The crucial roles of dermatan sulfate (DS) have been demonstrated in tissue development of the cutis, blood vessels, and bone through construction of the extracellular matrix and cell signaling. Although DS classically exerts physiological functions via interaction with collagens, growth factors, and heparin cofactor-II, new functions have been revealed through analyses of human genetic disorders as well as of knockout mice with loss of DS-synthesizing enzymes. Mutations in human genes encoding the epimerase and sulfotransferase responsible for the biosynthesis of DS chains cause connective tissue disorders including spondylodysplastic type Ehlers–Danlos syndrome, characterized by skin hyperextensibility, joint hypermobility, and tissue fragility. DS-deficient mice show perinatal lethality, skin fragility, vascular abnormalities, thoracic kyphosis, myopathy-related phenotypes, acceleration of nerve regeneration, and impairments in self-renewal and proliferation of neural stem cells.
1. Introduction
Dermatan sulfate (DS) was initially isolated from skin by Karl Myer in 1941
[1]. Although dermatan sulfate-proteoglycans (DS-PGs) ubiquitously exist in various tissues, they distribute abundantly throughout skin, cartilage, and the aorta
[2]. DS-PGs play important roles in anti-coagulation, binding to growth factors, wound healing, in the assembly of extracellular matrices, in tissue morphogenesis, and neuronal homeostasis
[3][4][5][6][7][8][9][3,4,5,6,7,8,9]. DS side chains are linear polysaccharides covalently attached to core proteins that form PGs
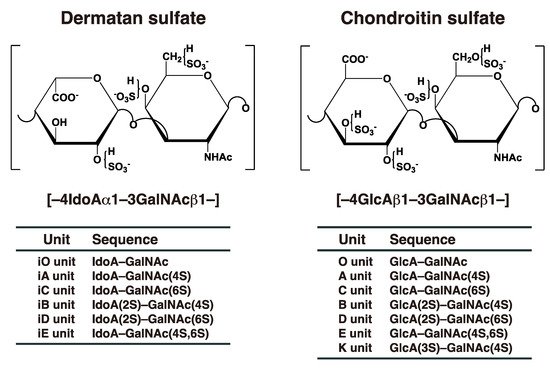
[2][10][2,10]. DS chains consist of alternating disaccharide units comprising
N-acetyl-D-galactosamine (GalNAc) and L-iduronic acid (IdoA) residues with 50–200 repeats (
Figure 1), constructed by specific glycosyltransferases and epimerase
[3]. The repeating disaccharide region of DS undergoes maturation by sulfation at the C-4 and C-2 positions on GalNAc and IdoA residues, respectively, thereby exerting a variety of biological functions
[3][4][5][6][7][8][9][3,4,5,6,7,8,9]. It should be noted that chondroitin sulfate (CS) consists of GalNAc and D-glucuronic acid (GlcA) (
Figure 1). After biosynthesis of the chondroitin precursor chain, the GlcA residue is epimerized to IdoA by DS-epimerase (DSE). Therefore, the ratio of IdoA to GlcA is distinct, and CS-DS hybrid chains are formed in each organ or developmental stage
[3][5][6][8][3,5,6,8].
Figure 1. Typical repeating disaccharide units in CS and DS, and their potential sulfation sites. The CS backbone consists of GlcA and GalNAc, whereas DS is a stereoisomer of CS that includes IdoA instead of GlcA. These sugar moieties may be esterified by sulfate at various positions indicated by “SO
3–”. The disaccharide units of CS and DS chains are classified as shown. The abbreviation “i” in DS units stands for IdoA, and 2S, 3S, 4S, and 6S stand for 2-
O-, 3-
O-, 4-
O-, and 6-
O-sulfate groups, respectively. The representative disaccharide compositions of CS/DS from various tissues and animal species are described in reference
[11].
Ehlers–Danlos syndrome (EDS) is a heterogenous group of heritable connective tissue disorders characterized by skin hyperextensibility, joint hypermobility, and tissue fragility
[12][13][12,13]. Various types of EDS are caused by defects in DS in addition to collagens, collagen-modifying enzymes, or Tenascin-X
[12][13][12,13]. The spondylodysplastic type of EDS that is characterized by kyphoscoliosis, hypermobile joints, generalized osteopenia, short stature, clubfeet, elbow malalignment, muscle hypotonia, wrinkled skin, a characteristic facial appearance, and defective wound healing, is caused by mutations in
B3GALT6 or
B4GALT7 [14][15][16][17][18][14,15,16,17,18].
B3GALT6 and
B4GALT7 encode distinct galactosyltransferase, responsible for glycosaminoglycans including CS, DS, and heparan sulfate (HS)
[19][20][19,20]. Furthermore, the musculocontractural type of EDS that is characterized by kyphoscoliosis, muscular hypotonia, joint hypermobility, multiple joint contracture, hyperextensible and thin skin, atrophic scars on the skin, characteristic craniofacial features, joint laxities, and recurrent dislocations, is caused by mutations in
DSE or
CHST14 [21][22][23][24][21,22,23,24].
DSE and
CHST14 encode DSE and dermatan 4-
O-sulfoteransferase (D4ST), respectively, which function to biosynthesize DS
[25][26][27][25,26,27]. Both knockout mice exhibited similar phenotypes such as skin fragility, thoracic kyphosis, and spina bifida, to patients with EDS of the musculocontractural type
[28][29][30][31][32][28,29,30,31,32]. Therefore, DS is an indispensable macromolecule for the normal development of various tissues. This review focuses on and discusses not only functions of DS, but also DS-defective model animals studied in the past decade.
2. Biosynthesis of DS Chains
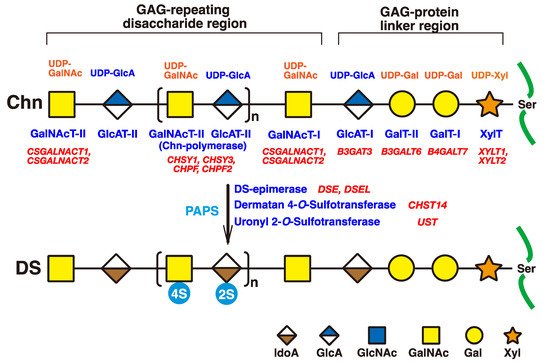
The initiation of GAGs is evoked by the transfer of D-xylose (Xyl) from uridine diphosphate (UDP)-Xyl to specific serine residues in the core proteins of PGs by xylosyltransferase (XYLT) in the endoplasmic reticulum and cis-Golgi compartments (
Figure 2)
[33][34][33,34]. Subsequently, two galactoses (Gals) and a GlcA residue are transferred from UDP-Gal and UDP-GlcA by galactosyltransferases-I (GalT-I)
[19], GalT-II
[20], and glucuronyltransferase-I (GlcAT-I)
[35], respectively, which results in the formation of the common glycosaminoglycan–protein linker region tetrasaccharide, GlcA-Gal-Gal-Xyl-
O- (
Figure 2)
[36][37][36,37].
Figure 2. Schematic presentation of biosynthesis of the DS chain. All glycosyltransferases require a corresponding UDP-sugar, such as UDP-Xyl, UDP-Gal, UDP-GlcA, and UDP-GalNAc, as a donor substrate. After specific core proteins have been translated, the GAG-protein linker region, GlcA-Gal-Gal-Xyl-, is constructed by XylT, GalT-I, GalT-II, and GlcAT-I. The fifth sugar moiety, GalNAc, is then transferred to the GlcA residue in the linker region by GalNAcT-I, thereby resulting in the formation of the repeating disaccharide region, [-GlcA-GalNAc-]n, which is the unsulfate backbone of CS, by Chn-polymerase that is formed by a hetero complex of any CHSY1, CHSY3, CHPF, or CHPF2. Then, DS-epimerase converts GlcA into IdoA by epimerizing the C-5 carboxy group in the chondroitin precursor, which results in the formation of the repeating disaccharide region of dermatan, [-IdoA-GalNAc-]n. After formation of the dermatan backbone, each sugar residue is modified by sulfation and catalyzed by sulfotransferases, as indicated in the figure. D4ST or UST transfers a sulfate group from PAPS to the C-4 position of the GalNAc or to the C-2 position of the IdoA residues in the dermatan chain, respectively. It should be noted that the 4-O-sulfation but not the 2-O-sulfation is predominant. Each enzyme and its coding gene are described under the respective sugar symbols. The abbreviations 2S and 4S stand for 2-O- and 4-O-sulfates, respectively.
The chondroitin precursor chain, [-4GlcAβ1-3GalNAcβ1-]
n, which is the unsulfate backbone of CS, is built up to the non-reducing terminal GlcA residue of the linker region tetrasaccharide by chondroitin synthase family members, using UDP-GlcA and UDP-GalNAc as the donor substrate (
Figure 2)
[38][39][40][41][42][43][38,39,40,41,42,43]. The GlcA residues in [-4GlcAβ1-3GalNAcβ1-]
n are converted into IdoA by epimerizing the carboxy group of GlcA by DSE during and/or after the formation of a chondroitin backbone
[25][44][25,44], resulting in formation of the repeating disaccharide region of dermatan, [-4IdoAα1-3GalNAcβ1-]
n. D4ST1 and UST transfer the sulfate group from the sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to the C-4 position of GalNAc and to the C-2 position of IdoA residues in dermatan, respectively
[26][27][45][26,27,45].
3. Classical and Additional Functions of DS
3.1. Classical Functions of DS
Although classical functions of DS have been described
[4][10][46]in the literature [4,10,46], they are briefly introduced in this section. The DS side chain of decorin binds to collagen to assemble the extracellular matrix
[47][48][47,48] (Table 1). A focused ion beam scanning electron microscope revealed that the DS side chain of decorin forms a ring-mesh like structure, with each ring surrounding a collagen fibril
[49][50][49,50].
Heparin cofactor II, like antithrombin III, inhibits proteolytic enzymes involved in blood coagulation via interaction with DS as well as heparin
[51] [51](Table 1). The DS-derived hexasaccharide, [-IdoA(2-
O-sulfate)-GalNAc(4-
O-sulfate)-]
3, from porcine skin has been identified as the smallest fragment of DS binding to heparin cofactor II with high affinity
[52].
DS regulates specific cell signaling through interactions with effector molecules such as fibroblast growth factors (FGFs) and hepatocyte growth factor (HGF)
[53][54][55][53,54,55] (Table 1). The minimum sizes for cell proliferation through FGF2 as well as FGF7 are octa- and decasaccharides, respectively
[56]. Furthermore, the disulfated disaccharide unit, [-IdoA(2-
O-sulfate)-GalNAc(4-
O-sulfate)-], from Ascidian,
S. plicata, shows greater activity than the monosulfated disaccharide unit, [-IdoA-GalNAc(4-
O-sulfate)-] from porcine intestinal mucosa
[56].
Highly sulfated DS containing characteristic disaccharide units such as [-IdoA(2-
O-sulfate)-GalNAc(4-
O-sulfate)-], [-IdoA(2-
O-sulfate)-GalNAc(6-
O-sulfate)-], or [-IdoA-GalNAc(4-
O-, 6-
O-disulfates)-] derived from Ascidian (
S. plicata and
A. nigra), embryonic sea urchin, notochord of hagfish, or shark skin, exerted neurite outgrowth-promoting activity of hippocampal neurons in vitro
[57][58][59][57,58,59] (Table 1). The activity might be mediated by neurotrophic factors including pleiotrophin, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), HGF, and/or FGFs
[59][60][61][59,60,61].
DS and/or DS-PGs are up-regulated in tumor cells as well as in the stroma
[62][63][64][65][62,63,64,65], which is consistent with up-regulations of glycosyltransferases, epimerases, and sulfotransferases responsible for the biosynthesis of DS
[65]. Furthermore, IdoA-deficient human esophagus squamous cell carcinoma by shRNA showed decreased migration and invasion capabilities in vitro, which was associated with reduced cellular interaction with HGF, inhibition of pERK-1/2 signaling, and deregulated actin cytoskeleton dynamics and focal adhesion formation
[65]. These findings suggest that DS and/or DS-PGs may contribute to proliferation, invasion, and metastasis via binding with effector proteins. However, what remains unclear is the ratio of CS/DS, the content of IdoA, chain length, sulfation pattern, binding molecules, and cell signaling. Further studies are required in order to clarify the molecular mechanisms involving DS-PGs through the use of model animals as well as clinical specimens.
3.2. Recent Additional Functions of DS
Recently, it was shown that molecules longer than tetrasaccharides derived from DS enhance the activation of anaplastic lymphoma kinase, a receptor tyrosine kinase, by clustering of anaplastic lymphoma kinase
[62].
Furthermore, it was demonstrated that the expressions of
Dsel and
D4st1 increased during formation of the embryonic body from mouse embryonic stem cells, and that an addition of DS to the culture medium promoted neuronal differentiation by activation of extracellular signal-regulated kinase 1/2, and also accelerated neurite outgrowth in mouse embryonic stem cells
[66][71]. On the other hand, knockdown or overexpression of D4ST1 in mouse embryonic stem cells led to the promotion or suppression of endodermal differentiation, respectively
[67][72]. These opposite effects of the addition of DS as well as knockdown or overexpression of D4ST1 on differentiation of mouse embryonic stem cells remain unclear; further study is required to explain these phenomena. In addition, DS promoted neuronal differentiation and neuronal migration, but not neurite outgrowth in human neuronal stem cells
[66][71]. These findings indicate that DS may modulate neuronal differentiation in both mouse and human stem cells.
Although knockdown of C4ST1 by antisense morpholino oligonucreotide accelerated regeneration of axons after spinal cord injury in zebrafish, knockdown of D4ST1 did not
[68][73], indicating that 4-
O-sulfation of CS, but not DS, inhibit axonal regrowth after spinal cord injury.