
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marianna Christodoulou | -- | 2285 | 2022-07-10 11:58:41 | | | |
| 2 | Jessie Wu | Meta information modification | 2285 | 2022-07-14 07:51:26 | | |
Video Upload Options
Immunogenic cell death (ICD) is a type of regulated cell death (RCD), increasingly studied in recent years, due to its therapeutic implication in several diseases associated with immune system dysfunction. The new and increasingly studied concept of immunogenic cell death (ICD) revealed a previously unknown perspective of the various regulated cell death (RCD) modalities, elucidating their immunogenic properties and rendering obsolete the notion that immune stimulation is solely the outcome of necrosis. A distinct characteristic of ICD is the release of danger-associated molecular patterns (DAMPs) by dying and/or dead cells. These are evolutionary conserved stress signals, recognized primarily by innate immune system receptors. The immunogenicity of DAMPs characterizes ICD, rendering them potential prognostic, diagnostic clinical tools and/or possible therapeutic targets.
1. Introduction
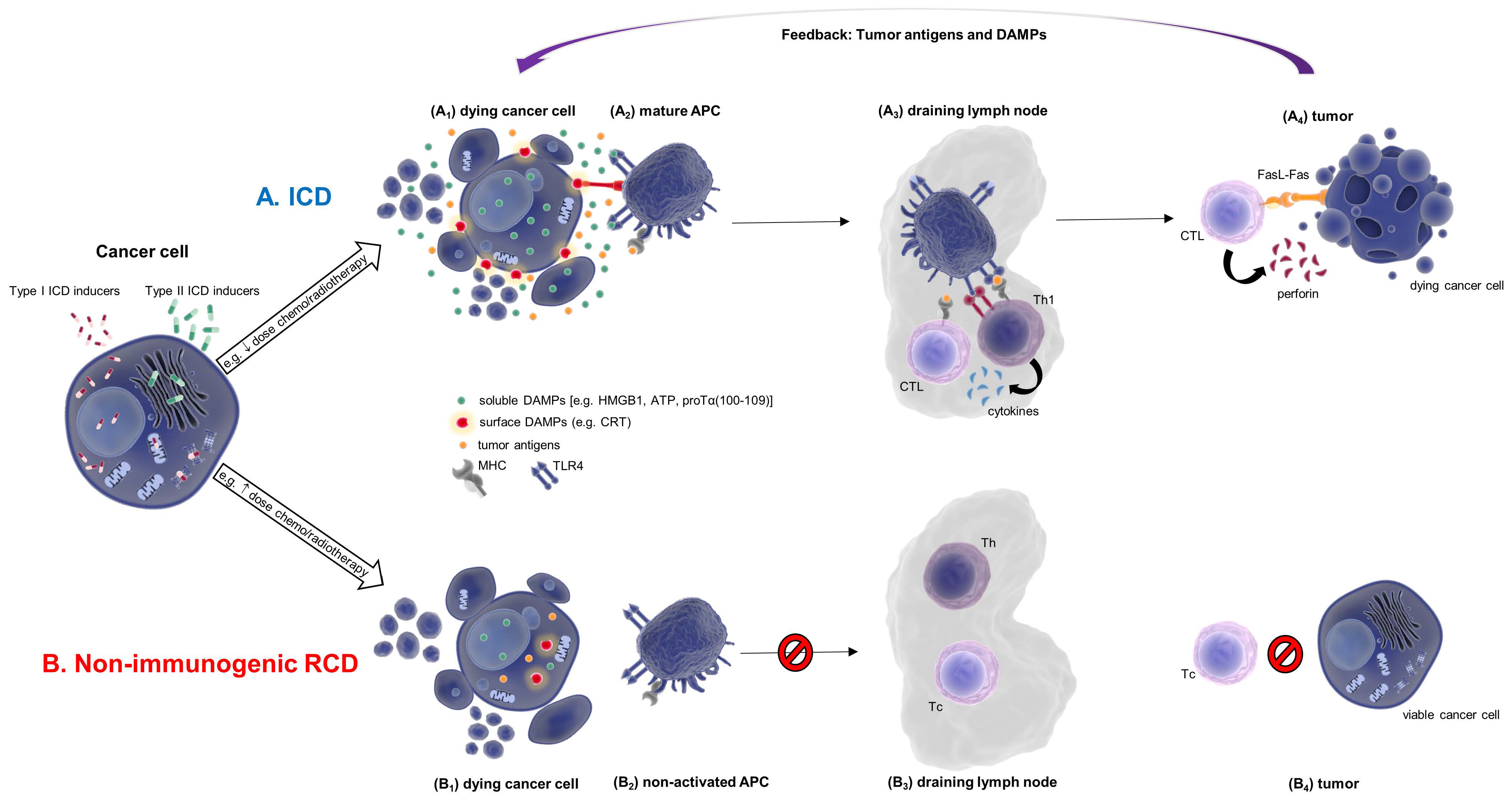
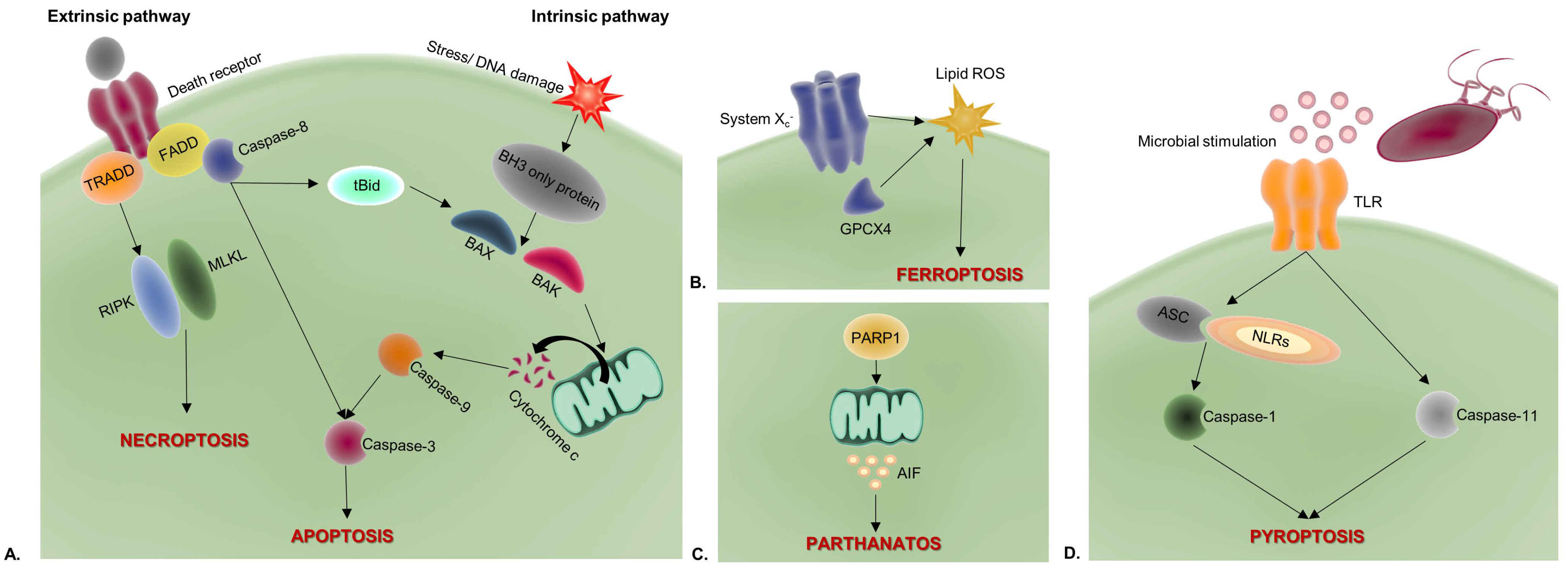
2. Apoptosis
3. Necroptosis
4. Pyroptosis
5. Ferroptosis
6. Parthanatos
| Cell Death Modality | Classification | Morphological Characteristics | Immunologic Profile | Regulators |
|---|---|---|---|---|
| Necrosis | ACD | cell swelling; DNA fragmentation; membrane rupture; loss of cell organelles | Tolerogenic/immunogenic | None |
| Apoptosis | RCD | cell shrinkage/rounding; nuclear condensation/fragmentation; nuclear membrane rupture; membrane blebbing; apoptotic body formation | Tolerogenic/immunogenic | Death receptors, BAX, BAK, AIF, caspases 2, 3, 6, 7, 8, and 9 |
| Necroptosis | RCD | cell/mitochondrial swelling; membrane rupture; chromatin condensation; loss of cell organelles | Immunogenic | TLRs, TCR, RIPK1, RIPK3, MLKL |
| Pyroptosis | RCD | cell swelling; membrane permeabilization/rupture; DNA condensation/ fragmentation | Immunogenic | CASP1, CASP11, GSDMD, NLRs, ALRs |
| Ferroptosis | RCD | mitochondrial shrinkage; reduced mitochondrial cristae; mitochondrial membrane rupture | Immunogenic | System XC−, GPX4, TFRC, ACSL4, LPCAT3, ALOX15, GLS2, DPP4, NCOA4, BAP1, BECN1, PEBP1, CARS, VDAC2/3, RAB7A, HSP90, ALK4/5 |
| Parthanatos | RCD | chromatin condensation; DNA fragmentation; membrane rupture; inconsistent mitochondrial membrane; no apoptotic body formation | Immunogenic | PARP-1, AIFM1, MIF, OGG1 |
| Anoikis | RCD | cell shrinkage/rounding; nuclear condensation/fragmentation; nuclear membrane rupture; membrane blebbing; apoptotic body formation; detachment from substrate/other cells | Tolerogenic/immunogenic | Death receptors, BAX, BAK, AIF, caspases 2, 3, 6, 7, 8, and 9 |
| MPT-driven necrosis | RCD | similar to necrosis; loss of mitochondrial inner membrane impermeability; mitochondrial membrane dissipation/breakdown | Immunogenic | CYPD (PPIF) |
| Entotic cell death (Entosis) |
RCD | cell-in-cell formation | Tolerogenic/immunogenic | RhoA, ROCKI/II, E-cadherin, α-catenin, actomyosin, LC3, ATGs |
| Neutrophil extracellular trap cell death (NETosis) | RCD | membrane rupture; nuclear membrane dissolvement; chromatin decondensation/release | Tolerogenic/immunogenic | NOX4, PAD4, ELANE, MMP, MPO, ELANE, MMP, MPO |
| Lysosome-dependent cell death (LDCD) |
RCD | lysosome/plasma membrane rupture | Immunogenic | BECN1, Na+/K+-ATPase, AMPK, Ras-like protein A |
| Autophagy-dependent cell death (ADCD) | RCD | vacuolization (large intracellular vesicles); enlargement of cell organelles; depletion of cell organelles | Immunogenic | UKL1, PI3KIII, ATGs, LC3 |
| Autosis | RCD | enhanced cell-substrate adherence; ER fragmentation/breakdown; cell swelling; chromatin condensation | Immunogenic | Na+/K+-ATPase |
| Alkaliptosis | RCD | similar to necrosis | Immunogenic | IKBKB, NF-κB |
| Oxeiptosis | RCD | similar to apoptosis | Tolerogenic | KEAP1, PGAM5, AIFM1 |
References
- Lucillia Bezu; Ligia C. Gomes-De-Silva; Heleen Dewitte; Karine Breckpot; Jitka Fucikova; Radek Spisek; Lorenzo Galluzzi; Oliver Kepp; Guido Kroemer; Combinatorial Strategies for the Induction of Immunogenic Cell Death. Frontiers in Immunology 2015, 6, 187, 10.3389/fimmu.2015.00187.
- Francesca Pentimalli; Sandro Grelli; Nicola Di Daniele; Gerry Melino; Ivano Amelio; Cell death pathologies: targeting death pathways and the immune system for cancer therapy. Genes & Immunity 2018, 20, 539-554, 10.1038/s41435-018-0052-x.
- Dmitri Krysko; Abhishek Garg; Agnieszka Kaczmarek; Olga Krysko; Patrizia Agostinis; Peter Vandenabeele; Immunogenic cell death and DAMPs in cancer therapy. Nature Cancer 2012, 12, 860-875, 10.1038/nrc3380.
- Lorenzo Galluzzi; Aitziber Buqué; Oliver Kepp; Lorenzo Galluzzi Aitziber Buqué Laurence Zitvogel; Guido Kroemer; Immunogenic cell death in cancer and infectious disease. Nature Reviews Immunology 2016, 17, 97-111, 10.1038/nri.2016.107.
- Irena Adkins; Lenka Sadilkova; Nada Hradilova; Jakub Tomala; Marek Kovar; Radek Spisek; Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. OncoImmunology 2017, 6, e1311433-e1311433, 10.1080/2162402x.2017.1311433.
- Alfonso Serrano-Del Valle; Alberto Anel; Javier Naval; Isabel Marzo; Immunogenic Cell Death and Immunotherapy of Multiple Myeloma. Frontiers in Cell and Developmental Biology 2019, 7, 50, 10.3389/fcell.2019.00050.
- Lorenzo Galluzzi; Ilio Vitale; Stuart A. Aaronson; John M. Abrams; Dieter Adam; Patrizia Agostinis; Emad S. Alnemri; Lucia Altucci; Ivano Amelio; David W. Andrews; et al.Margherita Annicchiarico-PetruzzelliAlexey V. AntonovEli AramaEric H. BaehreckeNickolai A. BarlevNicolas G. BazanFrancesca BernassolaMathieu J. M. BertrandKatiuscia BianchiMikhail V. BlagosklonnyKlas BlomgrenChristoph BornerPatricia BoyaCatherine BrennerMichelangelo CampanellaEleonora CandiDidac Carmona-GutierrezFrancesco CecconiFrancis K.-M. ChanNavdeep S. ChandelEmily H. ChengJerry E. ChipukJohn A. CidlowskiAaron CiechanoverGerald M. CohenMarcus ConradJuan R. Cubillos-RuizPeter CzabotarVincenzo D’AngiolellaTed M. DawsonValina L. DawsonVincenzo De LaurenziRuggero De MariaKlaus-Michael DebatinRalph J. DeBerardinisMohanish DeshmukhNicola Di DanieleFrancesco Di VirgilioVishva M. DixitScott J. DixonColin S. DuckettBrian D. DynlachtWafik S. El-DeiryJohn W. ElrodGian Maria FimiaSimone FuldaAna J. García-SáezAbhishek GargCarmen GarridoEvripidis GavathiotisPierre GolsteinEyal GottliebDouglas R. GreenLloyd A. GreeneHinrich GronemeyerAtan GrossGyorgy HajnoczkyJ. Marie HardwickIsaac S. HarrisMichael HengartnerClaudio HetzHidenori IchijoMarja JäätteläBertrand JosephPhilipp J. JostPhilippe P. JuinWilliam J. KaiserMichael KarinThomas KaufmannOliver KeppAdi KimchiRichard N. KitsisDaniel J. KlionskyRichard A. KnightSharad KumarSam W. LeeJohn J. LemastersBeth LevineAndreas LinkermannStuart A. LiptonRichard A. LockshinCarlos López-OtínScott W. LoweTom LueddeEnrico LugliMarion MacFarlaneFrank MadeoMichal MalewiczWalter MalorniGwenola ManicJean-Christophe MarineSeamus J. MartinJean-Claude MartinouJan Paul MedemaPatrick MehlenPascal MeierSonia MelinoEdward A. MiaoJeffery D. MolkentinUte M. MollCristina Muñoz-PinedoShigekazu NagataGabriel NuñezAndrew OberstMoshe OrenMichael OverholtzerMichele PaganoTheocharis PanaretakisManolis PasparakisJosef PenningerDavid M. PereiraShazib PervaizMarcus E. PeterMauro PiacentiniPaolo PintonJochen H.M. PrehnHamsa PuthalakathGabriel A. RabinovichMarkus RehmRosario RizzutoCecilia M.P. RodriguesDavid C. RubinszteinThomas RudelKevin M. RyanEmre SayanLuca ScorranoFeng ShaoYufang ShiJohn SilkeHans-Uwe SimonAntonella SistiguBrent R. StockwellAndreas StrasserGyorgy SzabadkaiStephen W.G. TaitDaolin TangNektarios TavernarakisAndrew ThorburnYoshihide TsujimotoBoris TurkTom Vanden BerghePeter VandenabeeleMatthew G. Vander HeidenAndreas VillungerHerbert W. VirginKaren H. VousdenDomagoj VucicErwin F. WagnerHenning WalczakDavid WallachYing WangJames A. WellsWill WoodJunying YuanZahra ZakeriBoris ZhivotovskyLaurence ZitvogelGerry MelinoGuido Kroemer Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death & Differentiation 2018, 25, 486-541, 10.1038/s41418-017-0012-4.
- Ivan Poon; Christopher Lucas; Adriano G. Rossi; Kodi Ravichandran; Apoptotic cell clearance: basic biology and therapeutic potential. Nature Reviews Immunology 2014, 14, 166-180, 10.1038/nri3607.
- Nader Yatim; Hélène Jusforgues-Saklani; Susana Orozco; Oliver Schulz; Rosa Barreira da Silva; Caetano Reis e Sousa; Douglas R. Green; Andrew Oberst; Matthew L. Albert; RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8 + T cells. Science 2015, 350, 328-334, 10.1126/science.aad0395.
- Dmitri V. Krysko; Peter Vandenabeele; Clearance of dead cells: mechanisms, immune responses and implication in the development of diseases. Apoptosis 2010, 15, 995-997, 10.1007/s10495-010-0524-6.
- Lynda M. Stuart; Mark Lucas; Cathy Simpson; Jonathan Lamb; John Savill; Adam Lacy-Hulbert; Inhibitory Effects of Apoptotic Cell Ingestion upon Endotoxin-Driven Myeloid Dendritic Cell Maturation. The Journal of Immunology 2002, 168, 1627-1635, 10.4049/jimmunol.168.4.1627.
- Reinhard E. Voll; Martin Herrmann; Edith A. Roth; Christian Stach; Joachim R. Kalden; Irute Girkontaite; Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350-351, 10.1038/37022.
- Nader Yatim; Hélène Jusforgues-Saklani; Susana Orozco; Oliver Schulz; Rosa Barreira da Silva; Caetano Reis e Sousa; Douglas R. Green; Andrew Oberst; Matthew L. Albert; RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8 + T cells. Science 2015, 350, 328-334, 10.1126/science.aad0395.
- Abhishek D. Garg; Dominika Nowis; Jakub Golab; Peter Vandenabeele; Dmitri V. Krysko; Patrizia Agostinis; Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochimica et Biophysica Acta 2010, 1805, 53-71, 10.1016/j.bbcan.2009.08.003.
- Michel Obeid; Antoine Tesniere; Francois Ghiringhelli; Gian Maria Fimia; Lionel Apetoh; Jean-Luc Perfettini; Maria Castedo; Grégoire Mignot; Theocharis Panaretakis; Noelia Casares; et al.Didier MétivierNathanael LarochettePeter van EndertFabiola CiccosantiMauro PiacentiniLaurence ZitvogelGuido Kroemer Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine 2006, 13, 54-61, 10.1038/nm1523.
- Lorenzo Galluzzi; Juliette Humeau; Aitziber Buqué; Laurence Zitvogel; Guido Kroemer; Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nature Reviews Clinical Oncology 2020, 17, 725-741, 10.1038/s41571-020-0413-z.
- Guido Kroemer; Claudia Galassi; Laurence Zitvogel; Lorenzo Galluzzi; Immunogenic cell stress and death. Nature Immunology 2022, 23, 487-500, 10.1038/s41590-022-01132-2.
- Davide Bedognetti; Society For Immunotherapy Of Cancer (Sitc) Cancer Immune Responsiveness Task Force And Working Groups; Michele Ceccarelli; Lorenzo Galluzzi; Rongze Lu; Karolina Palucka; Josue Samayoa; Stefani Spranger; Sarah Warren; Kwok Kin Wong; et al.Elad ZivDiego ChowellLisa M. CoussensDaniel D. De CarvalhoDavid G. DeNardoJérôme GalonHoward L. KaufmanTomas KirchhoffMichael T. LotzeJason J. LukeAndy J. MinnKaterina PolitiLeonard D. ShultzRichard SimonVésteinn ThórssonJoanne B. WeidhaasMaria Libera AsciertoPaolo Antonio AsciertoJames M. BarnesValentin BarsanPraveen K. BommareddyAdrian BotSarah E. ChurchGennaro CilibertoAndrea De MariaDobrin DraganovWinson S. HoHeather McGeeAnne MonetteJoseph F. MurphyPaola NisticòWungki ParkMaulik PatelMichael QuigleyLaszlo RadvanyiHarry RaftopoulosNils-Petter RudqvistAlexandra SnyderRandy F. SweisSara ValpioneRoberta ZappasodiLisa H. ButterfieldMary L. DisisBernard A. FoxAlessandra CesanoFrancesco M. Marincola Correction to: Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop. Journal for ImmunoTherapy of Cancer 2019, 7, 167, 10.1186/s40425-019-0640-y.
- Jitka Fucikova; Oliver Kepp; Lenka Kasikova; Giulia Petroni; Takahiro Yamazaki; Peng Liu; Liwei Zhao; Radek Spisek; Guido Kroemer; Lorenzo Galluzzi; et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death & Disease 2020, 11, 1-13, 10.1038/s41419-020-03221-2.
- Simone Fulda; Klaus‐Michael Debatin; Apoptosis Signaling in Tumor Therapy. Annals of the New York Academy of Sciences 2004, 1028, 150-156, 10.1196/annals.1322.016.
- S Fulda; K-M Debatin; Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798-4811, 10.1038/sj.onc.1209608.
- Henning Walczak; Peter H. Krammer; The CD95 (APO-1/Fas) and the TRAIL (APO-2L) Apoptosis Systems. Experimental Cell Research 2000, 256, 58-66, 10.1006/excr.2000.4840.
- Xavier Saelens; Nele Festjens; Lieselotte Vande Walle; Maria van Gurp; Geert van Loo; Peter Vandenabeele; Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861-2874, 10.1038/sj.onc.1207523.
- Nader Yatim; Sean Cullen; Matthew L. Albert; Dying cells actively regulate adaptive immune responses. Nature Reviews Immunology 2017, 17, 262-275, 10.1038/nri.2017.9.
- Matthew L. Albert; Birthe Sauter; Nina Bhardwaj; Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998, 392, 86-89, 10.1038/32183.
- Pisana Moroni Rawson; Caroline Molette; Melissa Videtta; Laura Altieri; Debora Franceschini; Tiziana Donato; Luigi Finocchi; Antonella Propato; Marino Paroli; Francesca Meloni; et al.Claudio Maria MastroianniGabriella D'ettorreJohn SidneyAlessandro SetteVincenzo Barnaba Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nature Medicine 2007, 13, 1431-1439, 10.1038/nm1679.
- Douglas R. Green; Thomas Ferguson; Laurence Zitvogel; Guido Kroemer; Immunogenic and tolerogenic cell death. Nature Reviews Immunology 2009, 9, 353-363, 10.1038/nri2545.
- Noelia Casares; Marie O. Pequignot; Antoine Tesniere; François Ghiringhelli; Stéphan Roux; Nathalie Chaput; Elise Schmitt; Ahmed Hamai; Sandra Hervas-Stubbs; Michel Obeid; et al.Frédéric CoutantDidier MétivierEvelyne PichardPierre AucouturierGérard PierronCarmen GarridoLaurence ZitvogelGuido Kroemer Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. Journal of Experimental Medicine 2005, 202, 1691-1701, 10.1084/jem.20050915.
- Jaba Gamrekelashvili; Tamar Kapanadze; Miaojun Han; Josef Wissing; Chi Ma; Lothar Jaensch; Michael P. Manns; Todd Armstrong; Elizabeth Jaffee; Ayla O. White; et al.Deborah E. CitrinFirouzeh KorangyTim F. Greten Peptidases released by necrotic cells control CD8+ T cell cross-priming. Journal of Clinical Investigation 2013, 123, 4755-4768, 10.1172/jci65698.
- Dominique Vercammen; Greet Brouckaert; Geertrui Denecker; Marc Van De Craen; Wim Declercq; Walter Fiers; Peter Vandenabeele; Dual Signaling of the Fas Receptor: Initiation of Both Apoptotic and Necrotic Cell Death Pathways. Journal of Experimental Medicine 1998, 188, 919-930, 10.1084/jem.188.5.919.
- William J Kaiser; Jason W Upton; Edward S Mocarski; Viral modulation of programmed necrosis. Current Opinion in Virology 2013, 3, 296-306, 10.1016/j.coviro.2013.05.019.
- Lieselotte Vande Walle; Mohamed Lamkanfi; Pyroptosis. Current Biology 2016, 26, R568-R572, 10.1016/j.cub.2016.02.019.
- Luigi Franchi; Tatjana Eigenbrod; Raúl Muñoz-Planillo; Gabriel Nuñez; The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology 2009, 10, 241-247, 10.1038/ni.1703.
- Lu Liu; Bingwei Sun; Neutrophil pyroptosis: new perspectives on sepsis. Cellular and Molecular Life Sciences 2019, 76, 2031-2042, 10.1007/s00018-019-03060-1.
- Brent R. Stockwell; Jose Pedro Friedmann Angeli; Hülya Bayir; Ashley Bush; Marcus Conrad; Scott J. Dixon; Simone Fulda; Sergio Gascón; Stavroula Hatzios; Valerian E. Kagan; et al.Kay NoelXuejun JiangAndreas LinkermannMaureen E. MurphyMichael OverholtzerAtsushi OyagiGabriela PagnussatJason ParkQitao RanCraig S. RosenfeldKonstantin SalnikowDaolin TangFrank M. TortiSuzy V. TortiShinya ToyokuniK.A. WoerpelDonna D. Zhang Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273-285, 10.1016/j.cell.2017.09.021.
- Howard O Fearnhead; Peter Vandenabeele; Tom Vanden Berghe; How do we fit ferroptosis in the family of regulated cell death?. Cell Death & Differentiation 2017, 24, 1991-1998, 10.1038/cdd.2017.149.
- Yitian Sun; Peng Chen; Bingtao Zhai; Mingming Zhang; Yu Xiang; Jiaheng Fang; Sinan Xu; Yufei Gao; Xin Chen; Xinbing Sui; et al.Guoxiong Li The emerging role of ferroptosis in inflammation. Biomedicine & Pharmacotherapy 2020, 127, 110108, 10.1016/j.biopha.2020.110108.
- Jing-Jie Peng; Wei-Tao Song; Fei Yao; Xuan Zhang; Jun Peng; Xiu-Ju Luo; Xiao-Bo Xia; Involvement of regulated necrosis in blinding diseases: Focus on necroptosis and ferroptosis. Experimental Eye Research 2020, 191, 107922, 10.1016/j.exer.2020.107922.
- Karen Kate David; Shaida Ahmad Andrabi; Ted Murray Dawson; Valina Lynn Dawson; Parthanatos, a messenger of death. Frontiers in Bioscience 2009, ume, 1116-28, 10.2741/3297.
- Nirmal Robinson; Raja Ganesan; Csaba Hegedűs; Katalin Kovács; Thomas A. Kufer; László Virág; Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biology 2019, 26, 101239, 10.1016/j.redox.2019.101239.
- G Kroemer; W S El-Deiry; P Golstein; Marcus Ernst Peter; David Vaux; Peter Vandenabeele; Boris Zhivotovsky; M V Blagosklonny; W Malorni; R A Knight; et al.M PiacentiniShigekazu NagataG Melino Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death & Differentiation 2005, 12, 1463-1467, 10.1038/sj.cdd.4401724.
- Ge Yan; Mohamed Elbadawi; Thomas Efferth; Multiple cell death modalities and their key features (Review). World Academy of Sciences Journal 2020, 2, 39-48, 10.3892/wasj.2020.40.
- Daolin Tang; Rui Kang; Tom Vanden Berghe; Peter Vandenabeele; Guido Kroemer; The molecular machinery of regulated cell death. Cell Research 2019, 29, 347-364, 10.1038/s41422-019-0164-5.
- Shida Yousefi; Darko Stojkov; Nina Germic; Dagmar Simon; Xiaoliang Wang; Charaf Benarafa; Hans‐Uwe Simon; Untangling “NETosis” from NETs. European Journal of Immunology 2019, 49, 221-227, 10.1002/eji.201747053.
- Y Liu; B Levine; Autosis and autophagic cell death: the dark side of autophagy. Cell Death & Differentiation 2014, 22, 367-376, 10.1038/cdd.2014.143.




