
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hongda Cheng | -- | 3360 | 2022-07-13 13:21:23 | | | |
| 2 | Camila Xu | Meta information modification | 3360 | 2022-07-14 03:14:25 | | |
Video Upload Options
Based on different permeation mechanism, membrane for hydrogen separation can be categorized as mixed protonic–electronic conducting (MPEC) membrane, dense metal membrane, and porous inorganic membrane. Each membrane also has its own advantages and disadvantages. Cermet membrane is composed of a ceramic phase and a metal phase. The ceramic phase is benefit to improve the mechanical stability and high protonic conductivity of the membrane while the metal phase is used to enhance the electronic conductivity and surface-exchange kinetic. In order to overcome the drawbacks of cermet membranes, researchers developed another kind of dual-phase membrane (cercer membranes), in which a ceramic with high electronic conductivity replaces the metal as the electronic conducting phase.
1. Introduction
2. Cermet Dual-Phase Membrane
2.1. Pd-Based Cermet Membranes
2.2. Ni-Based Cermet Membranes
| Composition | Hydrogen Flux (mL·min−1·cm−2) |
T (°C) | Thickness (mm) |
Feed/Sweep Gas | Note | Ref. |
|---|---|---|---|---|---|---|
| 50Pd-50YSZ | 20 | 900 | 0.22 | 100%H2/N2 | mechanical mixing | [33] |
| 50Pd-50YSZ | 5.5 | 900 | 0.218 | 80% H2-He/N2 | ball-milling | [37] |
| 50Pd-50YSZ | 52 | 900 | 0.018 | 100%H2/N2 | mechanical mixing; asymmetric | [38] |
| 50Pd-50Gd0.2Ce0.8O2-δ | 5.5 | 900 | 0.282 | 80% H2-He/N2 | ball-milling | [39] |
| 50Pd-50CaZr0.9Y0.1O3-δ | 2.3 | 900 | 0.50 | 80% H2-He/N2 | ball-milling | [34] |
| 50Pd-50BaCe0.4Zr0.4Gd0.1Dy0.1O3-δ | 2.77 | 700 | 0.40 | 100%H2/N2 | ball-milling | [40] |
| 40Ni-60BaZr0.7Pr0.1Y0.2O3-δ | 0.0162 | 950 | 0.40 | wet50%H2-N2/Ar | mechanical mixing | [41] |
| 50Ni-50BaCe0.85Te0.05 Zr0.1O3-δ | 0.17 | 800 | 0.50 | 50%H2-He/Ar | mechanical mixing | [42] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.805 | 900 | 0.266 | 100%H2/N2 | ball-milling | [43] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.056 | 900 | 1 | 100%H2/N2 | ball-milling | [44] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ | 0.087 | 900 | 0.5 | wet4%H2-Ar/N2 | ball-milling | [45] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ | 0.0174 | 900 | 0.75 | 20%H2-He/N2 | mechanical mixing | [46] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ | 0.215 | 900 | 0.40 | wet80%H2-He/N2 | mechanical mixing | [47] |
| 50Ni-50BaCe0.95Tb0.05O3-δ | 0.53 | 850 | 0.65 | 50%H2-He/N2 | ball-milling | [48] |
| 50Ni-50BaCe0.95Tb0.05O3-δ | 0.914 | 850 | 0.09 | 50%H2-He/N2 | ball-milling; asymmetric | [48] |
| 40Ni-60BaCe0.9Y0.1O3-δ | 0.24 | 900 | 0.08 | wet3.8%H2-He/N2 | ball-milling | [49] |
| 40Ni-60BaCe0.9Y0.1O3-δ | 0.76 | 800 | 0.23 | 100%H2/N2 | high-energy milling | [35] |
| 50Ni-50BaCe0.85Fe0.15O3-δ | 0.325 | 1000 | 0.50 | 50%H2-He/wet Ar | ball-milling | [50] |
| 40Ni-60BaCe0.7In0.2Ta0.1O3-δ | 0.15 | 900 | 1 | 20%H2-N2/Ar | mechanical mixing | [51] |
| 40Ni-60BaCe0.7Y0.2In0.1O3-δ | 0.013 | 850 | 0.86 | 20%H2-N2 /Ar | mechanical mixing | [52] |
| 40Ni-60SrCe0.8Yb0.2O3-δ | 0.105 | 900 | 0.25 | wet20%H2-He/N2 | ball-milling | [36] |
| 40Ni-60La0.5Ce0.5O2-δ | 0.088 | 900 | 0.048 | 20%H2-N2/Ar | mechanical mixing; asymmetric | [53] |
| 40Ni-60La0.5Ce0.5O2-δ | 0.021 | 900 | 0.6 | wet20%H2-N2/Ar | ball-milling | [54] |
| 40Ni-60La0.4875Ca0.0125Ce0.5O2-δ | 0.025 | 900 | 0.6 | wet20%H2-N2/Ar | ball-milling | [54] |
| 40Ni-60La1.95Sm0.05Ce2O7 | 0.037 | 900 | 0.6 | wet20%H2- Ar/N2 | ball-milling | [55] |
| 60Ni-40La5.5WO11.25-δ | 0.18 | 1000 | 0.5 | 50%H2-He/Ar | ball-milling | [56] |
| Cu-BaZr0.9Y0.1O3-δ | 0.00242 | 882 | 1.90 | 100%H2/Ar | molten-copper infiltration technique |
[57] |
| 60Ta-40YSZ | 1.2 | 500 | 0.50 | 100%H2/Ar | mechanical mixing | [58] |
 Figure 1. The contribution of two diffusion mechanisms to the total hydrogen permeation fluxes in Ni-SCYb cermet dual-phase membranes [36].
Figure 1. The contribution of two diffusion mechanisms to the total hydrogen permeation fluxes in Ni-SCYb cermet dual-phase membranes [36]. Figure 2. H2 fluxes through Ni-BZCYb membrane feeding with dry and wet 4% H2 [43].
Figure 2. H2 fluxes through Ni-BZCYb membrane feeding with dry and wet 4% H2 [43].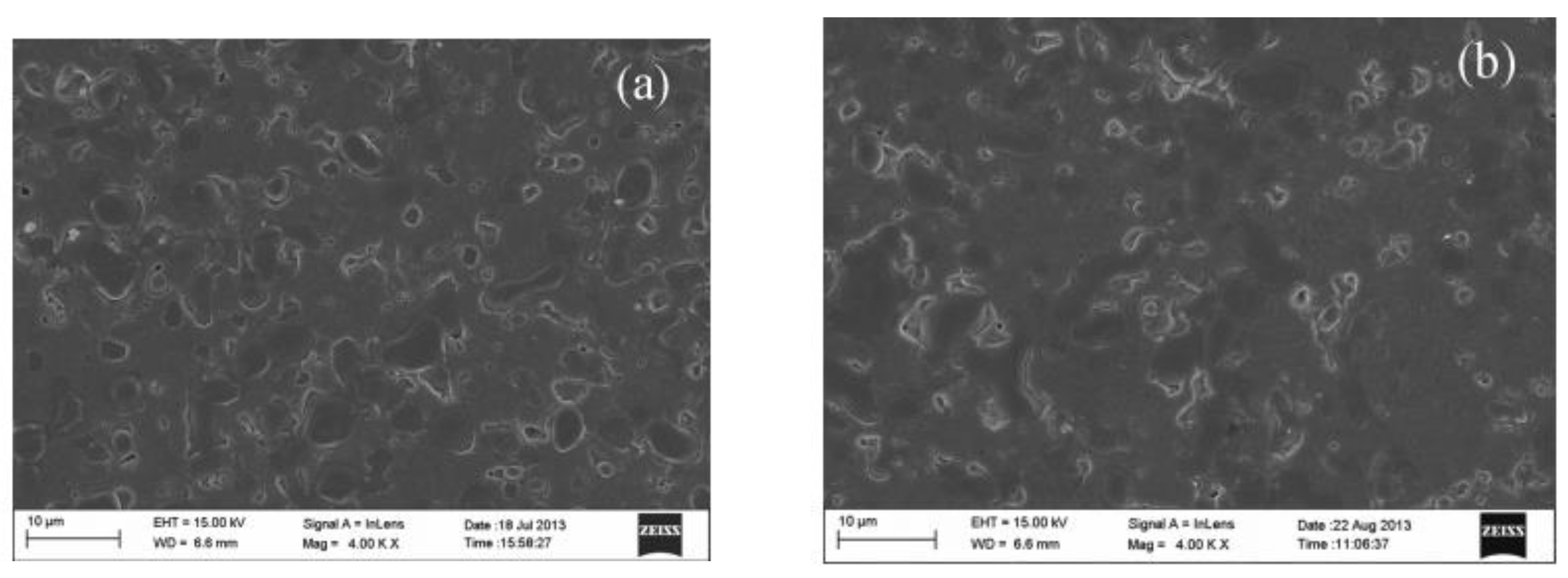 Figure 3. SEM images of Ni-BZCYYb sintered at 900 °C for 200 h in 20% CO2 containing atmosphere, (a) the original membrane, and (b) the sintered membrane [46].
Figure 3. SEM images of Ni-BZCYYb sintered at 900 °C for 200 h in 20% CO2 containing atmosphere, (a) the original membrane, and (b) the sintered membrane [46].3. Cercer Dual-Phase Membrane
3.1. Barium Cerate-Based Cercer Membranes
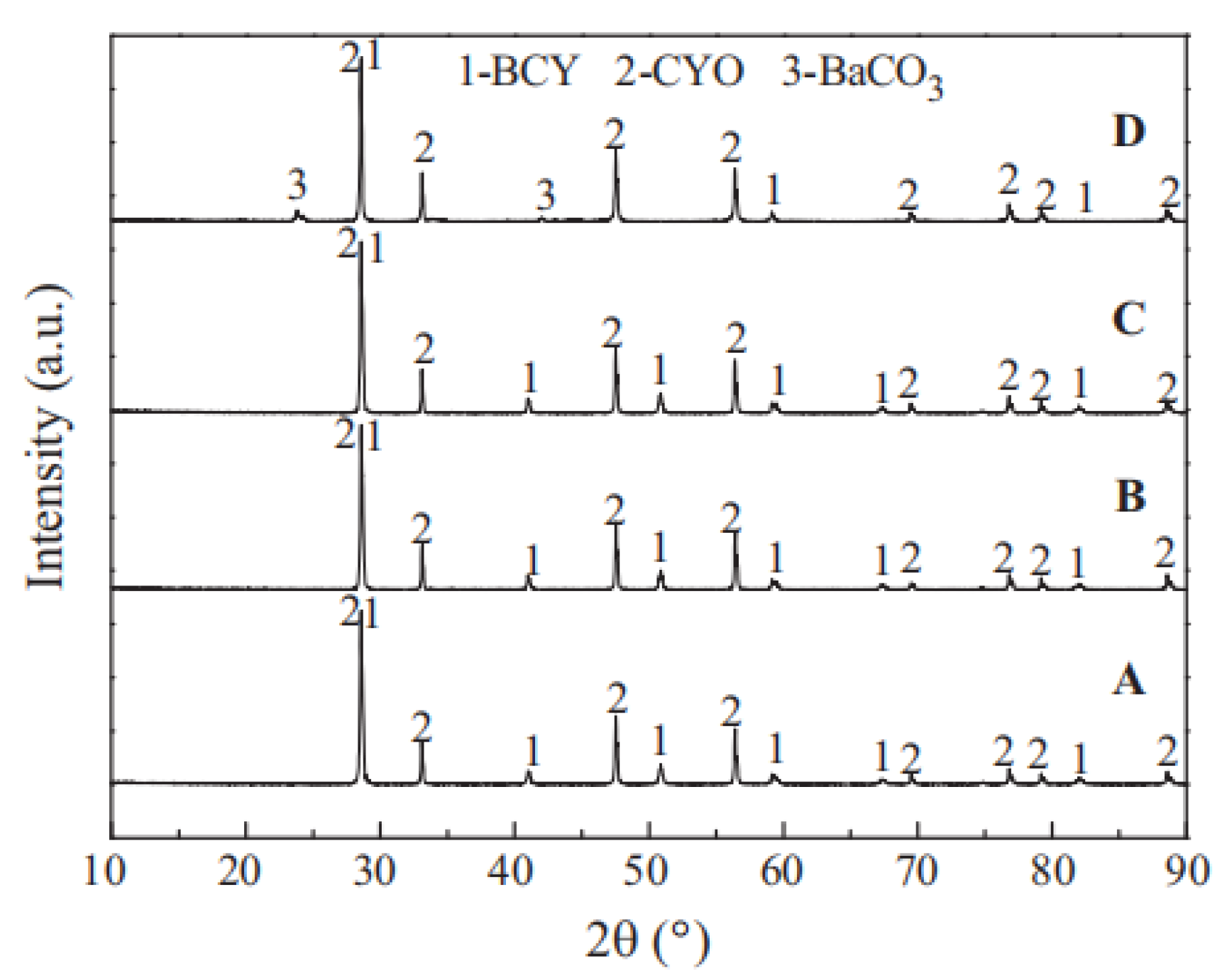 Figure 4. XRD patterns of BCY-CYO after the stability test in atmosphere containing 4% H2 (B), H2O (C), 100% CO2 (D); original membrane (A) [66].
Figure 4. XRD patterns of BCY-CYO after the stability test in atmosphere containing 4% H2 (B), H2O (C), 100% CO2 (D); original membrane (A) [66].3.2. Lanthanum Tungstate Cercer Membranes
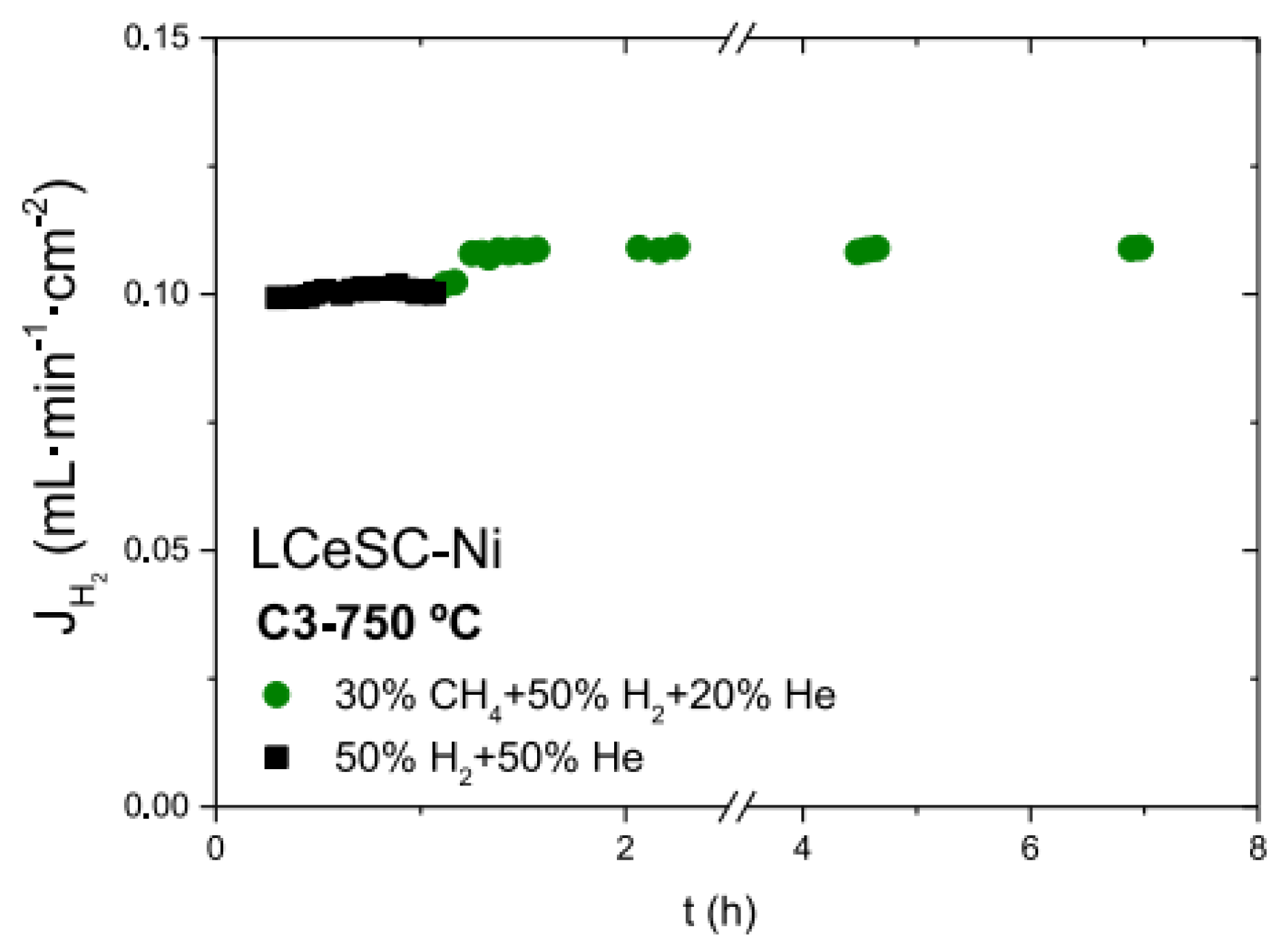 Figure 5. Hydrogen fluxes as a function of time for 60LWO-40LSC modified by La0.75Ce0.1Sr0.15CrO3-δ (LCeSC)-Ni feeding with different gases [68].
Figure 5. Hydrogen fluxes as a function of time for 60LWO-40LSC modified by La0.75Ce0.1Sr0.15CrO3-δ (LCeSC)-Ni feeding with different gases [68].3.3. Elimination of the Inter-Phase Reaction
3.4. Phase Composition Ratio
References
- Yang, M.; He, F.; Zhou, C.; Dong, F.; Yang, G.; Zhou, W.; Shao, Z. New perovskite membrane with improved sintering and self-reconstructed surface for efficient hydrogen permeation. J. Memb. Sci. 2021, 620, 118980.
- Saini, N.; Awasthi, K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2022, 282, 120029.
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087.
- Winter, C.J. Hydrogen energy-Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52.
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies-Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963.
- Li, W.; Li, Y.; Caro, J.; Huang, A. Fabrication of a flexible hydrogen-bonded organic framework based mixed matrix membrane for hydrogen separation. J. Memb. Sci. 2022, 643, 120021.
- Zadorozhnyy, V.; Soprunyuk, V.; Klyamkin, S.; Zadorozhnyy, M.; Berdonosova, E.; Savvotin, I.; Stepashkin, A.; Korol, A.; Kvaratskheliya, A.; Semenov, D.; et al. Mechanical spectroscopy of metal/polymer composite membranes for hydrogen separation. J. Alloys Compd. 2021, 866, 159014.
- Han, Z.; Xu, K.; Liao, N.; Xue, W. Theoretical investigations of permeability and selectivity of Pd-Cu and Pd-Ni membranes for hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 23715–23722.
- Duran, M.; Tüzün, F.N. Exploration of ceramic supports to be used in membrane reactors for hydrogen production and separation. Int. J. Hydrogen Energy 2021, 46, 29216–29229.
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230.
- Liu, J.; Liu, C.; Huang, A. Co-based zeolitic imidazolate framework ZIF-9 membranes prepared on α-Al2O3 tubes through covalent modification for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 703–711.
- Wey, M.-Y.; Chen, H.-H.; Lin, Y.-T.; Tseng, H.-H. Thin carbon hollow fiber membrane with Knudsen diffusion for hydrogen/alkane separation: Effects of hollow fiber module design and gas flow mode. Int. J. Hydrogen Energy 2020, 45, 7290–7302.
- Liu, J.; Li, X.; Liang, X.; Fu, H.; Rettenmayr, M.; Liu, D. Structure and properties of niobium carbide coated vanadium composite membranes for high temperature hydrogen separation. J. Alloys Compd. 2022, 900, 163530.
- Anggarini, U.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Structural two-phase evolution of aminosilica-based silver-coordinated membranes for increased hydrogen separation. J. Memb. Sci. 2022, 642, 119962.
- Liang, X.; Li, X.; Chen, R.; Nagaumi, H.; Guo, J.; Liu, D. Enhancement of hydrogen permeation stability at high temperatures for Pd/Nb30Ti35Co35/Pd composite membranes by HfN intermediate layer. J. Memb. Sci. 2022, 643, 120062.
- Pal, N.; Agarwal, M.; Maheshwari, K.; Solanki, Y.S. A review on types, fabrication and support material of hydrogen separation membrane. Mater. Today Proc. 2020, 28, 1386–1391.
- Wang, H.; Wang, X.; Meng, B.; Tan, X.; Loh, K.S.; Sunarso, J.; Liu, S. Perovskite-based mixed protonic-electronic conducting membranes for hydrogen separation: Recent status and advances. J. Ind. Eng. Chem. 2018, 60, 297–306.
- Zhang, Q.-Y.; Han, J.-J.; Huang, Y.; Chen, Y.; Yan, X.; Lang, W.-Z. Effect of Ba non-stoichiometry in Ba1-xZr0.1Ce0.7Y0.2O3-δ on its structure defect, sinterability and hydrogen permeability. Ceram. Int. 2020, 46, 19564–19573.
- Zhou, C.; Sunarso, J.; Dai, J.; Ran, R.; Song, Y.; He, F.; Zhou, W.; Shao, Z. Realizing stable high hydrogen permeation flux through BaCo0.4Fe0.4Zr0.1Y0.1O3-δ membrane using a thin Pd film protection strategy. J. Memb. Sci. 2020, 596, 117709.
- Xing, W.; Inge Dahl, P.; Valland Roaas, L.; Fontaine, M.-L.; Larring, Y.; Henriksen, P.P.; Bredesen, R. Hydrogen permeability of SrCe0.7Zr0.25Ln0.05O3-δ membranes (Ln = Tm and Yb). J. Memb. Sci. 2015, 473, 327–332.
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Asymmetric membrane structure: An efficient approach to enhance hydrogen separation performance. Sep. Purif. Technol. 2018, 207, 363–369.
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363.
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.; Zhou, W.; Sunarso, J. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrogen Energy 2018, 43, 15281–15305.
- Yang, Y.; Zeng, Y.; Amirkhiz, B.S.; Luo, J.-L.; Yan, N. Promoting the ambient-condition stability of Zr-doped barium cerate: Toward robust solid oxide fuel cells and hydrogen separation in syngas. Int. J. Hydrogen Energy 2018, 378, 134–138.
- Hossain, M.K.; Yamamoto, T.; Hashizume, K. Effect of sintering conditions on structural and morphological properties of Y- and Co-doped BaZrO3 proton conductors. Ceram. Int. 2021, 47, 27177–27187.
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748.
- Chen, Y.; Liu, H.; Zhuang, L.; Wei, Y.; Wang, H. Hydrogen permeability through Nd5.5W0.35Mo0.5Nb0.15O11.25-δ mixed protonic-electronic conducting membrane. J. Memb. Sci. 2019, 579, 33–39.
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Tailoring hydrogen separation performance through the ceramic lanthanum tungstate membranes by chlorine doping. J. Memb. Sci. 2019, 573, 117–125.
- Huang, Y.; Shi, G.F.; Liao, Q.; Chen, Y.; Lang, W.Z. Development of Mn and Mo double-substituted La5.5WO11.25-δ based membranes with enhanced hydrogen permeation flux. J. Eur. Ceram. Soc. 2021, 41, 5711–5720.
- Qi, X.; Lin, Y.S. Electrical conduction and hydrogen permeation through mixed proton-electron conducting strontium cerate membranes. Solid State Ion. 2000, 130, 149–156.
- Song, S.J.; Wachsman, E.D.; Rhodes, J.; Dorris, S. Hydrogen permeability of SrCe1-xMxO3-δ(x = 0.05, M=Eu, Sm). Solid State Ion. 2004, 167, 99–105.
- Phillips, R. Structural and electrical characterisation of SrCe1-xYxOξ. Solid State Ion. 1999, 125, 389–395.
- Balachandran, U.; Lee, T.; Chen, L.; Song, S.; Picciolo, J.; Dorris, S. Hydrogen separation by dense cermet membranes. Fuel 2006, 85, 150–155.
- Jeon, S.Y.; Lim, D.K.; Choi, M.B.; Wachsman, E.D.; Song, S.J. Hydrogen separation by Pd-CaZr0.9Y0.1O3-δ cermet composite membranes. Sep. Purif. Technol. 2011, 79, 337–341.
- Kim, H.; Kim, B.; Lee, J.; Ahn, K.; Kim, H.-R.; Yoon, K.J.; Kim, B.-K.; Cho, Y.W.; Lee, H.-W.; Lee, J.-H. Microstructural adjustment of Ni-BaCe0.9Y0.1O3-δ cermet membrane for improved hydrogen permeation. Ceram. Int. 2014, 40, 4117–4126.
- Song, S.J.; Lee, T.H.; Wachsman, E.D.; Chen, L.; Dorris, S.E.; Balachandran, U. Defect Structure and Transport Properties of Ni-SrCeO3-δ Cermet for Hydrogen Separation Membrane. J. Electrochem. Soc. 2005, 152, J125.
- Jeon, S.-Y.; Choi, M.-B.; Singh, B.; Song, S.-J. Hydrogen separation by dual functional cermet membranes with self-repairing capability against the damage by H2S. J. Memb. Sci. 2013, 428, 46–51.
- Balachandran, U.; Lee, T.H.; Park, C.Y.; Emerson, J.E.; Picciolo, J.J.; Dorris, S.E. Dense cermet membranes for hydrogen separation. Sep. Purif. Technol. 2014, 121, 54–59.
- Jeon, S.Y.; Choi, M.B.; Park, C.N.; Wachsman, E.D.; Song, S.J. High sulfur tolerance dual-functional cermet hydrogen separation membranes. J. Memb. Sci. 2011, 382, 323–327.
- Tsai, Y.C.; Lin, C.C.; Lin, W.L.; Wang, J.H.; Chen, S.Y.; Lin, P.; Wu, P.W. Palladium based cermet composite for hydrogen separation at elevated temperature. J. Power Sources 2015, 274, 965–970.
- Zhu, Z.; Sun, W.; Dong, Y.; Wang, Z.; Shi, Z.; Zhang, Q.; Liu, W. Evaluation of hydrogen permeation properties of Ni-Ba(Zr0.7Pr0.1Y0.2)O3-δ cermet membranes. Int. J. Hydrogen Energy 2014, 39, 11683–11689.
- Wei, Y.; Xue, J.; Fang, W.; Chen, Y.; Wang, H.; Caro, J. Enhanced stability of Zr-doped Ba(CeTb)O(3-δ)-Ni cermet membrane for hydrogen separation. Chem. Comm. 2015, 51, 11619–11621.
- Zuo, C.; Lee, T.H.; Dorris, S.E.; Balachandran, U.; Liu, M. Composite Ni-Ba(Zr0.1Ce0.7Y0.2)O3 membrane for hydrogen separation. J. Power Sources 2006, 159, 1291–1295.
- Yan, L.; Sun, W.; Bi, L.; Fang, S.; Tao, Z.; Liu, W. Influence of fabrication process of Ni-BaCe0.7Zr0.1Y0.2O3-δ cermet on the hydrogen permeation performance. J. Alloys Compd. 2010, 508, L5–L8.
- Zuo, C.; Dorris, S.E.; Balachandran, U.; Liu, M. Effect of Zr-doping on the chemical stability and hydrogen permeation of the Ni-BaCe0.8Y0.2O3-δ mixed protonic-electronic conductor. Chem. Mater. 2006, 18, 4647–4650.
- Fang, S.; Brinkman, K.; Chen, F. Unprecedented CO2-Promoted Hydrogen Permeation in Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3-δ Membrane. ACS Appl. Mater. Interfaces 2014, 6, 725–730.
- Fang, S.; Brinkman, K.S.; Chen, F. Hydrogen permeability and chemical stability of Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3-δ membrane in concentrated H2O and CO2. J. Memb. Sci. 2014, 467, 85–92.
- Meng, X.; Song, J.; Yang, N.; Meng, B.; Tan, X.; Ma, Z.; Li, K. Ni-BaCe0.95Tb0.05O3−δ cermet membranes for hydrogen permeation. J. Memb. Sci. 2012, 401, 300–305.
- Song, S.; Moon, J.; Lee, T.; Dorris, S.; Balachandran, U. Thickness dependence of hydrogen permeability for Ni-BaCe0.8Y0.2O3-δ. Solid State Ion. 2008, 179, 1854–1857.
- Zhuang, L.; Li, J.; Xue, J.; Jiang, Z.; Wang, H. Evaluation of hydrogen separation performance of Ni-BaCe0.85Fe0.15O3-δ cermet membranes. Ceram. Int. 2019, 45, 10120–10125.
- Ma, X.; Yang, C.; Chen, H.; Lv, Q.; Sun, K.; Li, W. Hydrogen permeation and chemical stability of Ni-BaCe0.7In0.2Ta0.1O3-δ cermet membrane. Sep. Purif. Technol. 2020, 236, 116276.
- Yang, C.; Ma, X.; Chen, H.; Lv, Q.; Sun, K.; Chen, J.; Yun, S. Chemical stability and hydrogen permeation performance of Ni-BaCe0.7Y0.3-xInxO3-δ cermet membranes. J. Alloys Compd. 2018, 762, 409–414.
- Zhu, Z.; Sun, W.; Wang, Z.; Cao, J.; Dong, Y.; Liu, W. A high stability Ni-La0.5Ce0.5O2-δ asymmetrical metal-ceramic membrane for hydrogen separation and generation. J. Power Sources 2015, 281, 417–424.
- Fang, S.; Lei, B.; Yan, L.; Sun, W.; Wei, L. CO2-Resistant Hydrogen Permeation Membranes Based on Doped Ceria and Nickel. J. Phys. Chem. C 2010, 114, 10986–10991.
- Yan, L.; Sun, W.; Bi, L.; Fang, S.; Tao, Z.; Liu, W. Effect of Sm-doping on the hydrogen permeation of Ni-La2Ce2O7 mixed protonic-electronic conductor. Int. J. Hydrogen Energy 2010, 35, 4508–4511.
- Xie, H.; Zhuang, L.; Wei, Y.; Xue, J.; Wang, H. CO2-tolerant Ni-La5.5WO11.25-δ dual-phase membranes with enhanced H2 permeability. Ceram. Int. 2017, 43, 14608–14615.
- Rosensteel, W.A.; Sullivan, N.P. Fabrication and hydrogen permeation through novel BaZr0.9Y0.1O3-δ-Cu composite ceramic-metallic membranes. Int. J. Hydrogen Energy 2017, 42, 4216–4223.
- Park, J.H.; Jeon, S.I.; Sim, W.J.; Baek, I.H.; Choi, S.H. Stability of Ta/YSZ cermet membrane for hydrogen separation. Energy Procedia 2011, 4, 756–762.
- Unemoto, A.; Kaimai, A.; Sato, K.; Yashiro, K.; Matsumoto, H.; Mizusaki, J.; Amezawa, K.; Kawada, T. Hydrogen permeability and electrical properties in oxide composites. Solid State Ion. 2008, 178, 1663–1667.
- Song, J.; Meng, B.; Tan, X. Stability and electrical conductivity of BaCe0.85Tb0.05M0.1O 3-δ (M=Co, Fe, Y, Zr, Mn) high temperature proton conductors. Ceram. Int. 2016, 42, 13278–13284.
- Rosensteel, W.A.; Ricote, S.; Sullivan, N.P. Hydrogen permeation through dense BaCe0.8Y0.2O3-δ-Ce0.8Y0.2O2-δ composite-ceramic hydrogen separation membranes. Int. J. Hydrogen Energy 2016, 41, 2598–2606.
- Ivanova, M.E.; Escolastico, S.; Balaguer, M.; Palisaitis, J.; Sohn, Y.J.; Meulenberg, W.A.; Guillon, O.; Mayer, J.; Serra, J.M. Hydrogen separation through tailored dual phase membranes with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ at intermediate temperatures. Sci. Rep. 2016, 6, 34773–34785.
- Fish, J.S.; Ricote, S.; Lenrick, F.; Wallenberg, L.R.; Holgate, T.C.; O’hayre, R.; Bonanos, N. Synthesis by spark plasma sintering of a novel protonic/electronic conductor composite: BaCe0.2Zr0.7Y0.1O3-δ /Sr0.95Ti0.9Nb0.1O3-δ (BCZY27/STN95). J. Mater. Sci. 2013, 48, 6177–6185.
- Sun, W.; Shi, Z.; Liu, W. Considerable Hydrogen Permeation Behavior through a Dense Ce0.8Sm0.2O2-δ(SDC) Asymmetric Thick Film. J. Electrochem. Soc. 2013, 160, F585–F590.
- Ricote, S.; Manerbino, A.; Sullivan, N.P.; Coors, W.G. Preparation of dense mixed electron- and proton-conducting ceramic composite materials using solid-state reactive sintering: BaCe0.8Y0.1M0.1O3-δ-Ce0.8Y0.1M0.1O2-δ (M=Y, Yb, Er, Eu). J. Mater. Sci. 2014, 49, 4332–4340.
- Liu, Y.; Dai, L.; Zhang, W.; Zhou, H.; Li, Y.; Wang, L. Preparation of dual-phase composite BaCe0.8Y0.2O3/Ce0.8Y0.2O2 and its application for hydrogen permeation. Ceram. Int. 2016, 42, 6391–6398.
- Escolástico, S.; Solís, C.; Kjølseth, C.; Serra, J.M. Outstanding hydrogen permeation through CO2-stable dual-phase ceramic membranes. Energy Environ. Sci. 2014, 7, 3736–3746.
- Escolástico, S.; Kjølseth, C.; Serra, J.M. Catalytic activation of ceramic H2 membranes for CMR processes. J. Memb. Sci. 2016, 517, 57–63.
- Rebollo, E.; Mortalò, C.; Escolástico, S.; Boldrini, S.; Barison, S.; Serra, J.M.; Fabrizio, M. Exceptional hydrogen permeation of all-ceramic composite robust membranes based on BaCe0.65Zr0.20Y0.15O3-δ and Y-or Gd-doped ceria. Energy Environ. Sci. 2015, 8, 3675–3686.
- Wang, T.; Zhang, H.; Meng, B.; Wang, X.; Sunarso, J.; Tan, X.; Liu, S. SrCe0.95Y0.05O3-δ-ZnO dual-phase membranes for hydrogen permeation. RSC Adv. 2016, 6, 36786–36793.
- Wang, Y.; Huang, J.; Su, T.; Liu, W.; Qi, H.; Yang, J. Synthesis, microstructure and electrical properties of BaZr0.9Y0.1O3-δ: BaCe0.86Y0.1Zn0.04O3-δ proton conductors. Mater. Sci. Eng. B 2015, 196, 35–39.
- Polfus, J.M.; Xing, W.; Fontaine, M.-L.; Denonville, C.; Henriksen, P.P.; Bredesen, R. Hydrogen separation membranes based on dense ceramic composites in the La27W5O55.5-LaCrO3 system. J. Memb. Sci. 2015, 479, 39–45.




