In recent years, dual-phase MPEC hydrogen separation membranes with high electronic and protonic conductivity have attracted great attention. The dual-phase MPEC membrane is fabricated by combing protonic conductor with an electronic conducting phase; in addition, according to the composition difference of the electronic conducting phase, dual-phase MPEC membranes are further divided into ceramic–metal (cermet) membranes and ceramic–ceramic (cercer) membranes, in which metal and ceramic with high electronic conductivity are used respectively.
Cermet membrane is composed of a ceramic phase and a metal phase. The ceramic phase is benefit to improve the mechanical stability and high protonic conductivity of the membrane while the metal phase is used to enhance the electronic conductivity and surface-exchange kinetic. Palladium (Pd) and nickel (Ni) are the common used metals in cermet membranes.
Dense Pd membrane is a kind of membrane with excellent hydrogen permeability, and it can be applied to prepare high purity and high flux hydrogen. However, the low mechanical stability, low chemical stability, and the high cost restrict its industry application. In 2006, Argonne National Laboratory developed a cermet dual-phase membrane using Pd as the hydrogen transport metal and yttria-stabilized ZrO
2 (YSZ) as the mechanically stable matrix [
33]. The membrane showed a high hydrogen permeability, of which the H
2 permeation rate reached 20 mL·min
−1·cm
−2 at 900 °C. Moreover, the membrane stability was investigated under H
2S atmosphere. Hydrogen flux showed no degradation for 270 h in atmosphere containing 400 ppm H
2S. The results indicated the combination of hydrogen separation metal and ceramic matrix can ensure the high hydrogen permeability and good mechanical stability of the membrane. Inspired by the excellent performance of Pd-YSZ, Jeon et al. fabricated a cermet dual-phase membrane by embedding Pd in protonic conducting ceramic matrix CaZr
0.9Y
0.1O
3-δ (CZY) [
34]. In this membrane, CZY is not only the matrix of Pd but also an active contributor for the hydrogen separation, facilitating the transport of protons through the membrane.
2.2. Ni-Based Cermet Membranes
Attributing to the unique properties, Ni is considered to be one of the most suitable metals used for conducting electrons in cermet membranes. As a well-known anode catalyst for fuel cells, Ni has high surface exchange kinetics, is cheaper, and has superior chemical and thermal stability compared with Pt. In addition, Ni has similar thermal expansion with BaCe0.9Y0.1O3-δ, which can enable Ni to have good compatibility with ceramic phases. Moreover, high sintering temperature is necessary to prepare a dense membrane, and the melting point of Ni is up to 1453 °C and can ensure the stability of the membrane during the sintering process.
The content of the metal phase in the composites is closely associated with the hydrogen permeability. To gain high hydrogen permeability, the amount of the metal phase needed in the composites should be firstly ascertained. Kim et al. studied the effect of membrane composition on the membrane electrical conductivity and hydrogen permeability [
48]. The results showed that only when the Ni content reached 40 vol%, the metal phase could form a three-dimensional network and cause sufficient Ni connectivity. The membrane containing 40 vol% Ni had higher electrical conductivity and H
2 permeability compared with the membranes with 30 and 35 vol% Ni. As shown in
Table 1, Ni content in most of the current Ni-based dual-phase membranes was set to 40 vol%. Song et al. prepared cermet composite Ni-SrCe
0.8Yb
0.2O
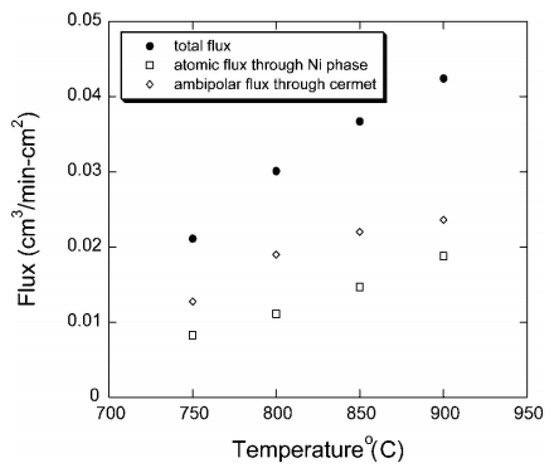
3-x (Ni-SCYb) containing 40 vol% Ni via ball-milling method. Both the membrane electronic conductivity and surface exchange kinetics were improved distinctly. The hydrogen flux was ten times that of single SCYb membrane. Hydrogen permeation due to the atomic diffusion through Ni phase was also detected (
Figure 4). However, compared with Pd-based composite membranes, the atomic hydrogen diffusion of Ni is much weaker, and the ambipolar diffusion of SCYb was dominant over the whole temperature range [
52].
Table 1. Cermet membranes with different hydrogen permeation performances.
| Composition |
Hydrogen Flux
(mL·min−1·cm−2) |
T (°C) |
Thickness
(mm) |
Feed/Sweep Gas |
Note |
Ref. |
| 50Pd-50YSZ |
20 |
900 |
0.22 |
100%H2/N2 |
mechanical mixing |
[33] |
| 50Pd-50YSZ |
5.5 |
900 |
0.218 |
80% H2-He/N2 |
ball-milling |
[36] |
| 50Pd-50YSZ |
52 |
900 |
0.018 |
100%H2/N2 |
mechanical mixing; asymmetric |
[37] |
| 50Pd-50Gd0.2Ce0.8O2-δ |
5.5 |
900 |
0.282 |
80% H2-He/N2 |
ball-milling |
[38] |
| 50Pd-50CaZr0.9Y0.1O3-δ |
2.3 |
900 |
0.50 |
80% H2-He/N2 |
ball-milling |
[34] |
| 50Pd-50BaCe0.4Zr0.4Gd0.1Dy0.1O3-δ |
2.77 |
700 |
0.40 |
100%H2/N2 |
ball-milling |
[35] |
| 40Ni-60BaZr0.7Pr0.1Y0.2O3-δ |
0.0162 |
950 |
0.40 |
wet50%H2-N2/Ar |
mechanical mixing |
[39] |
| 50Ni-50BaCe0.85Te0.05 Zr0.1O3-δ |
0.17 |
800 |
0.50 |
50%H2-He/Ar |
mechanical mixing |
[40] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ |
0.805 |
900 |
0.266 |
100%H2/N2 |
ball-milling |
[41] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ |
0.056 |
900 |
1 |
100%H2/N2 |
ball-milling |
[42] |
| 40Ni-60BaZr0.1Ce0.7Y0.2O3-δ |
0.087 |
900 |
0.5 |
wet4%H2-Ar/N2 |
ball-milling |
[43] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ |
0.0174 |
900 |
0.75 |
20%H2-He/N2 |
mechanical mixing |
[44] |
| 40Ni-60BaZr0.1Ce0.7Y0.1Yb0.1O3-δ |
0.215 |
900 |
0.40 |
wet80%H2-He/N2 |
mechanical mixing |
[45] |
| 50Ni-50BaCe0.95Tb0.05O3-δ |
0.53 |
850 |
0.65 |
50%H2-He/N2 |
ball-milling |
[46] |
| 50Ni-50BaCe0.95Tb0.05O3-δ |
0.914 |
850 |
0.09 |
50%H2-He/N2 |
ball-milling; asymmetric |
[46] |
| 40Ni-60BaCe0.9Y0.1O3-δ |
0.24 |
900 |
0.08 |
wet3.8%H2-He/N2 |
ball-milling |
[47] |
| 40Ni-60BaCe0.9Y0.1O3-δ |
0.76 |
800 |
0.23 |
100%H2/N2 |
high-energy milling |
[48] |
| 50Ni-50BaCe0.85Fe0.15O3-δ |
0.325 |
1000 |
0.50 |
50%H2-He/wet Ar |
ball-milling |
[49] |
| 40Ni-60BaCe0.7In0.2Ta0.1O3-δ |
0.15 |
900 |
1 |
20%H2-N2/Ar |
mechanical mixing |
[50] |
| 40Ni-60BaCe0.7Y0.2In0.1O3-δ |
0.013 |
850 |
0.86 |
20%H2-N2 /Ar |
mechanical mixing |
[51] |
| 40Ni-60SrCe0.8Yb0.2O3-δ |
0.105 |
900 |
0.25 |
wet20%H2-He/N2 |
ball-milling |
[52] |
| 40Ni-60La0.5Ce0.5O2-δ |
0.088 |
900 |
0.048 |
20%H2-N2/Ar |
mechanical mixing; asymmetric |
[53] |
| 40Ni-60La0.5Ce0.5O2-δ |
0.021 |
900 |
0.6 |
wet20%H2-N2/Ar |
ball-milling |
[54] |
| 40Ni-60La0.4875Ca0.0125Ce0.5O2-δ |
0.025 |
900 |
0.6 |
wet20%H2-N2/Ar |
ball-milling |
[54] |
| 40Ni-60La1.95Sm0.05Ce2O7 |
0.037 |
900 |
0.6 |
wet20%H2- Ar/N2 |
ball-milling |
[55] |
| 60Ni-40La5.5WO11.25-δ |
0.18 |
1000 |
0.5 |
50%H2-He/Ar |
ball-milling |
[56] |
| Cu-BaZr0.9Y0.1O3-δ |
0.00242 |
882 |
1.90 |
100%H2/Ar |
molten-copper infiltration
technique |
[57] |
| 60Ta-40YSZ |
1.2 |
500 |
0.50 |
100%H2/Ar |
mechanical mixing |
[58] |
Figure 4. The contribution of two diffusion mechanisms to the total hydrogen permeation fluxes in Ni-SCYb cermet dual-phase membranes [
52].
Since the electronic conductivity of the Ni phase decreases at elevated temperatures, enhancement of membrane permeability at high temperatures should be attributed to the improvement of the protonic conductivity of the ceramic phase. Therefore, for Ni-based dual-phase membranes, the hydrogen permeability mainly depends on the protonic conductivity of the ceramic phases. For the Ni phase, although the electronic conductivity decreases at high temperatures, the hydrogen permeability increases because of the improvement of the atomic diffusion.
The stability is important for hydrogen separation membranes, especially for dual-phase membranes under vapor or CO
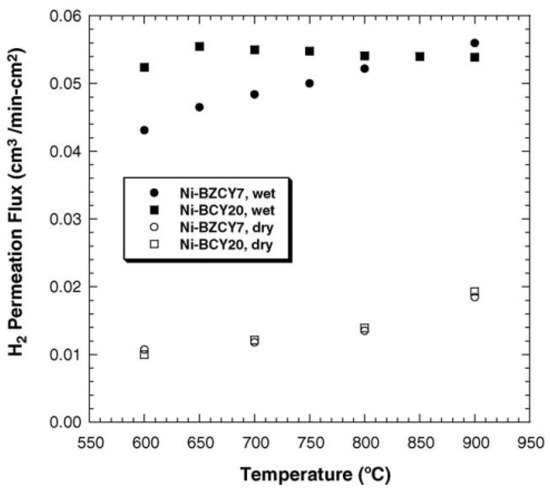
2-containing atmosphere. Humidity is favorable for the ceramic phase; both the catalytic activity and protonic conductivity of the ceramic phase will be enhanced owning to the hydration of the membrane. However, humidity is unfavorable for the metal phase; the conductivity of the metal phase will decrease in wet atmosphere due to the oxidizing of the surface. Zuo et al. studied the hydrogen permeability of Ni-Ba(Zr
0.8-xCe
xYb
0.2)O
3-α (Ni-BZCYb) membrane under wet conditions. As shown in
Figure 5, Ni-BZCYb had higher hydrogen fluxes under wet conditions [
41]. The stability of composite membrane Ni-BaZr
0.1Ce
0.7Y
0.1Yb
0.1O
3-δ (Ni-BZCYYb) was tested under wet CO
2 atmosphere. The hydrogen flux kept stable in wet 30 vol% CO
2. As shown in
Figure 6, no microstructure change happened on the membrane surface after the stability test. The authors attributed the excellent performance of the membrane to the Zr, Yb co-doping, and the modified membrane preparation method [
45].
Figure 5. H
2 fluxes through Ni-BZCYb membrane feeding with dry and wet 4% H
2 [
41].
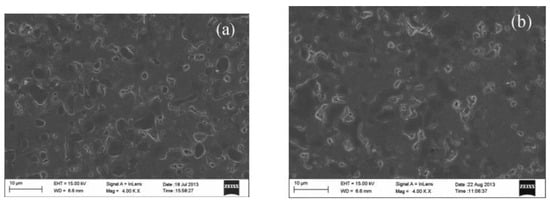
Figure 6. SEM images of Ni-BZCYYb sintered at 900 °C for 200 h in 20% CO
2 containing atmosphere, (
a) the original membrane, and (
b) the sintered membrane [
44].
3. Cercer Dual-Phase Membrane
Owing to the combination of a metal phase and a ceramic protonic conductor, the mixed protonic-electronic conductivity of cermet dual-phase membranes is significantly improved compared with ceramic single-phase membranes. However, cermet membranes still exhibit some unresolved issues, such as the oxidation of the metal phase under high temperatures and the thermal expansion coefficient mismatch between metal and ceramic. In order to overcome the drawbacks of cermet membranes, researchers developed another kind of dual-phase membrane (cercer membranes), in which a ceramic with high electronic conductivity replaces the metal as the electronic conducting phase. Because both the electronic and protonic conducting phases of the membrane are ceramic, the chemical and thermal property compatibility of the membrane can be effectively improved. Unemoto first proposed the concept of cercer dual-phase membrane and investigated the electronic conductivity and hydrogen permeation properties of a model membrane, SrZrO
3-SrFeO
3 [
59].
3.1. Barium Cerate-Based Cercer Membranes
As one of the advanced hydrogen-permeation materials, doped BaCeO
3 is the most commonly used protonic conducting phase in cercer membranes and has the highest protonic conductivity among the oxide protonic conductors. In addition, it also presents a nonnegligible electronic conductivity especially at high temperatures, and its electronic conductivity is the highest among the perovskite protonic conductors [
46,
60]. So far, the main electronic conducting phases used for barium cerate cercer membranes include fluorite oxides Y, Gd- or Sm-doped CeO
2, Nb-doped SrTiO
3, and SrFeO
3.
Rosensteel et al. designed a type of cercer hydrogen separation membrane using Y-doped BaCeO
3 (BCY) and CeO
2 (CYO) as the protonic and electronic conducting phase respectively. Fully dense dual-phase membranes were prepared via a single synthesis and sintering step. As a good electron conductor, CYO largely enhanced the membrane electronic conductivity [
61]. Ivanova et al. also reported a cercer membrane BaCe
0.8Eu
0.2O
3-δ-Ce
0.8Y
0.2O
2-δ (BCEO-CYO), which exhibited a remarkable high hydrogen permeation flux up to 0.61 mL·min
−1·cm
−2 at 700 °C. It was observed that Eu had a strong tendency to migrate from the BCEO phase to CYO phase, leading to a significant Eu-depleted BCEO [
62]. The electronic conductivity of three kinds of electronic conducting phases at 800 °C follows the trend of Sr
0.95Ti
0.9Nb
0.1O
3-δ (200 S·cm
−1) > Sm
0.8Y
0.2O
2-δ (0.39 S·cm
−1) > Ce
0.8Y
0.2O
2-δ (0.35 S·cm
−1) [
63,
64,
65].
The protonic conductivity of doped BaCeO
3 is excellent. Theoretically, its chemical stability should be poor under CO
2 atmosphere because there is a reaction between BaCeO
3 and CO
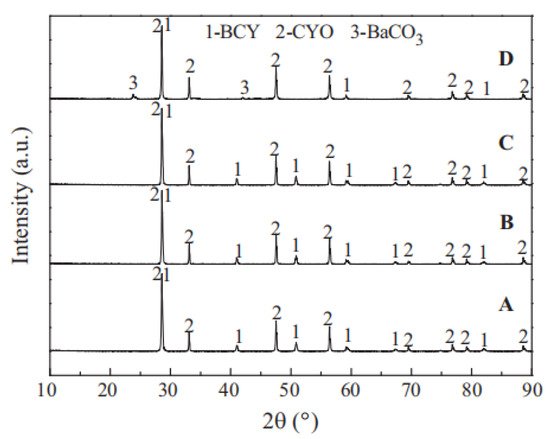
2 at high temperatures. However, as shown in the XRD images of BCY-CYO after the stability tests (
Figure 7), compared with BaCeO
3 single-phase membranes, the carbonate compounds generated reduced greatly under CO
2 atmosphere, and as a result, the chemical stability of the membrane was significantly improved. Liu et al. further speculated the improvement of chemical stability attributed to the formation of CeO
2; CeO
2 inhibited the reaction between CO
2 and BaCeO
3. As shown in Equation, the presence of CeO
2 makes the reaction proceed to the direction of forming BaCeO
3, thus protecting the perovskite structure [
66].
Figure 7. XRD patterns of BCY-CYO after the stability test in atmosphere containing 4% H
2 (
B), H
2O (
C), 100% CO
2 (
D); original membrane (
A) [
66].
3.2. Lanthanum Tungstate Cercer Membranes
Lanthanide tungstate oxides (LnWO, Ln = La, Nd, In, Sm) are important potential candidates used for hydrogen-separation membranes. These compounds present single defective fluorite structures while the ratio of Ln/W is in the range of 5.3 to 5.7. Besides the good protonic conductivity, lanthanide tungstate oxides exhibit better chemical stability against H
2O, CO
2, and H
2S than perovskite materials. Lanthanum tungstate (LWO) is one of the main lanthanide tungstate oxides; this material has significant hydrogen permeability at temperatures above 800 °C, but with insufficient permeability at temperatures less than 700 °C because of the low electronic conductivity. Escolástico et al. prepared a La
5.5WO
11.25-δ-La
0.87Sr
0.13CrO
3-δ (LWO-LSC) dual-phase membrane using LSC, in which LSC, used as the electronic conductivity phase, had both high p-type electronic conductivity and remarkable stability in reducing atmosphere. The dense membrane was obtained by sintering at 1400 °C and the H
2 flux at 700 °C reached 0.15 mL·min
−1·cm
−2, exhibiting a higher permeability than most of the BaCeO
3-based cercer membranes [
75]. The combination of LWO and LSC solved the abovementioned problem of LWO single-phase membranes well. Besides these, the stability and proton transport of composite membrane LWO-LSC was investigated under CH
4 atmosphere. Hydrogen flux remained unchanged for 5 h when 30% CH
4 was contained in the feed gas (
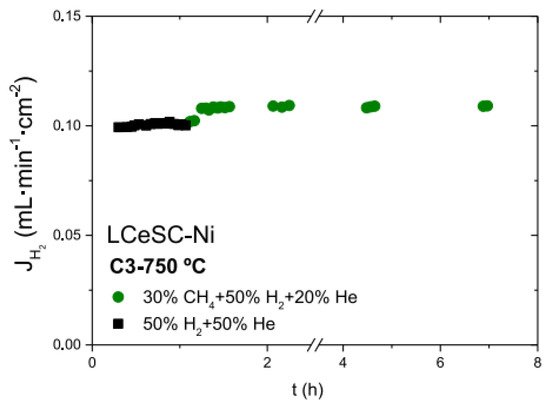
Figure 8). The membrane stability under high steam content methane was also evaluated. After annealing at 700 °C and 3 bars for 24 h in 50% CH
4-H
2O atmosphere, the membranes kept totally dense and no secondary phase was found [
77].
Figure 8. Hydrogen fluxes as a function of time for 60LWO-40LSC modified by La
0.75Ce
0.1Sr
0.15CrO
3-δ (LCeSC)-Ni feeding with different gases [
77].
3.3. Elimination of the Inter-Phase Reaction
To obtain a dense membrane, a high-temperature sintering process is inevitable for the preparation of composite membranes. However, high temperatures tend to cause inter-phase reactions inside the membrane, generating undesirable phases which may damage the performances of the membrane. Adding sintering aid is an efficient method to decrease the sintering temperature, and then decrease the inter-phase reaction. Rebollo prepared BaCe
0.65Zr
0.20Y
0.15O
3-δ-Ce
0.83Gd
0.15O
2-δ dual-phase membrane via a single synthesis and sintering step using ZnO as the sintering aid. Dense membranes without any cracks were obtained by sintering treatments at 1450 °C. The addition of the sintering aid decreases the sintering temperature and avoids the detrimental of high temperature to the membrane mechanical and electrical properties [
68]. Meng et al. fabricated a dual-phase membrane using ZnO and SrCe
0.95Y
0.05O
3-δ. ZnO was used as both sintering aid and electronic conductor in the membrane. The membrane was sintered at 1200 °C and there was no new phase formed during the sintering progress. The membrane possessed the highest conductivity when the content of ZnO reached 20 wt% [
73]. Wang et al. synthesized Zn doped BaZr
0.9Y
0.1O
3-δ-BaCe
0.86Y
0.1Zn
0.04O
3-δ dual-phase membranes by gel polymerization method; attributed to the doping of Zn, the membranes were well-sintered at 1350 °C and no extra phase was generated. In contrast, the non-doped membranes still kept their porous structure even when sintered at 1500 °C [
81]. Updating sintering technologies also is one of the important methods to decrease the sintering temperature and eliminate the inter-phase reaction.
3.4. Phase Composition Ratio
For cercer dual-phase membrane, both high protonic and electronic conductivity are necessary to obtain high hydrogen permeability, yet the phase ratio of the membranes have a great influence on the protonic and electronic conductivity. Therefore, to ensure high and comparable protonic and electronic conducting ability, the phase ratio of the membranes should be carefully investigated.
Escolástico et al. prepared different composite membranes using La
5.5WO
11.25-δ (LWO) and La
0.87Sr
0.13CrO
3-δ (LSC); the phase ratio was set as 2/8, 3/7, 4/6, and 7/3 respectively. The results showed that the 20%LWO-LSC composite had the highest total conductivity while the 50%LWO-LSC composite exhibited the highest hydrogen permeability [
75]. Polfus et al. prepared La
27W
3.5Mo
1.5O
55.5-δ-La
0.87Sr
0.13CrO
3-δ (LWMO-LSC) cercer composite membranes with different phase ratios. The results showed that the best permeability was obtained by the membrane of 70LWMO-30LSC in which complete electronically conducting phase percolation might not be formed [
78]. Similar to LWMO-LSC membrane, BCY-CYO membrane with phase ratio of 3/7 possessed the highest total conductivity and highest hydrogen permeability [
66]. It indicates that the electronic or protonic conducting phase in composite membrane may not be necessary to form a continuous network, which has ever been considered needed for high hydrogen permeability. Adding too much electronic conducting phase not only has no benefit to the hydrogen permeability but also can choke off the conductivity of the protonic conducting phase. The hydrogen permeability enhancement should be attributed to the effective combination of the protonic and electronic phases, which brings about high ambipolar conductivity.