGs play important roles in anti-coagulation, binding to growth factors, wound healing, in the assembly of extracellular matrices, in tissue morphogenesis, and neuronal homeostasis [3][4][5][6][7][8][9]. DS side chains are linear polysaccharides covalently attached to core proteins that form PGs [2][10]. DS chains consist of alternating disaccharide units comprising N-acetyl-D-galactosamine (GalNAc) and L-iduronic acid (IdoA) residues with 50–200 repeats (Figure 1), constructed by specific glycosyltransferases and epimerase [3]. The repeating disaccharide region of DS undergoes maturation by sulfation at the C-4 and C-2 positions on GalNAc and IdoA residues, respectively, thereby exerting a variety of biological functions [3][4][5][6][7][8][9]. It should be noted that chondroitin sulfate (CS) consists of GalNAc and D-glucuronic acid (GlcA) (Figure 1). After biosynthesis of the chondroitin precursor chain, the GlcA residue is epimerized to IdoA by DS-epimerase (DSE). Therefore, the ratio of IdoA to GlcA is distinct, and CS-DS hybrid chains are formed in each organ or developmental stage [3][5][6][8].
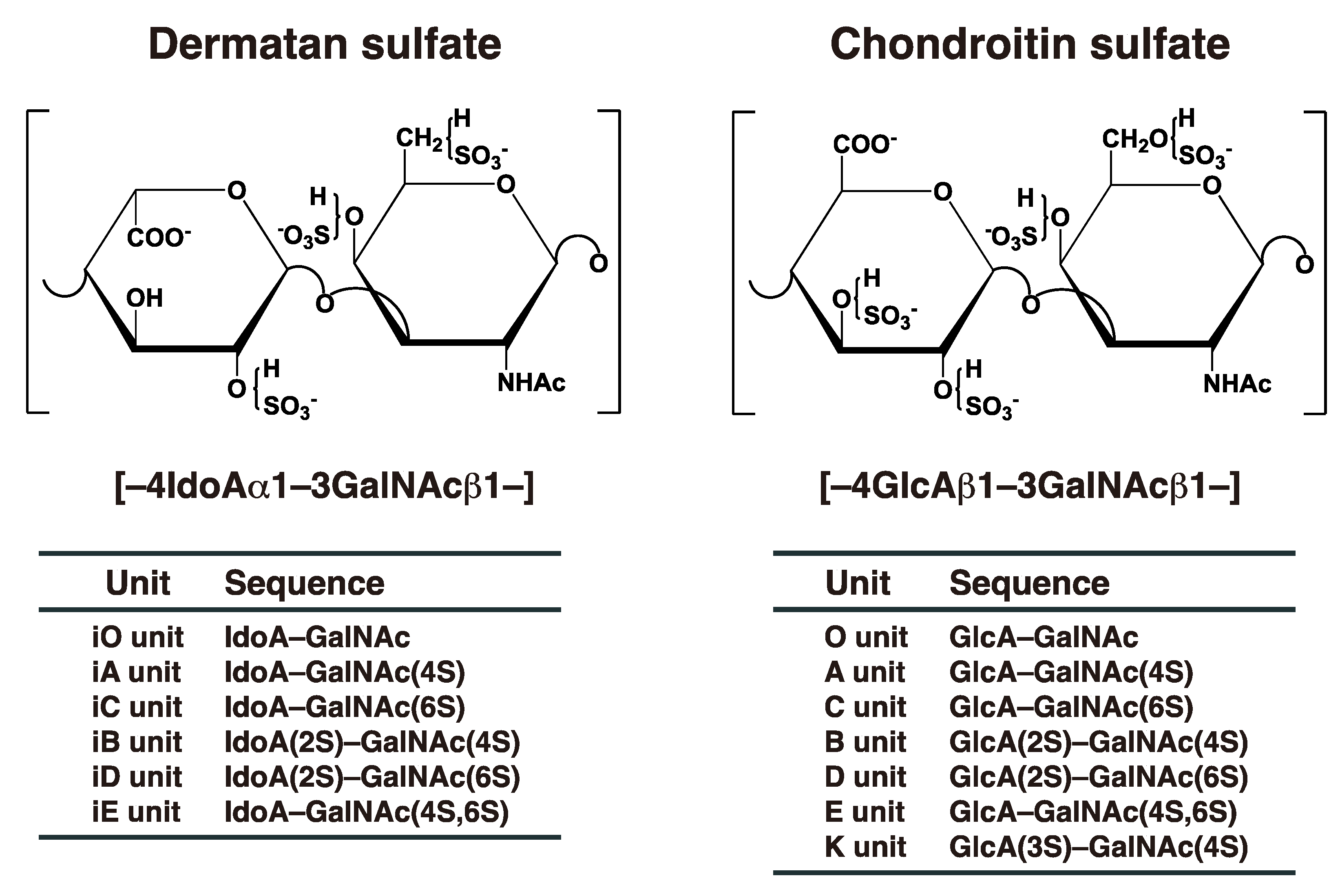
Figure 1. Typical repeating disaccharide units in CS and DS, and their potential sulfation sites. The CS backbone consists of GlcA and GalNAc, whereas DS is a stereoisomer of CS that includes IdoA instead of GlcA. These sugar moieties may be esterified by sulfate at various positions indicated by “SO3–”. The disaccharide units of CS and DS chains are classified as shown. The abbreviation “i” in DS units stands for IdoA, and 2S, 3S, 4S, and 6S stand for 2-O-, 3-O-, 4-O-, and 6-O-sulfate groups, respectively. The representative disaccharide compositions of CS/DS from various tissues and animal species are described in reference [11].
Ehlers–Danlos syndrome (EDS) is a heterogenous group of heritable connective tissue disorders characterized by skin hyperextensibility, joint hypermobility, and tissue fragility [12][13]. Various types of EDS are caused by defects in DS in addition to collagens, collagen-modifying enzymes, or Tenascin-X [12][13]. The spondylodysplastic type of EDS that is characterized by kyphoscoliosis, hypermobile joints, generalized osteopenia, short stature, clubfeet, elbow malalignment, muscle hypotonia, wrinkled skin, a characteristic facial appearance, and defective wound healing, is caused by mutations in B3GALT6 or B4GALT7 [14][15][16][17][18]. B3GALT6 and B4GALT7 encode distinct galactosyltransferase, responsible for glycosaminoglycans including CS, DS, and heparan sulfate (HS) [19][20]. Furthermore, the musculocontractural type of EDS that is characterized by kyphoscoliosis, muscular hypotonia, joint hypermobility, multiple joint contracture, hyperextensible and thin skin, atrophic scars on the skin, characteristic craniofacial features, joint laxities, and recurrent dislocations, is caused by mutations in DSE or CHST14 [21][22][23][24]. DSE and CHST14 encode DSE and dermatan 4-O-sulfoteransferase (D4ST), respectively, which function to biosynthesize DS [25][26][27]. Both knockout mice exhibited similar phenotypes such as skin fragility, thoracic kyphosis, and spina bifida, to patients with EDS of the musculocontractural type [28][29][30][31][32]. Therefore, DS is an indispensable macromolecule for the normal development of various tissues. This review focuses on and discusses not only functions of DS, but also DS-defective model animals studied in the past decade.
2. Biosynthesis of DS Chains
The initiation of GAGs is evoked by the transfer of D-xylose (Xyl) from uridine diphosphate (UDP)-Xyl to specific serine residues in the core proteins of PGs by xylosyltransferase (XYLT) in the endoplasmic reticulum and cis-Golgi compartments (Figure 2) [33][34]. Subsequently, two galactoses (Gals) and a GlcA residue are transferred from UDP-Gal and UDP-GlcA by galactosyltransferases-I (GalT-I) [19], GalT-II [20], and glucuronyltransferase-I (GlcAT-I) [35], respectively, which results in the formation of the common glycosaminoglycan–protein linker region tetrasaccharide, GlcA-Gal-Gal-Xyl-O- (Figure 2) [36][37].
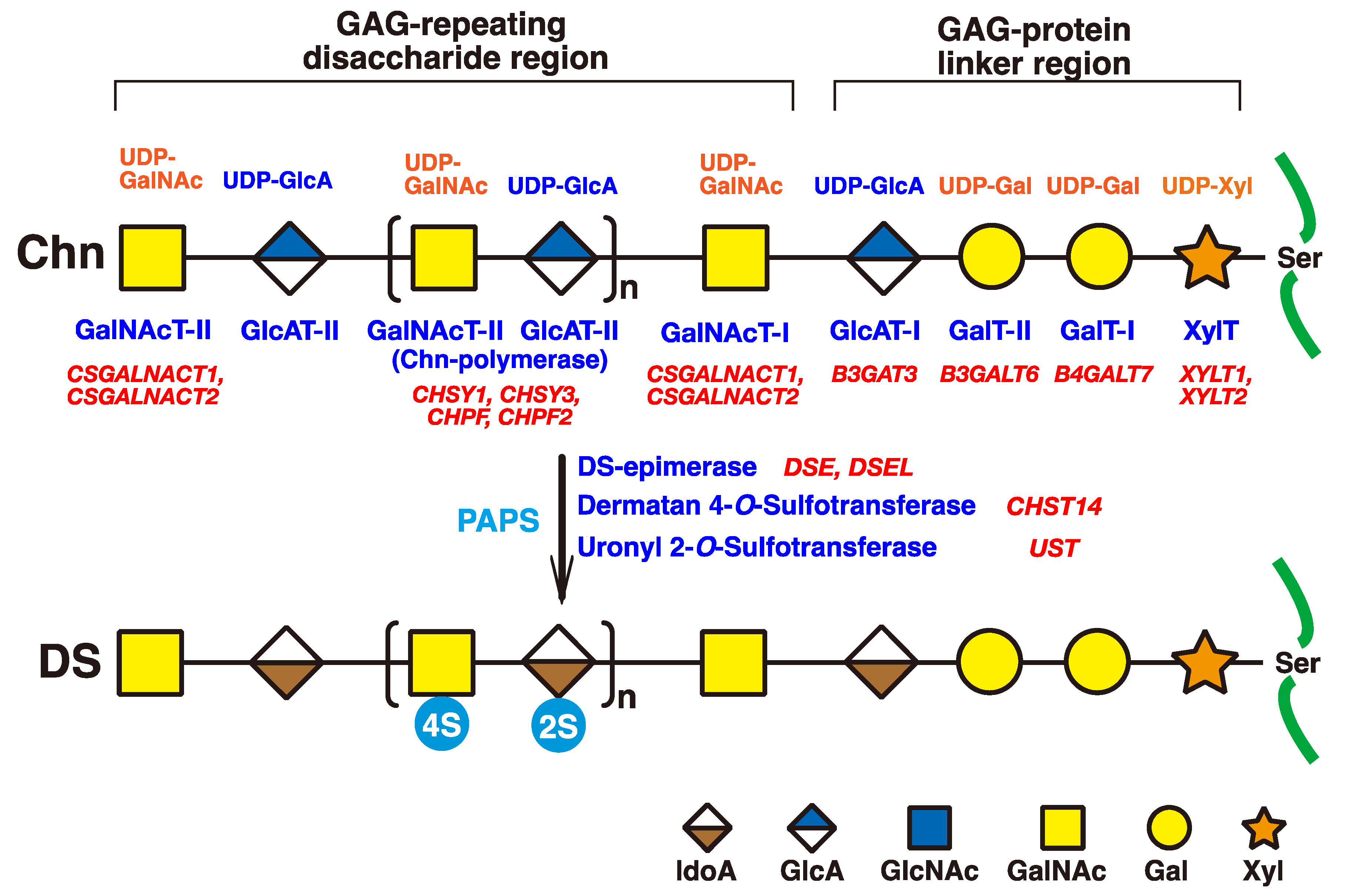
Figure 2. Schematic presentation of biosynthesis of the DS chain. All glycosyltransferases require a corresponding UDP-sugar, such as UDP-Xyl, UDP-Gal, UDP-GlcA, and UDP-GalNAc, as a donor substrate. After specific core proteins have been translated, the GAG-protein linker region, GlcA-Gal-Gal-Xyl-, is constructed by XylT, GalT-I, GalT-II, and GlcAT-I. The fifth sugar moiety, GalNAc, is then transferred to the GlcA residue in the linker region by GalNAcT-I, thereby resulting in the formation of the repeating disaccharide region, [-GlcA-GalNAc-]n, which is the unsulfate backbone of CS, by Chn-polymerase that is formed by a hetero complex of any CHSY1, CHSY3, CHPF, or CHPF2. Then, DS-epimerase converts GlcA into IdoA by epimerizing the C-5 carboxy group in the chondroitin precursor, which results in the formation of the repeating disaccharide region of dermatan, [-IdoA-GalNAc-]n. After formation of the dermatan backbone, each sugar residue is modified by sulfation and catalyzed by sulfotransferases, as indicated in the figure. D4ST or UST transfers a sulfate group from PAPS to the C-4 position of the GalNAc or to the C-2 position of the IdoA residues in the dermatan chain, respectively. It should be noted that the 4-O-sulfation but not the 2-O-sulfation is predominant. Each enzyme and its coding gene are described under the respective sugar symbols. The abbreviations 2S and 4S stand for 2-O- and 4-O-sulfates, respectively.
The chondroitin precursor chain, [-4GlcAβ1-3GalNAcβ1-]n, which is the unsulfate backbone of CS, is built up to the non-reducing terminal GlcA residue of the linker region tetrasaccharide by chondroitin synthase family members, using UDP-GlcA and UDP-GalNAc as the donor substrate (Figure 2) [38][39][40][41][42][43]. The GlcA residues in [-4GlcAβ1-3GalNAcβ1-]n are converted into IdoA by epimerizing the carboxy group of GlcA by DSE during and/or after the formation of a chondroitin backbone [25][44], resulting in formation of the repeating disaccharide region of dermatan, [-4IdoAα1-3GalNAcβ1-]n. D4ST1 and UST transfer the sulfate group from the sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to the C-4 position of GalNAc and to the C-2 position of IdoA residues in dermatan, respectively [26][27][45].
3. Classical and Additional Functions of DS
3.1. Classical Functions of DS
Although classical functions of DS have been described [4][10][46], they are briefly introduced in this section. The DS side chain of decorin binds to collagen to assemble the extracellular matrix [47][48]. A focused ion beam scanning electron microscope revealed that the DS side chain of decorin forms a ring-mesh like structure, with each ring surrounding a collagen fibril [49][50].
Heparin cofactor II, like antithrombin III, inhibits proteolytic enzymes involved in blood coagulation via interaction with DS as well as heparin [51]. The DS-derived hexasaccharide, [-IdoA(2-O-sulfate)-GalNAc(4-O-sulfate)-]3, from porcine skin has been identified as the smallest fragment of DS binding to heparin cofactor II with high affinity [52].
DS regulates specific cell signaling through interactions with effector molecules such as fibroblast growth factors (FGFs) and hepatocyte growth factor (HGF) [53][54][55]. The minimum sizes for cell proliferation through FGF2 as well as FGF7 are octa- and decasaccharides, respectively [56]. Furthermore, the disulfated disaccharide unit, [-IdoA(2-O-sulfate)-GalNAc(4-O-sulfate)-], from Ascidian, S. plicata, shows greater activity than the monosulfated disaccharide unit, [-IdoA-GalNAc(4-O-sulfate)-] from porcine intestinal mucosa [56].
Highly sulfated DS containing characteristic disaccharide units such as [-IdoA(2-O-sulfate)-GalNAc(4-O-sulfate)-], [-IdoA(2-O-sulfate)-GalNAc(6-O-sulfate)-], or [-IdoA-GalNAc(4-O-, 6-O-disulfates)-] derived from Ascidian (S. plicata and A. nigra), embryonic sea urchin, notochord of hagfish, or shark skin, exerted neurite outgrowth-promoting activity of hippocampal neurons in vitro [57][58][59] . The activity might be mediated by neurotrophic factors including pleiotrophin, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), HGF, and/or FGFs [59][60][61].
DS and/or DS-PGs are up-regulated in tumor cells as well as in the stroma [62][63][64][65], which is consistent with up-regulations of glycosyltransferases, epimerases, and sulfotransferases responsible for the biosynthesis of DS [65]. Furthermore, IdoA-deficient human esophagus squamous cell carcinoma by shRNA showed decreased migration and invasion capabilities in vitro, which was associated with reduced cellular interaction with HGF, inhibition of pERK-1/2 signaling, and deregulated actin cytoskeleton dynamics and focal adhesion formation [65]. These findings suggest that DS and/or DS-PGs may contribute to proliferation, invasion, and metastasis via binding with effector proteins. However, what remains unclear is the ratio of CS/DS, the content of IdoA, chain length, sulfation pattern, binding molecules, and cell signaling. Further studies are required in order to clarify the molecular mechanisms involving DS-PGs through the use of model animals as well as clinical specimens.
3.2. Recent Additional Functions of DS
Recently, it was shown that molecules longer than tetrasaccharides derived from DS enhance the activation of anaplastic lymphoma kinase, a receptor tyrosine kinase, by clustering of anaplastic lymphoma kinase [62].
Furthermore, it was demonstrated that the expressions of Dsel and D4st1 increased during formation of the embryonic body from mouse embryonic stem cells, and that an addition of DS to the culture medium promoted neuronal differentiation by activation of extracellular signal-regulated kinase 1/2, and also accelerated neurite outgrowth in mouse embryonic stem cells [66]. On the other hand, knockdown or overexpression of D4ST1 in mouse embryonic stem cells led to the promotion or suppression of endodermal differentiation, respectively [67]. These opposite effects of the addition of DS as well as knockdown or overexpression of D4ST1 on differentiation of mouse embryonic stem cells remain unclear; further study is required to explain these phenomena. In addition, DS promoted neuronal differentiation and neuronal migration, but not neurite outgrowth in human neuronal stem cells [66]. These findings indicate that DS may modulate neuronal differentiation in both mouse and human stem cells.
Although knockdown of C4ST1 by antisense morpholino oligonucreotide accelerated regeneration of axons after spinal cord injury in zebrafish, knockdown of D4ST1 did not [68], indicating that 4-O-sulfation of CS, but not DS, inhibit axonal regrowth after spinal cord injury.
References
- Karl, M.; Chaffee, E. The mucopolysaccharides of skin. J. Biol. Chem. 1941, 138, 491–499.
- Fransson, L.-A.; Cheng, F.; Yoshida, K.; Heinegård, D.; Malmström, A.; Schmidtchen, A. Patterns of epimerization and sulphation in dermatan sulphate chains. In Dermatan Sulphate Proteoglycans: Chemistry, Biology, Chemical Pathology; Scott, J.E., Ed.; Portland Press: London, UK, 1993; pp. 11–25.
- Thelin, M.A.; Bartolini, B.; Axelsson, J.; Gustafsson, R.; Tykesson, E.; Pera, E.; Oldberg, Å.; Maccarana, M.; Malmström, A. Biological functions of iduronic acid in chondroitin/dermatan sulfate. FEBS J. 2013, 280, 2431–2446.
- Trowbridge, J.M.; Gallo, R.L. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002, 12, 117R–125R.
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42.
- Sugahara, K.; Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 2007, 17, 536–545.
- Hayes, A.J.; Melrose, J. Glycans and glycosaminoglycans in neurobiology: Key regulators of neuronal cell function and fate. Biochem. J. 2018, 475, 2511–2545.
- Hayes, A.; Sugahara, K.; Farrugia, B.; Whitelock, J.M.; Caterson, B.; Melrose, J. Biodiversity of CS-proteoglycan sulphation motifs: Chemical messenger recognition modules with roles in information transfer, control of cellular behaviour and tissue morphogenesis. Biochem. J. 2018, 475, 587–620.
- Hayes, A.J.; Melrose, J. Neural Tissue Homeostasis and Repair Is Regulated via CS and DS Proteoglycan Motifs. Front. Cell Dev. Biol. 2021, 9, 696640.
- Iozzo, R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998, 67, 609–652.
- Kinoshita, A.; Sugahara, K. Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: Application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal. Biochem. 1999, 269, 367–378.
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26.
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Primers 2020, 6, 64.
- Quentin, E.; Gladen, A.; Rodén, L.; Kresse, H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: Galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc. Natl. Acad. Sci. USA 1990, 87, 1342–1346.
- Almeida, R.; Levery, S.B.; Mandel, U.; Kresse, H.; Schwientek, T.; Bennett, E.P.; Clausen, H. Cloning and expression of a proteoglycan UDP-galactose:beta-xylose beta1,4-galactosyltransferase I. A seventh member of the human beta4-galactosyltransferase gene family. J. Biol. Chem. 1999, 274, 26165–26171.
- Okajima, T.; Fukumoto, S.; Furukawa, K.; Urano, T. Molecular basis for the progeroid variant of Ehlers-Danlos syndrome. Identification and characterization of two mutations in galactosyltransferase I gene. J. Biol. Chem. 1999, 274, 28841–28844.
- Nakajima, M.; Mizumoto, S.; Miyake, N.; Kogawa, R.; Iida, A.; Ito, H.; Kitoh, H.; Hirayama, A.; Mitsubuchi, H.; Miyazaki, O.; et al. Mutations in B3GALT6, which encodes a glycosaminoglycan linker region enzyme, cause a spectrum of skeletal and connective tissue disorders. Am. J. Hum. Genet. 2013, 92, 927–934.
- Malfait, F.; Kariminejad, A.; Van Damme, T.; Gauche, C.; Syx, D.; Merhi-Soussi, F.; Gulberti, S.; Symoens, S.; Vanhauwaert, S.; Willaert, A.; et al. Defective initiation of glycosaminoglycan synthesis due to B3GALT6 mutations causes a pleiotropic Ehlers-Danlos-syndrome-like connective tissue disorder. Am. J. Hum. Genet. 2013, 92, 935–945.
- Okajima, T.; Yoshida, K.; Kondo, T.; Furukawa, K. Human homolog of Caenorhabditis elegans sqv-3 gene is galactosyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 1999, 274, 22915–22918.
- Bai, X.; Zhou, D.; Brown, J.R.; Crawford, B.E.; Hennet, T.; Esko, J.D. Biosynthesis of the linkage region of glycosaminoglycans: Cloning and activity of galactosyltransferase II, the sixth member of the β1,3-galactosyltransferase family (beta3GalT6). J. Biol. Chem. 2001, 276, 48189–48195.
- Müller, T.; Mizumoto, S.; Suresh, I.; Komatsu, Y.; Vodopiutz, J.; Dundar, M.; Straub, V.; Lingenhel, A.; Melmer, A.; Lechner, S.; et al. Loss of dermatan sulfate epimerase (DSE) function results in musculocontractural Ehlers-Danlos syndrome. Hum. Mol. Genet. 2013, 22, 3761–3772.
- Dündar, M.; Müller, T.; Zhang, Q.; Pan, J.; Steinmann, B.; Vodopiutz, J.; Gruber, R.; Sonoda, T.; Krabichler, B.; Utermann, G.; et al. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am. J. Hum. Genet. 2009, 85, 873–882.
- Miyake, N.; Kosho, T.; Mizumoto, S.; Furuichi, T.; Hatamochi, A.; Nagashima, Y.; Arai, E.; Takahashi, K.; Kawamura, R.; Wakui, K.; et al. Loss-of-function mutations of CHST14 in a new type of Ehlers-Danlos syndrome. Hum. Mutat. 2010, 31, 966–974.
- Malfait, F.; Syx, D.; Vlummens, P.; Symoens, S.; Nampoothiri, S.; Hermanns-Lê, T.; Van Laer, L.; De Paepe, A. Musculocontractural Ehlers-Danlos Syndrome (former EDS type VIB) and adducted thumb clubfoot syndrome (ATCS) represent a single clinical entity caused by mutations in the dermatan-4-sulfotransferase 1 encoding CHST14 gene. Hum. Mutat. 2010, 31, 1233–1239.
- Maccarana, M.; Olander, B.; Malmström, J.; Tiedemann, K.; Aebersold, R.; Lindahl, U.; Li, J.P.; Malmström, A. Biosynthesis of dermatan sulfate: Chondroitin-glucuronate C5-epimerase is identical to SART2. J. Biol. Chem. 2006, 281, 11560–11568.
- Evers, M.R.; Xia, G.; Kang, H.G.; Schachner, M.; Baenziger, J.U. Molecular cloning and characterization of a dermatan-specific N-acetylgalactosamine 4-O-sulfotransferase. J. Biol. Chem. 2001, 276, 36344–36353.
- Mikami, T.; Mizumoto, S.; Kago, N.; Kitagawa, H.; Sugahara, K. Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: Implication of differential roles in dermatan sulfate biosynthesis. J. Biol. Chem. 2003, 278, 36115–36127.
- Maccarana, M.; Kalamajski, S.; Kongsgaard, M.; Magnusson, S.P.; Oldberg, A.; Malmström, A. Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol. Cell. Biol. 2009, 29, 5517–5528.
- Akyüz, N.; Rost, S.; Mehanna, A.; Bian, S.; Loers, G.; Oezen, I.; Mishra, B.; Hoffmann, K.; Guseva, D.; Laczynska, E.; et al. Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp. Neurol. 2013, 247, 517–530.
- Hirose, T.; Mizumoto, S.; Hashimoto, A.; Takahashi, Y.; Yoshizawa, T.; Nitahara-Kasahara, Y.; Takahashi, N.; Nakayama, J.; Takehana, K.; Okada, T.; et al. Systematic investigation of the skin in Chst14-/- mice: A model for skin fragility in musculocontractural Ehlers-Danlos syndrome caused by CHST14 variants (mcEDS-CHST14). Glycobiology 2021, 31, 137–150.
- Nitahara-Kasahara, Y.; Mizumoto, S.; Inoue, Y.U.; Saka, S.; Posadas-Herrera, G.; Nakamura-Takahashi, A.; Takahashi, Y.; Hashimoto, A.; Konishi, K.; Miyata, S.; et al. Muscle pathophysiology in mouse models of musculocontractural Ehlers-Danlos syndrome due to CHST14 mutations (mcEDS-CHST14), generated through CRISPR/Cas9-mediated genomic editing. Dis. Model. Mech. 2021, 14, dmm048963.
- Nitahara-Kasahara, Y.; Posadas-Herrera, G.; Mizumoto, S.; Nakamura-Takahashi, A.; Inoue, Y.U.; Inoue, T.; Nomura, Y.; Takeda, S.; Yamada, S.; Kosho, T.; et al. Myopathy associated with dermatan sulfate-deficient decorin and myostatin in musculocontractural Ehlers-Danlos syndrome: A mouse model investigation. Front. Cell Dev. Biol. 2021, 9, 695021.
- Götting, C.; Kuhn, J.; Zahn, R.; Brinkmann, T.; Kleesiek, K. Molecular cloning and expression of human UDP-D-Xylose:proteoglycan core protein beta-D-xylosyltransferase and its first isoform XT-II. J. Mol. Biol. 2000, 304, 517–528.
- Pönighaus, C.; Ambrosius, M.; Casanova, J.C.; Prante, C.; Kuhn, J.; Esko, J.D.; Kleesiek, K.; Götting, C. Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J. Biol. Chem. 2007, 282, 5201–5206.
- Kitagawa, H.; Tone, Y.; Tamura, J.; Neumann, K.W.; Ogawa, T.; Oka, S.; Kawasaki, T.; Sugahara, K. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 1998, 273, 6615–6618.
- Lindahl, U.; Rodén, L. Carbohydrate-protein linkages in proteoglycans of animal, plant and bacterial origin. In Glycoproteins: Their Composition, Structure and Function; Gottschalk, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1972; pp. 491–517.
- Kjellén, L.; Lindahl, U. Proteoglycans: Structures and interactions. Annu. Rev. Biochem. 1991, 60, 443–475.
- Kitagawa, H.; Uyama, T.; Sugahara, K. Molecular cloning and expression of a human chondroitin synthase. J. Biol. Chem. 2001, 276, 38721–38726.
- Kitagawa, H.; Izumikawa, T.; Uyama, T.; Sugahara, K. Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J. Biol. Chem. 2003, 278, 23666–23671.
- Izumikawa, T.; Uyama, T.; Okuura, Y.; Sugahara, K.; Kitagawa, H. Involvement of chondroitin sulfate synthase-3 (chondroitin synthase-2) in chondroitin polymerization through its interaction with chondroitin synthase-1 or chondroitin polymerizing factor. Biochem. J. 2007, 403, 545–552.
- Izumikawa, T.; Koike, T.; Shiozawa, S.; Sugahara, K.; Tamura, J.; Kitagawa, H. Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization: Chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J. Biol. Chem. 2008, 283, 11396–11406.
- Uyama, T.; Kitagawa, H.; Tamura, J.; Sugahara, K. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: The key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 2002, 277, 8841–8846.
- Uyama, T.; Kitagawa, H.; Tanaka, J.; Tamura, J.; Ogawa, T.; Sugahara, K. Molecular cloning and expression of a second chondroitin N-acetylgalactosaminyltransferase involved in the initiation and elongation of chondroitin/dermatan sulfate. J. Biol. Chem. 2003, 278, 3072–3078.
- Pacheco, B.; Malmström, A.; Maccarana, M. Two dermatan sulfate epimerases form iduronic acid domains in dermatan sulfate. J. Biol. Chem. 2009, 284, 9788–9795.
- Kobayashi, M.; Sugumaran, G.; Liu, J.; Shworak, N.W.; Silbert, J.E.; Rosenberg, R.D. Molecular cloning and characterization of a human uronyl 2-sulfotransferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J. Biol. Chem. 1999, 274, 10474–10480.
- Scott, J.E. (Ed.) Dermatan Sulphate Proteoglycans: Chemistry, Biology, Chemical Pathology; Portland Press: London, UK, 1993.
- Toole, B.P.; Lowther, D.A. Dermatan sulfate-protein: Isolation from and interaction with collagen. Arch. Biochem. Biophys. 1968, 128, 567–578.
- Scott, J.E.; Orford, C.R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem. J. 1981, 197, 213–216.
- Watanabe, T.; Kametani, K.; Koyama, Y.I.; Suzuki, D.; Imamura, Y.; Takehana, K.; Hiramatsu, K. Ring-Mesh Model of Proteoglycan Glycosaminoglycan Chains in Tendon based on Three-dimensional Reconstruction by Focused Ion Beam Scanning Electron Microscopy. J. Biol. Chem. 2016, 291, 23704–23708.
- Hirose, T.; Takahashi, N.; Tangkawattana, P.; Minaguchi, J.; Mizumoto, S.; Yamada, S.; Miyake, N.; Hayashi, S.; Hatamochi, A.; Nakayama, J.; et al. Structural alteration of glycosaminoglycan side chains and spatial disorganization of collagen networks in the skin of patients with mcEDS-CHST14. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 623–631.
- Tollefsen, D.M.; Pestka, C.A.; Monafo, W.J. Activation of heparin cofactor II by dermatan sulfate. J. Biol. Chem. 1983, 258, 6713–6716.
- Maimone, M.M.; Tollefsen, D.M. Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. J. Biol. Chem. 1990, 265, 18263–18271.
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 1998, 273, 28116–28121.
- Trowbridge, J.M.; Rudisill, J.A.; Ron, D.; Gallo, R.L. Dermatan sulfate binds and potentiates activity of keratinocyte growth factor (FGF-7). J. Biol. Chem. 2002, 277, 42815–42820.
- Lyon, M.; Deakin, J.A.; Rahmoune, H.; Fernig, D.G.; Nakamura, T.; Gallagher, J.T. Hepatocyte growth factor/scatter factor binds with high affinity to dermatan sulfate. J. Biol. Chem. 1998, 273, 271–278.
- Taylor, K.R.; Rudisill, J.A.; Gallo, R.L. Structural and sequence motifs in dermatan sulfate for promoting fibroblast growth factor-2 (FGF-2) and FGF-7 activity. J. Biol. Chem. 2005, 280, 5300–5306.
- Hikino, M.; Mikami, T.; Faissner, A.; Vilela-Silva, A.C.; Pavão, M.S.; Sugahara, K. Oversulfated dermatan sulfate exhibits neurite outgrowth-promoting activity toward embryonic mouse hippocampal neurons: Implications of dermatan sulfate in neuritogenesis in the brain. J. Biol. Chem. 2003, 278, 43744–43754.
- Nandini, C.D.; Mikami, T.; Ohta, M.; Itoh, N.; Akiyama-Nambu, F.; Sugahara, K. Structural and functional characterization of oversulfated chondroitin sulfate/dermatan sulfate hybrid chains from the notochord of hagfish. Neuritogenic and binding activities for growth factors and neurotrophic factors. J. Biol. Chem. 2004, 279, 50799–50809.
- Nandini, C.D.; Itoh, N.; Sugahara, K. Novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chains with a unique heterogeneous sulfation pattern from shark skin, which exhibit neuritogenic activity and binding activities for growth factors and neurotrophic factors. J. Biol. Chem. 2005, 280, 4058–4069.
- Bao, X.; Mikami, T.; Yamada, S.; Faissner, A.; Muramatsu, T.; Sugahara, K. Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J. Biol. Chem. 2005, 280, 9180–9191.
- Li, F.; Nandini, C.D.; Hattori, T.; Bao, X.; Murayama, D.; Nakamura, T.; Fukushima, N.; Sugahara, K. Structure of pleiotrophin- and hepatocyte growth factor-binding sulfated hexasaccharide determined by biochemical and computational approaches. J. Biol. Chem. 2010, 285, 27673–27685.
- Machino, M.; Gong, Y.; Ozaki, T.; Suzuki, Y.; Watanabe, E.; Imagama, S.; Kadomatsu, K.; Sakamoto, K. Dermatan sulphate is an activating ligand of anaplastic lymphoma kinase. J. Biochem. 2021, 170, 631–637.
- Fukatsu, T.; Sobue, M.; Nagasaka, T.; Ohiwa, N.; Fukata, S.; Nakashima, N.; Takeuchi, J. Immunohistochemical localization of chondroitin sulphate and dermatan sulphate proteoglycans in tumour tissues. Br. J. Cancer 1988, 57, 74–78.
- Ten Dam, G.B.; Yamada, S.; Kobayashi, F.; Purushothaman, A.; van de Westerlo, E.M.; Bulten, J.; Malmström, A.; Sugahara, K.; Massuger, L.F.; van Kuppevelt, T.H. Dermatan sulfate domains defined by the novel antibody GD3A12, in normal tissues and ovarian adenocarcinomas. Histochem. Cell. Biol. 2009, 132, 117–127.
- Thelin, M.A.; Svensson, K.J.; Shi, X.; Bagher, M.; Axelsson, J.; Isinger-Ekstrand, A.; van Kuppevelt, T.H.; Johansson, J.; Nilbert, M.; Zaia, J.; et al. Dermatan sulfate is involved in the tumorigenic properties of esophagus squamous cell carcinoma. Cancer Res. 2012, 72, 1943–1952.
- Ogura, C.; Hirano, K.; Mizumoto, S.; Yamada, S.; Nishihara, S. Dermatan sulphate promotes neuronal differentiation in mouse and human stem cells. J. Biochem. 2021, 169, 55–64.
- Ogura, C.; Nishihara, S. Dermatan-4-O-Sulfotransferase-1 Contributes to the Undifferentiated State of Mouse Embryonic Stem Cells. Front. Cell Dev. Biol. 2021, 9, 733964.
- Sahu, S.; Li, R.; Loers, G.; Schachner, M. Knockdown of chondroitin-4-sulfotransferase-1, but not of dermatan-4-sulfotransferase-1, accelerates regeneration of zebrafish after spinal cord injury. FASEB J. 2019, 33, 2252–2262.
More
Information
Subjects:
Biology; Biochemistry & Molecular Biology; Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
14 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





