Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marni E Shoemaker | -- | 5391 | 2022-07-11 18:30:28 | | | |
| 2 | Sirius Huang | Meta information modification | 5391 | 2022-07-12 04:49:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shoemaker, M.E.; Salmon, O.F.; Smith, C.M.; Duarte-Gardea, M.O.; Cramer, J.T. Vitamin D and Iron on Skeletal Muscle Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/25019 (accessed on 08 February 2026).
Shoemaker ME, Salmon OF, Smith CM, Duarte-Gardea MO, Cramer JT. Vitamin D and Iron on Skeletal Muscle Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/25019. Accessed February 08, 2026.
Shoemaker, Marni E., Owen F. Salmon, Cory M. Smith, Maria O. Duarte-Gardea, Joel T. Cramer. "Vitamin D and Iron on Skeletal Muscle Health" Encyclopedia, https://encyclopedia.pub/entry/25019 (accessed February 08, 2026).
Shoemaker, M.E., Salmon, O.F., Smith, C.M., Duarte-Gardea, M.O., & Cramer, J.T. (2022, July 11). Vitamin D and Iron on Skeletal Muscle Health. In Encyclopedia. https://encyclopedia.pub/entry/25019
Shoemaker, Marni E., et al. "Vitamin D and Iron on Skeletal Muscle Health." Encyclopedia. Web. 11 July, 2022.
Copy Citation
Many nutrients are essential for skeletal muscle health including vitamin D and iron. These nutrients have been well-established to play a role in improving muscular strength, muscle mass, and muscle function in various populations such as youth, athletes, and older adults. Together, muscular strength, muscle mass, and muscle function are important components that make up “skeletal muscle health”. Beyond the role that these nutrients have on skeletal muscle health, emerging literature has reported a link between vitamin D action on pro-inflammatory cytokines and mechanisms behind iron regulation, which may physiologically play a role in skeletal muscle health.
animal food sources
iron
muscle function
muscle mass
muscle strength
vitamin D
1. Vitamin D and Skeletal Muscle Health
1.1. Vitamin D and Skeletal Muscle Physiology
Vitamin D is a fat-soluble prohormone that provides key functions in multiple endocrine and autocrine processes throughout the body. Vitamin D can be obtained by both food sources and solar ultraviolet B (UVB) of 290–315 nm and in conjunction, is utilized to synthesize cholecalciferol, or vitamin D3 [1][2]. This production of vitamin D is dependent upon the solar elevation in which vitamin D is predominately produced when the elevation angle is greater than 45 degrees [3]. Once synthesized in the skin from sunlight or absorbed from food, vitamin D3 binds to vitamin D-binding protein (VDBP) and is transferred to the liver where it is converted to 25-hydroxycholecalciferol (25(OH)D) and transported to the kidney for further processing. Within the kidney, 25(OH)D is activated to the form of 1,25-dihydroxycholecalciferol [1,25(OH)2D], or calcitriol [4][5][6]. This active form can then perform functions such as calcium and phosphate regulation through the binding of vitamin D receptor (VDR). Vitamin D receptor is present on many body tissues including skeletal muscle, intestines, myocardium, bone, nervous system, as well as immune cells, indicating inadequate vitamin D effects multiple tissues within the body, resulting in a potential link to multiple pathological diseases including cardiovascular disease, inflammatory conditions, and respiratory illness [7][8][9][10][11].
The mechanism for the role vitamin D has on skeletal muscle involves VDR expression found in skeletal muscle cells [12][13][14]. Expression of VDR in the nucleus of skeletal muscle cells is necessary for vitamin D uptake [15], and reduced VDR concentrations have affected the contractility of muscle cells and may affect skeletal muscle repair and recovery [16][17]. Animal studies have demonstrated that mice without the VDR gene had smaller muscle fibers, lower body size and weight, and impaired movement compared to mice with VDR gene [18]. Additionally, VDR concentrations have been shown to increase after supplementation of 1,25(OH)2D3 and 25(OH)D3 in muscle cells, which was suggested to be linked to muscle cell regeneration [19].
Further support for the importance of VDR in skeletal muscle health was demonstrated in human studies. Expression of VDR primarily has been found to be located on fast-twitch muscle fibers [20], and interestingly, it has been identified that fast-twitch muscle fibers following vitamin D supplementation [21]. Thus, this provides support for the importance of VDR concentration, related to vitamin D status, for improving skeletal muscle health in humans. Supplementation with vitamin D3 in vitamin D insufficient females resulted increased VDR concentration [22], indicating that adequate VDR and vitamin D concentrations can support muscle fiber growth. Additionally, vitamin D actions on skeletal muscle through VDR may also influence calcium regulation and muscle contraction, anabolic or growth pathways, oxidative phosphorylation and mitochondrial function, and muscle inflammation [23].
1.2. Vitamin D Status and Skeletal Muscle Health
Vitamin D mechanistically appears to influence skeletal muscle health. Therefore, this effect on skeletal muscle health in conjunction with a high prevalence of vitamin D deficiency in certain populations such as youth, athletes, and older adults warrants an examination of the associations between vitamin D and measurements of muscle mass, muscle strength, and muscle function. Many studies in athletic populations reported associations between concentrations of 25(OH)D and measurements of performance [24][25][26][27][28]; however, other studies reported no associations between vitamin D and performance outcomes [29][30]. Koundorakis et al. reported correlations between serum 25(OH)D concentrations and tests of anaerobic and aerobic performance. These included moderate to high positive associations between serum 25(OH)D and jumping ability and VO2max (r = 0.394–0.740) and moderate negative associations between serum 25(OH)D and sprint times (r = −410–−0.649) during soccer pre- and post-season [24]. However, in male hockey players, there were no associations between serum 25(OH)D and aerobic exercise variables determined during a graded exercise test [29]. Forney et al. reported a positive association between serum 25(OH)D and VO2max (r = 0.360) but not with tests of anaerobic performance or muscle strength [30]. Additionally, submaximal aerobic performance and aerobic power was better in athletes with higher serum 25(OH)D levels compared to those with levels considered deficient (<35 ng·mL−1 and <30 ng·mL−1, respectively) [27][28]. Peak torque was found to be 12–17% higher in those with higher serum 25(OH)D levels [25][26]. These results suggest that while evidence supporting a positive association between vitamin D and athletic performance is inconclusive, many studies suggest that vitamin D levels are moderately related to performance. It is important to note that a majority of the studies were performed in young adult male athletes with varying tests of performance. Future studies inclusive of male and female athletes from a variety of athletic backgrounds is necessary.
In older adults, vitamin D showed consistent low to moderate relationships with muscle mass [31][32][33], muscle strength [31][34][35][36][37][38] and muscle function [32][34][35][37][38][39], with only a few of the studies finding no associations with skeletal muscle health. For example, while Conzade et al. reported a positive relationship between serum 25(OH)D concentrations and changes in muscle mass and an inverse relationship with time to complete TUG, there were no relationships with handgrip strength and gait speed [33]. Furthermore, Vaes et al. reported that older adults with serum 25(OH)D levels <50 nmol·L−1 and 50–75 nmol·L−1 had lower scores for tests of muscle function compared to those with levels >75 nmol·L−1 but observed no relationships with tests of muscle strength [39]. Similarly, in youth, positive relationships were consistently reported with tests of anaerobic (broad jump, vertical jump, sprints) performance and aerobic performance (estimated VO2max) [40][41][42][43]. However, there were mixed results with tests of muscle strength and serum 25(OH)D concentrations [44][45][46]. These results indicate that vitamin D levels are impactful on skeletal muscle mass and function across the lifespan, but the effects on muscle strength is inconclusive. In addition to these findings, it is reported that 25(OH)D metabolite accumulates in skeletal muscle cells, suggesting that maintaining muscle mass can also play a role in preserving vitamin D status in times when deficiency may become prevalent, suggesting that skeletal muscle health may have an influence on vitamin D levels [47][48].
1.3. Vitamin D Interventions and Skeletal Muscle Health
Multiple studies have examined the effects of vitamin D supplementation on muscle mass, strength, and function, typically with doses ranging from 1000 to 4000 IU·d−1 over 4–12 weeks to over 60,000 IU·week−1 for up to 4 months. Contrasting results of the effects vitamin D supplementation has on muscle health have been reported, potentially due to the large variety in dosage, type of vitamin D supplementation, duration of study, and target population [49][50]. In athletes, vitamin D supplementation has shown conflicting results with measurements of athletic performance. While some studies reported an increase in maximal strength performance for leg extensions, leg curls, and chin ups [51][52], other studies found no change in strength when compared to a control [53][54][55][56][57]. Close et al. demonstrated an increase in anaerobic performance (vertical jump height and 10 m sprint) after 6 weeks of 5000 IU vitamin D supplementation [54], while other studies showed no effect on anaerobic performance after supplementation [53][55][57]. One study reported an increase in VO2 max after 5000 IU·d−1 for 8 weeks [56], while another reported no changes in VO2 max after 12 weeks of 3000 IU·d−1 [57]. No studies in athletes examined the effects of vitamin D supplementation on muscle mass.
In older adults, the effects of vitamin D supplementation on strength were overall positive. Hajj et al. reported a greater change in handgrip strength compared to a placebo (10.13 to 27.98 ng·mL−1, p < 0.001 versus 10.56 to 15.71 ng·mL−1, p < 0.001) [58]. Bauer et al. observed an increase of 0.79 kg in handgrip strength over 13 weeks (p = 0.005); however, this supplementation also included whey protein and leucine, which may have contributed to the increase in strength [59]. Multiple studies have examined the effects of supplementation on tests of muscle function including the Short Physical Performance Battery (SPPB), chair stand, time-up and go, postural sways, and tests of walking speed. Increases in SPPB and chair stand performances were observed after 800–1000 IU of vitamin D compared to a placebo [59][60], but no differences were reported in other studies [61][62][63][64][65]. Similarly, studies that examined the effects of vitamin D on muscle mass were generally positive. In those receiving vitamin D supplementation, appendicular muscle mass increased [58][59] and lean mass was maintained over 9 months while decreasing in the placebo group [60]. Additionally, Ceglia et al. reported a 10.6% increase in muscle fiber cross-sectional area and 29.7% increase in VDR concentration, indicating a potential mechanistic influence of vitamin D on skeletal muscle [22]. These results indicate that while vitamin D supplementation may have a potential positive influence on muscle strength and function in older adults, there is more evidence supporting a beneficial effect on muscle mass in this population. Additionally, in all populations reviewed, the dosage and duration of treatment varied, ensuing in inconclusive results.
Few studies were observed reporting the effects of vitamin D supplementation on skeletal muscle health in a youth population [66][67][68]. These studies also varied in age, duration, and dosage of vitamin D supplementation. While each intervention resulted in an increase in serum 25(OH)D levels, there were no positive effects in muscle mass, strength, or function when compared to a placebo [66][67][68]. This suggests that vitamin D supplementation may be more effective and necessary as an individual ages, potentially due to reduced dietary intake, reduced sun exposure and ability of the skin to produce vitamin D, and impaired absorption [69][70], as well as impairments in vitamin D actions on skeletal muscle through mitochondrial dysfunction and compromised anabolic pathways with age [71]. Several studies indicate a relationship between serum 25(OH)D levels and muscle mass and strength, suggesting a mechanistic link between low vitamin D levels and declines in muscle mass, strength, and function. Vitamin D deficiency has been extensively researched in sarcopenic older adults, suggesting that while deficiency of vitamin D may lead to sarcopenia and related adverse outcomes such as higher risk of falls, muscle fiber atrophy, and disability during hospitalization [72][73], supplementation has shown conflicting results in improving sarcopenia [74][75] However, the conflicting results in intervention studies cannot confirm vitamin D supplementation has an effective way to increase performance or prevent and/or treat sarcopenia in older adults. It is more likely that protein sources including a variety of nutrients including vitamin D have a greater effect. For example, 12–13-week interventions of supplementation including vitamin D and leucine-enriched whey protein showed improvements in muscle mass and lower extremity strength and function in sarcopenic older adults [59][76][77], specifically if adequate protein was consumed [59][76][77].
Although many prospective cross-sectional and intervention studies have examined vitamin D and skeletal muscle health, conflicting results indicate that while there is potential for positive benefits of maintaining an adequate vitamin D status, there is no conclusive evidence that vitamin D levels beyond optimal range provide any enhancement to skeletal muscle health in a variety of populations. However, there appears to be a greater response in an older population, suggesting that maintaining levels through dietary intake and supplementation becomes more important with age. Additionally, the studies showed great diversity in participant characteristics, outcome measurements, and/or dosage amount and duration even within the separate population groups, which may have influenced the response or association between vitamin D and skeletal muscle health.
2. Iron and Skeletal Muscle Health
2.1. Iron and Skeletal Muscle Physiology
Iron is an essential mineral for multiple processes in the body that influence skeletal muscle performance such as oxygen transport, electron transport, and red blood cell production [78][79][80]. There is approximately 3–4 g of iron within the human body, in which about 70% of the body’s iron is found within hemoglobin (Hb) in red blood cells and myoglobin (Mb) within skeletal muscle [81]. Specifically, skeletal muscle contains about 10–15% of the iron in the body, mainly within oxidative fibers high in myoglobin [82]. The iron within the body is meticulously recycled to replace iron losses that occur within the gastrointestinal tract, skin, hair, sweat, and menses [83][84]. However, despite this efficient regulatory process, iron deficiency remains the most common nutritional deficiency in the world [81][85][86], typically from diminished iron absorption or increased iron loss, which is greater in females compared to males due to loss during menstruation [87]. Additionally, a vegan or vegetarian diet can be a risk factor for developing anemia, suggesting that dietary choices can be impactful for maintaining optimal iron status [88]. However, iron overload in thalassemia is an opposing challenge of iron deficiency that is influenced by iron regulation [89][90]. With the potential adverse health outcomes of iron overload, such as increased morbidity, maintaining an optimal iron status is necessary.
A number of biomarkers are utilized to reflect parts of the iron metabolism process, and therefore, are useful in isolation and in conjunction with one another. A common marker used to determine iron deficiency is ferritin. Ferritin is reflective of the body iron stores [91][92] and is used to determine the first stage of iron deficiency [81][93] defined as a lack of body iron stores. Ferritin levels <12–15 μg·L−1 indicate depleted iron stores; however, cutoff criteria between 15 and 35 μg·L−1 have often been utilized to diagnosis iron deficiency [94][95][96]. It is important to note that ferritin is an acute phase protein and is elevated with the presence of inflammation, so diagnosis with ferritin alone warrants caution in those with inflammation [97]. Correction for inflammation and measurement of inflammatory status are recommended when examining ferritin [98][99].
Decreased transferrin saturation or increased soluble transferrin receptor (sTfR) portrays the second stage of iron deficiency, reflecting reduced erythropoiesis. Transferrin saturation of <15–20% is considered indicative of iron deficiency [81]. An elevated sTfR indicates tissue iron deficiency and shows an inverse relationship with iron deficiency severity [100]. Together, the ratio of serum sTfR and serum ferritin can provide an index that reflects body iron. This measurement is effective for monitoring fluctuations in iron status [101].
The last stage of iron deficiency results in iron deficiency anemia with the addition of Hb as a biomarker [102]. Concentrations of Hb is a commonly measured parameter due to its affordability and accessibility; however, Hb is not specific to iron due to other potential contributors such as folate or vitamin B12 deficiency or anemia of chronic inflammation [81][103]. The inclusion of a second biomarker, such as ferritin or sTfR, simultaneously with Hb can confirm the diagnosis of iron deficiency anemia. Cutoff values range based on sex, age, and ethnicity [104]. According to the World Health Organization [102], diagnosis of anemia utilizing Hb concentrations have cutoff criteria of <115 g·L−1 for ages 5 to 11 years, <120 g·L−1 for ages 12–14 years, and <120 g·L−1 and 130 g·L−1 for females and males, respectively, 15 years or older [102][105].
Iron is essential for skeletal muscle function largely due to several pathways. While known for its necessary role for Hb production in red blood cells, iron is required for many processes for energy metabolism [106][107]. In particular, oxidative metabolism requires iron for adequate oxygen supply and the transfer of electrons during redox reactions [108][109]. Additionally, the majority of iron in skeletal muscle is within slow twitch muscle fibers that are abundant in myoglobin, in which oxidative metabolism occurs [109][110]. Enzymatic complexes within the electron transport chain rely on iron to function, indicating that an adequate supply of iron is essential for the oxidation of fuel sources for energy [107][108]. This indicates that the aerobic capacity of an individual greatly relies on the oxygen-carrying capacity of the blood as well as the muscle oxidative capacity [82], both of which are heavily dependent upon iron.
2.2. Iron and Skeletal Muscle Health
Multiple studies have examined associations between iron status and parameters of skeletal muscle health. In youth populations, the association between iron biomarkers and performance seemed to largely depend on what performance metric were utilized. Wang et al. reported that iron deficiency was related to lower fat-free associated VO2max within females, and both males and females with iron deficiencies had lower energy expenditure at leisure compared to adequate iron group [111]. Arsenault et al. reported that females with low ferritin levels had lower levels of performance during a shuttle run test, whereas males with low ferritin levels had lower long jump scores compared to those of normal ferritin levels [112]. Lastly, in a study conducted by Gracia-Marco et al. Hb concentrations were associated with estimated VO2max results in male adolescents only [41]. These varying outcomes provide insight into iron deficiency playing a role in oxygen transport directly influencing aerobic activities. The influence of iron status on anaerobic activities is further warranted to understand the role of iron on muscle strength, health, and function within youth populations.
Similar observations were observed in athletic populations where iron status seems to be more influential to the performance of aerobic-based activities. In a study conducted by DellaValle & Hass, female rower athletes that were categorized as iron depleted without anemia (IDNA) (serum ferritin < 20.0 μg·L−1), had lower VO2max and higher blood lactate concentrations during a 4 km rowing test [113]. In addition, the authors suggested that iron status also likely played a role in training load of the athletes where those athletes categorized as IDNA had lower training times than the non-anemic group during a 4-week observation. In addition, Tsai et al. reported that mildly anemic males enlisted within the Taiwan Military were likely to be the worst 10% performers during a standard 3000 m run test but were likely to be the best 10% performers during an anaerobic test such as the 2 min push-up test [114]. Shoemaker et al. also indicated within youth athletic population that performance during aerobic fitness tests such as vertical jump, broad jump, agility drill times, 20-yard dash time, power push up force, were related to Hb status in males and with sTfR and iron intake in females [115]. Together these studies further support the notion that iron plays a role in aerobic metabolism and cardiorespiratory fitness; however, the role on iron status and anaerobic fitness tests require further investigation.
Within the older adult population, iron status seems to play a role in frailty. For example, multiple studies reported that lower Hb concentrations were associated with higher frailty scores or associated with a greater risk of frailty than non-frail individuals [116][117][118][119]. However, in older adults, iron status seemed to vary based on muscle strength metrics. For example, low Hb levels were reported to not be associated with grip strength in older adults in Brazil [117]; however, in hospitalized older adults there was a positive association between Hb levels at baseline and at discharge within those with iron deficiency. Together, these associations suggest that low iron status is related to decreased aerobic and anaerobic performance. While few studies examined the associations between iron status and muscle mass, low Hb concentrations were related to lower muscle mass and the presence of frailty in older adults [116][117][118][120][121]. However, there was no concluding evidence on muscle strength and function measurements.
2.3. Iron Interventions and Skeletal Muscle Health
Iron supplementation is a common method utilized to correct iron deficiency, particularly in athletic populations. Oral supplementation doses ranging from 40 to 400 mg·d−1 for treatment durations of 6–24 weeks has been effective in improving iron status [122][123][124][125]. However, there are contrasting results regarding the effectiveness of iron supplementation for performance measurements reflective of skeletal muscle health. Multiple studies have examined the effects of iron supplementation on performance in athletes, yet there is a lack of studies examining if iron supplementation influences skeletal muscle mass or is effective in other healthy populations such as youth or older adults. In aerobic-based athletes (rowers, runners, and cyclists), iron supplementation over 6 weeks was effective in improving aerobic performance such as 4 km time trial and VO2max for rowers and runners [123][124]. However, a shorter intervention of 80 mg·d−1 showed no improvement in muscle recovery from cycling performance [126]. One study examined muscle strength measurements in female volleyball players. Iron supplementation of 325 mg·d−1 over 11 weeks improved strength in two power exercises and total strength over six different exercises [125]. These studies indicate that improving iron status via supplementation may be effective in improving performance-based measurements of skeletal muscle health. Future research should examine the effects of improving iron status on skeletal muscle mass, strength, and function in other vulnerable populations such as youth and older adults.
3. Interrelationship between Vitamin D, Iron, and Chronic Inflammation
3.1. Anti-Inflammatory Role of Vitamin D
In addition to its role on skeletal muscle health, vitamin D is thought to have anti-inflammatory actions through activation and differentiation of inflammatory cells, leading to a reduction in risk of infection and inflammation [127][128][129][130]. Vitamin D has immunomodulatory benefits including the enhancement of antimicrobial peptides and defensins to improve cellular immunity and reduce cytokine storms linked to infection. Adequate levels of vitamin D are related to the decrease in production of pro-inflammatory cytokines and an increase in anti-inflammatory cytokines [131][132][133][134][135]. Additionally, Shoemaker et al. recently reviewed the effects of vitamin D supplementation on reducing the risks of respiratory tract infections and viral infections including SARS-CoV-2 and indicate that sub-optimal vitamin D status increases risk for incidence, complication, and mortality due to infection and the presence of inflammation (manuscript in press). Therefore, vitamin D is a potential nutritional strategy that may reduce chronic inflammation.
The relationship between vitamin D status and inflammation has been studied previously in older adults [136][137]. Furthermore, vitamin D supplementation has demonstrated beneficial effects on chronic inflammation [131][132][133][138][139]. For example, Liberman et al. reported that 13 weeks of vitamin D and protein supplementation was effective in preventing increases in inflammatory cytokines compared to a placebo in older adults [138]. Similarly, Pereira et al. reported that 12 weeks of oral nutritional supplementation rich in vitamin D, HMB, and protein improved multiple biomarkers related to inflammation, immune function, and overall muscle health [140].
3.2. Connection between Vitamin D and Iron Status
Vitamin D and iron are both essential nutrients for skeletal muscle health, suggesting that optimal status in both micronutrients may interactively benefit skeletal muscle health. Vitamin D is important for the regulation of iron metabolism; therefore, low vitamin D status may result in low iron status [141][142][143]. Due to the relationship observed between vitamin D action on pro-inflammatory cytokines and mechanisms behind iron regulation [141][144], examining the physiological functions of vitamin D and iron status on skeletal muscle health and inflammation is an important next step in promoting health and performance. This theorized interaction is displayed in Figure 1. The connection between vitamin D and iron status is thought to be associated with hepcidin, an antimicrobial peptide that is essential for the regulation of iron metabolism [141][144]. Iron absorption and excretion is a highly regulated process, in which iron absorption increases with deficiency and decreases when iron stores are full. Systemic iron status, erythropoiesis requirements, and presence of inflammation can all influence this regulatory process [78]. The presence of high inflammation results in an increase in hepcidin production, causing iron to sequester and limit iron-supported erythropoiesis [145]. This leads to diminished ability to absorb iron, thus leading to iron deficiency anemia.
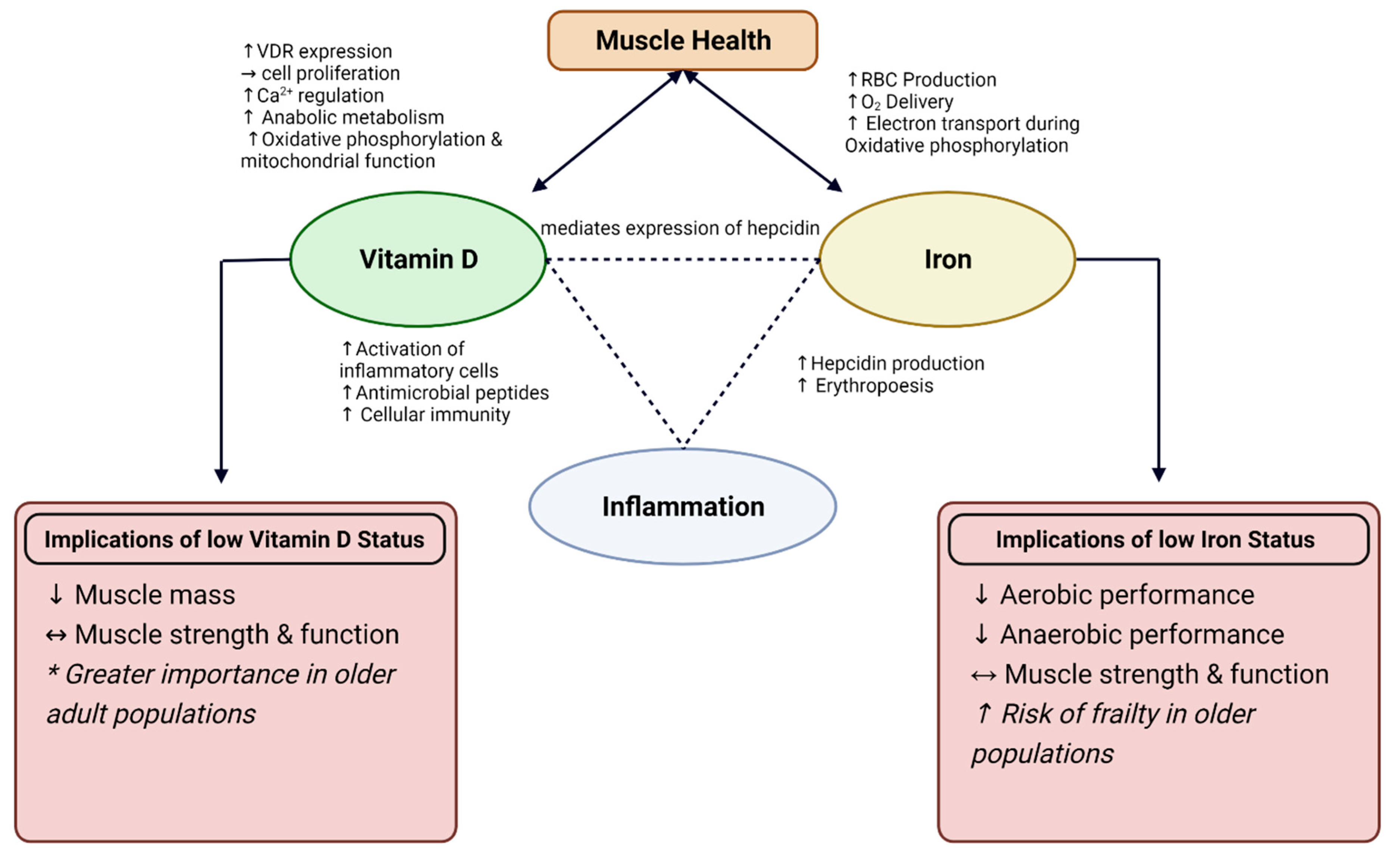
Figure 1. Interaction between Vitamin D and Iron on muscle health and role for inflammation. Depicted implications of low Vitamin D and Iron status on muscle health and performance. VDR = Vitamin D Receptor; RBC = red blood cell, Ca2+ = calcium; O2 = oxygen; ↑ = increase; ↓ = decrease; ↔ = conflicting results. * More profound results observed in interventions with older adults.
Additionally, vitamin D can mediate the expression of hepcidin through the binding of VDR with a gene promotor called HAMP gene to downregulate hepcidin production [144]. Furthermore, the role vitamin D has in decreasing expression of inflammatory cytokines that have a stimulating role on hepcidin production may indirectly contribute to this integration. Through in vitro studies, there is evidence that adequate levels of vitamin D are associated with reduced concentrations of hepcidin due to the suppression of the HAMP gene, as well as due to reduced concentrations of pro-inflammatory cytokines such as IL-1β and IL-6 [142]. This suggests the potential for vitamin D levels to influence iron regulation through hepcidin, specifically in the presence of inflammation. Additionally, vitamin D supplementation has been found to decrease hepcidin, and thus may have benefits in altered iron status, particularly in those with chronic inflammation [142]. While typically associated with anemia related to inflammation, it is possible that this mechanism may also be related to iron deficiency with or without anemia due to the reduction in iron necessary to support erythropoiesis. This suggests that those with chronic inflammation may have greater iron requirements to increase circulating iron concentrations and promote red blood cell production, indicating the need for nutritional support with both vitamin D and iron.
Relationships between low vitamin D status and low iron status are reported, providing further support for this nutritional interaction [146][147][148]. Vitamin D deficiency and low iron status are prevalent in multiple populations including older adults [149][150][151][152][153], children [154][155][156][157], and athletes [94][158][159], emphasizing the potential influence these nutrients may have on skeletal muscle health throughout the lifespan. Additionally, associations between vitamin D deficiency and low iron status have been demonstrated. For example, Malczewska-Lenczowska et al. reported that female athletes with iron deficiency also had lower vitamin D concentrations. Female athletes with vitamin D deficiency also had lower ferritin and iron concentrations and higher total iron binding capacity and sTfR, indicating low iron status [146]. Additionally, vitamin D supplementation (3000 IU·day−1) was effective in preventing a decline in Hb and hematocrit and improve transferrin levels, as well as concentrations of vitamin D in elite male rowers [160]. These findings support the association between vitamin D and iron status in athletes, although it is unclear which of the nutrients is the cause or the effect in the relationship. Further research is needed to examine if this nutrient interaction is influential to skeletal muscle health.
A retrospective study in children aged 10–20 years demonstrated an association between vitamin D deficiency and both anemia and iron deficiency when accounting for contributing factors [161]. The relationship between Hb and vitamin D was more prominent in female children, compared to males, suggesting that those vulnerable to nutritional deficiencies may be most affected by this nutritional interaction with skeletal muscle health through the growth and development stage. Similarly, in a pediatric population of inflammatory bowel disease patients, children with vitamin D concentrations ≥ 30 ng mL−1 had lower hepcidin and higher Hb concentrations when controlling for inflammation [162]. In older adults, the prevalence of vitamin D deficiency was higher in those with anemia due to inflammation (56%) and nutritional deficiency (48%) [153]. These findings suggest that children and older adults are at risk for compounding nutritional deficiencies, along with inflammation, that may be influential to the muscle growth and atrophy typically observed at each life stage. Therefore, adequate consumption of nutrients such as iron and vitamin D is essential for these populations.
4. Animal Food Sources
Adequate consumption of vitamin D and iron may be key in enhancing muscle mass, strength, and performance. Animal-source foods are abundant in vitamin D and iron, which may help individuals reach optimal, bioavailable intakes of these nutrients to support skeletal muscle health [163][164][165]. Specifically, beef is rich in bioavailable heme iron that may reduce the risk of iron deficiency and anemia [166][167]. Heme iron is found only in animal-source foods and are better absorbed than plant sources containing non-heme iron [167]. In particular, beef sources including ground beef, beef liver, and bottom round beef cuts are abundant sources of iron, containing 2–5 g of heme iron per 3 oz. serving. Previous studies have indicated the consumption of iron-rich red meat, along with resistance training, have shown beneficial effects on muscle mass, muscle strength, and reduce inflammatory markers [163][164], providing support for iron’s role in muscle health.
While vitamin D originates from sunlight exposure, dietary intake of vitamin D can be obtained from a variety of food sources in which approximately 60% of intake comes from animal-source foods such as fish, meat, and eggs [168]. Dietary intake of vitamin D range from 3.8 to 7.2 µg·d−1 in youth, 3.6 to 5.4 µg·d−1 in adults, and 3.9 to 5.1 µg·d−1 in older adults [169], which is lower than the recommended intake between 10 and 15 µg·d−1 (400–600 IU) [170]. Fortified milk provides a majority of the vitamin D within the American diet [171], with approximately 3 µg per cup [172]. Additionally, other key sources of vitamin D include beef liver and other beef sources, fatty fish such as salmon, eggs, and chicken [173]. Previous studies have observed positive results in vitamin D status after supplementing with vitamin D-fortified milk [174], suggesting potential for increasing muscle health through food sources.
Adequate nutritional intake is essential for muscle growth, performance, and preventing of sarcopenia. In particular, nutrients abundant in animal food sources such as vitamin D, iron, and protein, have been related to athletic performance, functional performance, and muscle growth [163][164][175][176][177]. Criticism of the current Recommended Daily Allowance (RDA) of 0.8 g·kg−1·d−1 warrants an increase specifically when protein anabolism is effected such as during the aging process and during exercise [178][179]. Sarcopenic older adults had lower intakes of protein, lipids, and micronutrients including iron and vitamin D [180][181]. Additionally, oral nutritional supplementation rich in protein and vitamin D resulted in improvement of markers of health, strength, and inflammation in malnourished, sarcopenic older adults [140]. These results indicate that an animal-source food matrix may be optimal when trying to enhance skeletal muscle function and reduce the risk of chronic inflammation.
Dietary protein from animal sources has long been established as beneficial for skeletal muscle by increasing muscle protein synthesis due to the essential amino acid content [182][183]. Animal protein sources effectively improved skeletal muscle strength and mass in healthy young adults and older adults [163][164][175][184][185], indicating the functional benefits of including dietary animal-source foods. Red meat, such as beef, and dairy products are two groups of animal-source foods that show promise for improving skeletal muscle health, potentially due to the nutrient content of these foods.
Multiple studies have examined the effects of beef intake on skeletal muscle-based outcomes in older adults [163][164][186][187][188]. Recent reviews have concluded that beef and/or the nutrients found within beef may improve muscle function [186][189]. Asp et al. investigated the relationship between beef intake and muscle mass in older adults ages 60–88 years, reporting that beef intake was positively related to mid-arm muscle area. Furthermore, regression analysis predicted that a 1 oz increase in beef consumption per day would result in a 2.23 cm2 increase in mid-arm muscle area [163]. In agreement, Morris & Jacques also predicted a linear increase in muscle mass (appendicular skeletal muscle index) in association with a 100 g per week increase in beef intake [185]. Lean red meat enhanced the effects of resistance training on muscle mass and strength in older females [164] and in 1 repetition maximum leg extension strength in older males [187]. In contrast, when examining the effects of lean beef in addition to resistance training, no additional benefits were observed in older adults when beef was consumed twice a week for 24 weeks [188]. Additionally, lean beef and protein supplementation had no positive effect on fat-free mass in older adults [187][190]. Meals containing pork, beef, or chicken showed similar impact on body composition and strength, indicating that high-quality protein sources, in general, have the same effect on skeletal muscle health [191][192]. Furthermore, consumption of beef protein isolate, chicken protein isolate, or whey protein all resulted in increases in lean body mass, regardless of the source of protein [175]. Higher intake of protein source foods including red meat, poultry, fish, dairy, soy, nuts, seeds, and legumes were positively associated with higher percent skeletal muscle mass over time in older adults, indicating that higher intake of animal-source foods can help maintain skeletal muscle with age [184]. These studies indicate conflicting skeletal muscle health outcomes with animal protein consumption; however, adequate consumption of protein sources in general appears to be a beneficial method for preserving muscle mass and strength. Future random controlled trials are required to provide further support of animal-source foods such as beef and poultry as dietary sources to promote skeletal muscle health.
Fortified dairy products are also nutrient-dense foods with the potential to improve skeletal muscle health [176][177]. In addition to many dairy products being fortified with vitamin D, they are rich in protein and many micronutrients including vitamin B12, calcium, riboflavin, and zinc, that are important for muscle health and function [193]. Consumption of dairy milk with additional protein improved fat-free mass, strength, and power in young males when consumed following resistance training [194]. However, in older adults, consuming higher protein dairy milk did not further improve the benefits obtained from resistance training alone on fat-free mass, power, or physical performance, but did improve maximal strength measurements [195].
References
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-Analysis123. Am. J. Clin. Nutr. 2012, 95, 1357–1364.
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95.
- Engelsen, O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients 2010, 2, 482–495.
- Hollis, B.W.; Wagner, C.L. Clinical Review: The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628.
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of Vitamin D by the Liver. J. Clin. Investig. 1969, 48, 2032–2037.
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell 1999, 96, 507–515.
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Vitamin D Treatment Modulates Immune Activation in Cystic Fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371.
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and Cardiovascular Disease Prevention. Nat. Rev. Cardiol. 2016, 13, 404–417.
- Dozio, E.; Briganti, S.; Vianello, E.; Dogliotti, G.; Barassi, A.; Malavazos, A.E.; Ermetici, F.; Morricone, L.; Sigruener, A.; Schmitz, G.; et al. Epicardial Adipose Tissue Inflammation Is Related to Vitamin D Deficiency in Patients Affected by Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 267–273.
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D Deficiency Contributes Directly to the Acute Respiratory Distress Syndrome (ARDS). Thorax 2015, 70, 617–624.
- Treiber, G.; Prietl, B.; Fröhlich-Reiterer, E.; Lechner, E.; Ribitsch, A.; Fritsch, M.; Rami-Merhar, B.; Steigleder-Schweiger, C.; Graninger, W.; Borkenstein, M.; et al. Cholecalciferol Supplementation Improves Suppressive Capacity of Regulatory T-Cells in Young Patients with New-Onset Type 1 Diabetes Mellitus—A Randomized Clinical Trial. Clin. Immunol. 2015, 161, 217–224.
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In Situ Detection of 1,25-Dihydroxyvitamin D3 Receptor in Human Skeletal Muscle Tissue. Histochem. J. 2001, 33, 19–24.
- Ceglia, L.; da Silva Morais, M.; Park, L.K.; Morris, E.; Harris, S.S.; Bischoff-Ferrari, H.A.; Fielding, R.A.; Dawson-Hughes, B. Multi-Step Immunofluorescent Analysis of Vitamin D Receptor Loci and Myosin Heavy Chain Isoforms in Human Skeletal Muscle. J. Mol. Histol. 2010, 41, 137–142.
- Costa, E.M.; Blau, H.M.; Feldman, D. 1,25-Dihydroxyvitamin D3 Receptors and Hormonal Responses in Cloned Human Skeletal Muscle Cells. Endocrinology 1986, 119, 2214–2220.
- Abboud, M.; Rybchyn, M.S.; Ning, Y.J.; Brennan-Speranza, T.C.; Girgis, C.M.; Gunton, J.E.; Fraser, D.R.; Mason, R.S. 1,25-Dihydroxycholecalciferol (Calcitriol) Modifies Uptake and Release of 25-Hydroxycholecalciferol in Skeletal Muscle Cells in Culture. J. Steroid Biochem. Mol. Biol. 2018, 177, 109–115.
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-Hydroxyvitamin D Concentrations Are Associated with Better Lower-Extremity Function in Both Active and Inactive Persons Aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758.
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111.
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of Vitamin D Receptor Gene in Mice Results in Abnormal Skeletal Muscle Development with Deregulated Expression of Myoregulatory Transcription Factors. Endocrinology 2003, 144, 5138–5144.
- Srikuea, R.; Zhang, X.; Park-Sarge, O.-K.; Esser, K.A. VDR and CYP27B1 Are Expressed in C2C12 Cells and Regenerating Skeletal Muscle: Potential Role in Suppression of Myoblast Proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405.
- Suzuki, T.; Kwon, J.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Yoshida, H. Low Serum 25-Hydroxyvitamin D Levels Associated with Falls among Japanese Community-Dwelling Elderly. J. Bone Miner. Res. 2008, 23, 1309–1317.
- Sørensen, O.H.; Lund, B.; Saltin, B.; Lund, B.; Andersen, R.B.; Hjorth, L.; Melsen, F.; Mosekilde, L. Myopathy in Bone Loss of Ageing: Improvement by Treatment with 1 Alpha-Hydroxycholecalciferol and Calcium. Clin. Sci. 1979, 56, 157–161.
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A Randomized Study on the Effect of Vitamin D₃ Supplementation on Skeletal Muscle Morphology and Vitamin D Receptor Concentration in Older Women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935.
- Pojednic, R.M.; Ceglia, L. The Emerging Biomolecular Role of Vitamin D in Skeletal Muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81.
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE 2014, 9, e101659.
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D Concentration in 342 Professional Football Players and Association with Lower Limb Isokinetic Function. J. Sci. Med. Sport 2014, 17, 139–143.
- Książek, A.; Zagrodna, A.; Dziubek, W.; Pietraszewski, B.; Ochmann, B.; Słowińska-Lisowska, M. 25(OH)D3 Levels Relative to Muscle Strength and Maximum Oxygen Uptake in Athletes. J. Hum. Kinet. 2016, 50, 71–77.
- Zeitler, C.; Fritz, R.; Smekal, G.; Ekmekcioglu, C. Association between the 25-Hydroxyvitamin D Status and Physical Performance in Healthy Recreational Athletes. Int. J. Environ. Res. Public Health 2018, 15, 2724.
- Most, A.; Dörr, O.; Nef, H.; Hamm, C.; Bauer, T.; Bauer, P. Influence of 25-Hydroxy-Vitamin D Insufficiency on Maximal Aerobic Power in Elite Indoor Athletes: A Cross-Sectional Study. Sports Med. Open 2021, 7, 74.
- Fitzgerald, J.S.; Peterson, B.J.; Warpeha, J.M.; Wilson, P.B.; Rhodes, G.S.; Ingraham, S.J. Vitamin D Status and VO2peak during a Skate Treadmill Graded Exercise Test in Competitive Ice Hockey Players. J. Strength Cond. Res. 2014, 28, 3200–3205.
- Forney, L.A.; Earnest, C.P.; Henagan, T.M.; Johnson, L.E.; Castleberry, T.J.; Stewart, L.K. Vitamin D Status, Body Composition, and Fitness Measures in College-Aged Students. J. Strength Cond. Res. 2014, 28, 814–824.
- Marantes, I.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J.; Amin, S. Is Vitamin D a Determinant of Muscle Mass and Strength? J. Bone Miner. Res. 2011, 26, 2860–2871.
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.; van Loon, L.J.C.; De Groot, L.C.P.G.M. Low Vitamin D Status Is Associated with Reduced Muscle Mass and Impaired Physical Performance in Frail Elderly People. Eur. J. Clin. Nutr. 2013, 67, 1050–1055.
- Conzade, R.; Grill, E.; Bischoff-Ferrari, H.A.; Ferrari, U.; Horsch, A.; Koenig, W.; Peters, A.; Thorand, B. Vitamin D in Relation to Incident Sarcopenia and Changes in Muscle Parameters among Older Adults: The KORA-Age Study. Calcif. Tissue Int. 2019, 105, 173–182.
- Mastaglia, S.R.; Seijo, M.; Muzio, D.; Somoza, J.; Nuñez, M.; Oliveri, B. Effect of Vitamin D Nutritional Status on Muscle Function and Strength in Healthy Women Aged over Sixty-Five Years. J. Nutr. Health Aging 2011, 15, 349–354.
- Toffanello, E.D.; Perissinotto, E.; Sergi, G.; Zambon, S.; Musacchio, E.; Maggi, S.; Coin, A.; Sartori, L.; Corti, M.-C.; Baggio, G.; et al. Vitamin D and Physical Performance in Elderly Subjects: The Pro.V.A Study. PLoS ONE 2012, 7, e34950.
- Gumieiro, D.N.; Murino Rafacho, B.P.; Buzati Pereira, B.L.; Cavallari, K.A.; Tanni, S.E.; Azevedo, P.S.; Polegato, B.F.; Mamede Zornoff, L.A.; Dinhane, D.I.; Innocenti Dinhane, K.G.; et al. Vitamin D Serum Levels Are Associated with Handgrip Strength but Not with Muscle Mass or Length of Hospital Stay after Hip Fracture. Nutrition 2015, 31, 931–934.
- Iolascon, G.; de Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, R.; Gimigliano, F. Hypovitaminosis D Is Associated with a Reduction in Upper and Lower Limb Muscle Strength and Physical Performance in Post-Menopausal Women: A Retrospective Study. Aging Clin. Exp. Res. 2015, 27 (Suppl. 1), S23–S30.
- Aspell, N.; Laird, E.; Healy, M.; Lawlor, B.; O’Sullivan, M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength and Physical Performance in Community-Dwelling Older Adults: Findings from the English Longitudinal Study of Ageing. Clin. Interv. Aging 2019, 14, 1751–1761.
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. The Association between 25-Hydroxyvitamin D Concentration, Physical Performance and Frailty Status in Older Adults. Eur. J. Nutr. 2019, 58, 1173–1181.
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-Hydroxyvitamin D Levels in Adolescents: Race, Season, Adiposity, Physical Activity, and Fitness. Pediatrics 2010, 125, 1104–1111.
- Gracia-Marco, L.; Valtueña, J.; Ortega, F.B.; Pérez-López, F.R.; Vicente-Rodríguez, G.; Breidenassel, C.; Ferrari, M.; Molnar, D.; Widhalm, K.; de Henauw, S.; et al. Iron and Vitamin Status Biomarkers and Its Association with Physical Fitness in Adolescents: The HELENA Study. J. Appl. Physiol. 2012, 113, 566–573.
- Valtueña, J.; Gracia-Marco, L.; Huybrechts, I.; Breidenassel, C.; Ferrari, M.; Gottrand, F.; Dallongeville, J.; Sioen, I.; Gutierrez, A.; Kersting, M.; et al. Cardiorespiratory Fitness in Males, and Upper Limbs Muscular Strength in Females, Are Positively Related with 25-Hydroxyvitamin D Plasma Concentrations in European Adolescents: The HELENA Study. QJM Int. J. Med. 2013, 106, 809–821.
- Bezrati, I.; Hammami, R.; Ben Fradj, M.K.; Martone, D.; Padulo, J.; Feki, M.; Chaouachi, A.; Kaabachi, N. Association of Plasma 25-Hydroxyvitamin D with Physical Performance in Physically Active Children. Appl. Physiol. Nutr. Metab. 2016, 41, 1124–1128.
- Carson, E.L.; Pourshahidi, L.K.; Hill, T.R.; Cashman, K.D.; Strain, J.J.; Boreham, C.A.; Mulhern, M.S. Vitamin D, Muscle Function, and Cardiorespiratory Fitness in Adolescents From the Young Hearts Study. J. Clin. Endocrinol. Metab. 2015, 100, 4621–4628.
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S.; Sacheck, J.M. Relationship between Muscle Strength and Dyslipidemia, Serum 25(OH)D, and Weight Status among Diverse Schoolchildren: A Cross-Sectional Analysis. BMC Pediatr. 2018, 18, 23.
- Wakayo, T.; Belachew, T.; Whiting, S.J. Serum Vitamin D Level Associates With Handgrip Muscle Strength among Ethiopian Schoolchildren: A Cross-Sectional Study. Food Nutr. Bull 2018, 39, 54–64.
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087.
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270.
- Bislev, L.S.; Grove-Laugesen, D.; Rejnmark, L. Vitamin D and Muscle Health: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Bone Miner. Res. 2021, 36, 1651–1660.
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of Vitamin D Supplementation on Upper and Lower Body Muscle Strength Levels in Healthy Individuals. A Systematic Review with Meta-Analysis. J. Sci. Med. Sport 2015, 18, 575–580.
- Wyon, M.A.; Wolman, R.; Nevill, A.M.; Cloak, R.; Metsios, G.S.; Gould, D.; Ingham, A.; Koutedakis, Y. Acute Effects of Vitamin D3 Supplementation on Muscle Strength in Judoka Athletes: A Randomized Placebo-Controlled, Double-Blind Trial. Clin. J. Sport Med. 2016, 26, 279–284.
- Fairbairn, K.A.; Ceelen, I.J.M.; Skeaff, C.M.; Cameron, C.M.; Perry, T.L. Vitamin D3 Supplementation Does Not Improve Sprint Performance in Professional Rugby Players: A Randomized, Placebo-Controlled, Double-Blind Intervention Study. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 1–9.
- Shanely, R.A.; Nieman, D.C.; Knab, A.M.; Gillitt, N.D.; Meaney, M.P.; Jin, F.; Sha, W.; Cialdella-Kam, L. Influence of Vitamin D Mushroom Powder Supplementation on Exercise-Induced Muscle Damage in Vitamin D Insufficient High School Athletes. J. Sports Sci. 2014, 32, 670–679.
- Close, G.L.; Russell, J.; Cobley, J.N.; Owens, D.J.; Wilson, G.; Gregson, W.; Fraser, W.D.; Morton, J.P. Assessment of Vitamin D Concentration in Non-Supplemented Professional Athletes and Healthy Adults during the Winter Months in the UK: Implications for Skeletal Muscle Function. J. Sports Sci. 2013, 31, 344–353.
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The Effects of Vitamin D(3) Supplementation on Serum Total 25D Concentration and Physical Performance: A Randomised Dose-Response Study. Br. J. Sports Med. 2013, 47, 692–696.
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J. Strength Cond. Res. 2016, 30, 2648–2655.
- Todd, J.J.; McSorley, E.M.; Pourshahidi, L.K.; Madigan, S.M.; Laird, E.; Healy, M.; Magee, P.J. Vitamin D3 Supplementation Using an Oral Spray Solution Resolves Deficiency but Has No Effect on VO2 Max in Gaelic Footballers: Results from a Randomised, Double-Blind, Placebo-Controlled Trial. Eur. J. Nutr. 2017, 56, 1577–1587.
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D Supplementation and Muscle Strength in Pre-Sarcopenic Elderly Lebanese People: A Randomized Controlled Trial. Arch. Osteoporos. 2018, 14, 4.
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747.
- Cangussu, L.M.; Nahas-Neto, J.; Orsatti, C.L.; Bueloni-Dias, F.N.; Nahas, E.a.P. Effect of Vitamin D Supplementation Alone on Muscle Function in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Osteoporos Int. 2015, 26, 2413–2421.
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-Weekly Dose of 8400 IU Vitamin D(3) Compared with Placebo: Effects on Neuromuscular Function and Tolerability in Older Adults with Vitamin D Insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991.
- Lagari, V.; Gómez-Marín, O.; Levis, S. The Role of Vitamin D in Improving Physical Performance in the Elderly. J. Bone Miner. Res. 2013, 28, 2194–2201.
- Vaes, A.M.M.; Tieland, M.; Toussaint, N.; Nilwik, R.; Verdijk, L.B.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Cholecalciferol or 25-Hydroxycholecalciferol Supplementation Does Not Affect Muscle Strength and Physical Performance in Prefrail and Frail Older Adults. J. Nutr. 2018, 148, 712–720.
- Mølmen, K.S.; Hammarström, D.; Pedersen, K.; Lian Lie, A.C.; Steile, R.B.; Nygaard, H.; Khan, Y.; Hamarsland, H.; Koll, L.; Hanestadhaugen, M.; et al. Vitamin D3 Supplementation Does Not Enhance the Effects of Resistance Training in Older Adults. J. Cachexia Sarcopenia Muscle 2021, 12, 599–628.
- Janssen, H.C.J.P.; Samson, M.M.; Verhaar, H.J.J. Muscle Strength and Mobility in Vitamin D-Insufficient Female Geriatric Patients: A Randomized Controlled Trial on Vitamin D and Calcium Supplementation. Aging Clin. Exp. Res. 2010, 22, 78–84.
- Ward, K.A.; Das, G.; Roberts, S.A.; Berry, J.L.; Adams, J.E.; Rawer, R.; Mughal, M.Z. A Randomized, Controlled Trial of Vitamin D Supplementation upon Musculoskeletal Health in Postmenarchal Females. J. Clin. Endocrinol. Metab. 2010, 95, 4643–4651.
- Wright, C.S.; Laing, E.M.; Pollock, N.K.; Hausman, D.B.; Weaver, C.M.; Martin, B.R.; McCabe, G.P.; Peacock, M.; Warden, S.J.; Hill Gallant, K.M.; et al. Serum 25-Hydroxyvitamin D and Intact Parathyroid Hormone Influence Muscle Outcomes in Children and Adolescents. J. Bone Miner. Res. 2018, 33, 1940–1947.
- Mortensen, C.; Mølgaard, C.; Hauger, H.; Kristensen, M.; Damsgaard, C.T. Winter Vitamin D3 Supplementation Does Not Increase Muscle Strength, but Modulates the IGF-Axis in Young Children. Eur. J. Nutr. 2019, 58, 1183–1192.
- van der Wielen, R.P.J.; de Groot, L.C.P.G.M.; van Staveren, W.A.; Löwik, M.R.H.; van den Berg, H.; Haller, J.; Moreiras, O. Serum Vitamin D Concentrations among Elderly People in Europe. Lancet 1995, 346, 207–210.
- Wagatsuma, A.; Sakuma, K. Vitamin D Signaling in Myogenesis: Potential for Treatment of Sarcopenia. BioMed Res. Int. 2014, 2014, 121254.
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189.
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861.
- Abiri, B.; Vafa, M. Vitamin D and Muscle Sarcopenia in Aging. Methods Mol. Biol. 2020, 2138, 29–47.
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247.
- Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333.
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055.
- Lin, C.-C.; Shih, M.-H.; Chen, C.-D.; Yeh, S.-L. Effects of Adequate Dietary Protein with Whey Protein, Leucine, and Vitamin D Supplementation on Sarcopenia in Older Adults: An Open-Label, Parallel-Group Study. Clin. Nutr. 2021, 40, 1323–1329.
- Ganz, T. Molecular Control of Iron Transport. J. Am. Soc. Nephrol. 2007, 18, 394–400.
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-Dependent Changes in Cellular Energy Metabolism: Influence on Citric Acid Cycle and Oxidative Phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107.
- Xu, W.; Barrientos, T.; Andrews, N.C. Iron and Copper in Mitochondrial Diseases. Cell Metab. 2013, 17, 319–328.
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron Status in the Elderly. Mech. Ageing Dev. 2014, 136–137, 22–28.
- Buratti, P.; Gammella, E.; Rybinska, I.; Cairo, G.; Recalcati, S. Recent Advances in Iron Metabolism: Relevance for Health, Exercise, and Performance. Med. Sci. Sports Exerc. 2015, 47, 1596–1604.
- Telford, R.D.; Sly, G.J.; Hahn, A.G.; Cunningham, R.B.; Bryant, C.; Smith, J.A. Footstrike Is the Major Cause of Hemolysis during Running. J. Appl. Physiol. 2003, 94, 38–42.
- Monsen, E.R. Iron Nutrition and Absorption: Dietary Factors Which Impact Iron Bioavailability. J. Am. Diet Assoc. 1988, 88, 786–790.
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916.
- Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers/.—Version Details. Available online: https://trove.nla.gov.au/version/31276429 (accessed on 5 March 2018).
- Harvey, L.J.; Armah, C.N.; Dainty, J.R.; Foxall, R.J.; John Lewis, D.; Langford, N.J.; Fairweather-Tait, S.J. Impact of Menstrual Blood Loss and Diet on Iron Deficiency among Women in the UK. Br. J. Nutr. 2005, 94, 557–564.
- Waldmann, A.; Koschizke, J.W.; Leitzmann, C.; Hahn, A. Dietary Iron Intake and Iron Status of German Female Vegans: Results of the German Vegan Study. Ann. Nutr. Metab. 2004, 48, 103–108.
- Taher, A.T.; Saliba, A.N. Iron Overload in Thalassemia: Different Organs at Different Rates. Hematology Am. Soc. Hematol. Educ. Program 2017, 2017, 265–271.
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771.
- Jacobs, P.; Dommisse, J. The Plasma Ferritin Level as a Reliable Index of Body Iron Stores Following Intravenous Iron Dextran. J. Med. 1982, 13, 309–321.
- Yang, Z.; Dewey, K.G.; Lönnerdal, B.; Hernell, O.; Chaparro, C.; Adu-Afarwuah, S.; McLean, E.D.; Cohen, R.J.; Domellöf, M.; Allen, L.H.; et al. Comparison of Plasma Ferritin Concentration with the Ratio of Plasma Transferrin Receptor to Ferritin in Estimating Body Iron Stores: Results of 4 Intervention Trials. Am. J. Clin. Nutr. 2008, 87, 1892–1898.
- Pfeiffer, C.M.; Looker, A.C. Laboratory Methodologies for Indicators of Iron Status: Strengths, Limitations, and Analytical Challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S.
- Shoemaker, M.E.; Gillen, Z.M.; McKay, B.D.; Koehler, K.; Cramer, J.T. High Prevalence of Poor Iron Status Among 8- to 16-Year-Old Youth Athletes: Interactions Among Biomarkers of Iron, Dietary Intakes, and Biological Maturity. J. Am. Coll. Nutr. 2019, 39, 155–162.
- Soppi, E.T. Iron Deficiency without Anemia—A Clinical Challenge. Clin. Case Rep. 2018, 6, 1082–1086.
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843.
- Feelders, R.A.; Vreugdenhil, G.; Eggermont, A.M.; Kuiper-Kramer, P.A.; van Eijk, H.G.; Swaak, A.J. Regulation of Iron Metabolism in the Acute-Phase Response: Interferon Gamma and Tumour Necrosis Factor Alpha Induce Hypoferraemia, Ferritin Production and a Decrease in Circulating Transferrin Receptors in Cancer Patients. Eur. J. Clin. Investig. 1998, 28, 520–527.
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting Ferritin Concentrations for Inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Am. J. Clin. Nutr. 2017, 106, 359S–371S.
- Thurnham, D.I.; Northrop-Clewes, C.A.; Knowles, J. The Use of Adjustment Factors to Address the Impact of Inflammation on Vitamin A and Iron Status in Humans123. J. Nutr. 2015, 145, 1137S–1143S.
- Bainton, D.F.; Finch, C.A. The diagnosis of iron deficiency anemia. Am. J. Med. 1964, 37, 62–70.
- Skikne, B.S.; Punnonen, K.; Caldron, P.H.; Bennett, M.T.; Rehu, M.; Gasior, G.H.; Chamberlin, J.S.; Sullivan, L.A.; Bray, K.R.; Southwick, P.C. Improved Differential Diagnosis of Anemia of Chronic Disease and Iron Deficiency Anemia: A Prospective Multicenter Evaluation of Soluble Transferrin Receptor and the STfR/Log Ferritin Index. Am. J. Hematol. 2011, 86, 923–927.
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron Deficiency or Anemia of Inflammation?: Differential Diagnosis and Mechanisms of Anemia of Inflammation. Wien Med. Wochenschr. 2016, 166, 411–423.
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123.
- WHO|Worldwide Prevalence of Anaemia 1993–2005. Available online: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596657/en/ (accessed on 6 March 2018).
- Beard, J.L. Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–579S, discussion 580S.
- Dziegala, M.; Josiak, K.; Kasztura, M.; Kobak, K.; von Haehling, S.; Banasiak, W.; Anker, S.D.; Ponikowski, P.; Jankowska, E. Iron Deficiency as Energetic Insult to Skeletal Muscle in Chronic Diseases. J. Cachexia Sarcopenia Muscle 2018, 9, 802–815.
- Galy, B.; Ferring-Appel, D.; Sauer, S.W.; Kaden, S.; Lyoumi, S.; Puy, H.; Kölker, S.; Gröne, H.-J.; Hentze, M.W. Iron Regulatory Proteins Secure Mitochondrial Iron Sufficiency and Function. Cell Metab. 2010, 12, 194–201.
- Robach, P.; Cairo, G.; Gelfi, C.; Bernuzzi, F.; Pilegaard, H.; Viganò, A.; Santambrogio, P.; Cerretelli, P.; Calbet, J.A.L.; Moutereau, S.; et al. Strong Iron Demand during Hypoxia-Induced Erythropoiesis Is Associated with down-Regulation of Iron-Related Proteins and Myoglobin in Human Skeletal Muscle. Blood 2007, 109, 4724–4731.
- Boulton, F.E. The Myoglobin Content of Human Skeletal Muscle. Br. J. Haematol. 1973, 25, 281.
- Wang, J.; Huo, J.-S.; Sun, J.; Ning, Z.-X. Physical Performance of Migrant Schoolchildren with Marginal and Severe Iron Deficiency in the Suburbs of Beijing. Biomed. Environ. Sci. 2009, 22, 333–339.
- Arsenault, J.E.; Mora-Plazas, M.; Forero, Y.; Lopez-Arana, S.; Jáuregui, G.; Baylin, A.; Gordon, P.M.; Villamor, E. Micronutrient and Anthropometric Status Indicators Are Associated with Physical Fitness in Colombian Schoolchildren. Br. J. Nutr. 2011, 105, 1832–1842.
- Dellavalle, D.M.; Haas, J.D. Iron Status Is Associated with Endurance Performance and Training in Female Rowers. Med. Sci. Sports Exerc. 2012, 44, 1552–1559.
- Tsai, K.-Z.; Lai, S.-W.; Hsieh, C.-J.; Lin, C.-S.; Lin, Y.-P.; Tsai, S.-C.; Chung, P.-S.; Lin, Y.-K.; Lin, T.-C.; Ho, C.-L.; et al. Association between Mild Anemia and Physical Fitness in a Military Male Cohort: The CHIEF Study. Sci. Rep. 2019, 9, 11165.
- Shoemaker, M.E.; Gillen, Z.M.; Mckay, B.D.; Bohannon, N.A.; Gibson, S.M.; Koehler, K.; Cramer, J.T. Sex-Specific Relationships among Iron Status Biomarkers, Athletic Performance, Maturity, and Dietary Intakes in Pre-Adolescent and Adolescent Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 42.
- Juárez-Cedillo, T.; Basurto-Acevedo, L.; Vega-García, S.; Manuel-Apolinar, L.; Cruz-Tesoro, E.; Rodríguez-Pérez, J.M.; García-Hernández, N.; Pérez-Hernández, N.; Fragoso, J.M. Prevalence of Anemia and Its Impact on the State of Frailty in Elderly People Living in the Community: SADEM Study. Ann. Hematol. 2014, 93, 2057–2062.
- Pires Corona, L.; Drumond Andrade, F.C.; de Oliveira Duarte, Y.A.; Lebrao, M.L. The Relationship between Anemia, Hemoglobin Concentration and Frailty in Brazilian Older Adults. J. Nutr. Health Aging 2015, 19, 935–940.
- Ruan, Y.; Guo, Y.; Kowal, P.; Lu, Y.; Liu, C.; Sun, S.; Huang, Z.; Zheng, Y.; Wang, W.; Li, G.; et al. Association between Anemia and Frailty in 13,175 Community-Dwelling Adults Aged 50 Years and Older in China. BMC Geriatr. 2019, 19, 327.
- Neidlein, S.; Wirth, R.; Pourhassan, M. Iron Deficiency, Fatigue and Muscle Strength and Function in Older Hospitalized Patients. Eur. J. Clin. Nutr. 2020, 75, 456–463.
- Kim, T.H.; Hwang, H.-J.; Kim, S.-H. Relationship between Serum Ferritin Levels and Sarcopenia in Korean Females Aged 60 Years and Older Using the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLoS ONE 2014, 9, e90105.
- Moon, J.-H.; Kong, M.-H.; Kim, H.-J. Relationship between Low Muscle Mass and Anemia in Korean Elderly Men: Using the Korea National Health and Nutrition Examination Survey (KNHANES IV–V). J. Clin. Gerontol. Geriatr. 2015, 6, 115–119.
- Burden, R.J.; Pollock, N.; Whyte, G.P.; Richards, T.; Moore, B.; Busbridge, M.; Srai, S.K.; Otto, J.; Pedlar, C.R. Effect of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med. Sci. Sports Exerc. 2015, 47, 1399–1407.
- DellaValle, D.M.; Haas, J.D. Iron Supplementation Improves Energetic Efficiency in Iron-Depleted Female Rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215.
- Garvican, L.A.; Saunders, P.U.; Cardoso, T.; Macdougall, I.C.; Lobigs, L.M.; Fazakerley, R.; Fallon, K.E.; Anderson, B.; Anson, J.M.; Thompson, K.G.; et al. Intravenous Iron Supplementation in Distance Runners with Low or Suboptimal Ferritin. Med. Sci. Sports Exerc. 2014, 46, 376–385.
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S. Iron Supplementation Prevents a Decline in Iron Stores and Enhances Strength Performance in Elite Female Volleyball Players during the Competitive Season. Appl. Physiol. Nutr. Metab. 2015, 40, 615–622.
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.I.; Caballero-García, A.; Roche, E.; Fernández-Lázaro, D. Effect of Iron Supplementation on the Modulation of Iron Metabolism, Muscle Damage Biomarkers and Cortisol in Professional Cyclists. Nutrients 2019, 11, 500.
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D Deficiency Promotes Epithelial Barrier Dysfunction and Intestinal Inflammation. J. Infect. Dis. 2014, 210, 1296–1305.
- Gombart, A.F. The Vitamin D-Antimicrobial Peptide Pathway and Its Role in Protection against Infection. Future Microbiol. 2009, 4, 1151–1165.
- Zhao, Y.; Ran, Z.; Jiang, Q.; Hu, N.; Yu, B.; Zhu, L.; Shen, L.; Zhang, S.; Chen, L.; Chen, H.; et al. Vitamin D Alleviates Rotavirus Infection through a Microrna-155-5p Mediated Regulation of the TBK1/IRF3 Signaling Pathway In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 3562.
- Ramos-Martínez, E.; López-Vancell, M.R.; Fernández de Córdova-Aguirre, J.C.; Rojas-Serrano, J.; Chavarría, A.; Velasco-Medina, A.; Velázquez-Sámano, G. Reduction of Respiratory Infections in Asthma Patients Supplemented with Vitamin D Is Related to Increased Serum IL-10 and IFNγ Levels and Cathelicidin Expression. Cytokine 2018, 108, 239–246.
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65.
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.-R.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Khalaji, N.; Gharavi, A. Improvement of Vitamin D Status Resulted in Amelioration of Biomarkers of Systemic Inflammation in the Subjects with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2012, 28, 424–430.
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518.
- De Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M.; et al. Relationship between Vitamin D and Inflammatory Markers in Older Individuals. Age 2014, 36, 9694.
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Henriksen, V.T.; Dixon, B.M.; Schneider, E.D.; Dern, A.; Weaver, L.K. Different Doses of Supplemental Vitamin D Maintain Interleukin-5 without Altering Skeletal Muscle Strength: A Randomized, Double-Blind, Placebo-Controlled Study in Vitamin D Sufficient Adults. Nutr. Metab. 2012, 9, 16.
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D Deficiency Is Associated with Inflammation in Older Irish Adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815.
- Alves, A.S.; Ishimura, M.E.; de Oliveira Duarte, Y.A.; Bueno, V. Parameters of the Immune System and Vitamin D Levels in Old Individuals. Front. Immunol. 2018, 9, 1122.
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen Weeks of Supplementation of Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement Attenuates Chronic Low-Grade Inflammation in Sarcopenic Older Adults: The PROVIDE Study. Aging Clin. Exp. Res. 2019, 31, 845–854.
- Han, J.E.; Alvarez, J.A.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Hao, L.; Brown, L.A.S.; Martin, G.S.; Ziegler, T.R. Impact of High-Dose Vitamin D3 on Plasma Free 25-Hydroxyvitamin D Concentrations and Antimicrobial Peptides in Critically Ill Mechanically Ventilated Adults. Nutrition 2017, 38, 102–108.
- Pereira, S.L.; Shoemaker, M.E.; Gawel, S.; Davis, G.J.; Luo, M.; Mustad, V.A.; Cramer, J.T. Biomarker Changes in Response to a 12-Week Supplementation of an Oral Nutritional Supplement Enriched with Protein, Vitamin D and HMB in Malnourished Community Dwelling Older Adults with Sarcopenia. Nutrients 2022, 14, 1196.
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-Dose Vitamin D3 Reduces Circulating Hepcidin Concentrations: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Adults. Clin. Nutr. 2017, 36, 980–985.
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The Role of Vitamin D in Regulating the Iron-Hepcidin-Ferroportin Axis in Monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25.
- Kell, D.B.; Pretorius, E. No Effects without Causes: The Iron Dysregulation and Dormant Microbes Hypothesis for Chronic, Inflammatory Diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1518–1557.
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572.
- Song, S.-N.J.; Tomosugi, N.; Kawabata, H.; Ishikawa, T.; Nishikawa, T.; Yoshizaki, K. Down-Regulation of Hepcidin Resulting from Long-Term Treatment with an Anti-IL-6 Receptor Antibody (Tocilizumab) Improves Anemia of Inflammation in Multicentric Castleman Disease. Blood 2010, 116, 3627–3634.
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167.
- Atkinson, M.A.; Melamed, M.L.; Kumar, J.; Roy, C.N.; Miller, E.R.; Furth, S.L.; Fadrowski, J.J. Vitamin D, Race, and Risk for Anemia in Children. J. Pediatr. 2014, 164, 153–158.e1.
- Sim, J.J.; Lac, P.T.; Liu, I.L.A.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D Deficiency and Anemia: A Cross-Sectional Study. Ann. Hematol. 2010, 89, 447–452.
- Orces, C.; Lorenzo, C.; Guarneros, J.E. The Prevalence and Determinants of Vitamin D Inadequacy among U.S. Older Adults: National Health and Nutrition Examination Survey 2007–2014. Cureus 2019, 11, e5300.
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and Predictors of Vitamin D-Deficiency in Frail Older Hospitalized Patients. BMC Geriatr. 2018, 18, 219.
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of Anemia in Persons 65 Years and Older in the United States: Evidence for a High Rate of Unexplained Anemia. Blood 2004, 104, 2263–2268.
- Robalo Nunes, A.; Fonseca, C.; Marques, F.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of Anemia and Iron Deficiency in Older Portuguese Adults: An EMPIRE Substudy. Geriatr. Gerontol. Int. 2017, 17, 1814–1822.
- Perlstein, T.S.; Pande, R.; Berliner, N.; Vanasse, G.J. Prevalence of 25-Hydroxyvitamin D Deficiency in Subgroups of Elderly Persons with Anemia: Association with Anemia of Inflammation. Blood 2011, 117, 2800–2806.
- Ferrari, M.; Mistura, L.; Patterson, E.; Sjöström, M.; Díaz, L.E.; Stehle, P.; Gonzalez-Gross, M.; Kersting, M.; Widhalm, K.; Molnár, D.; et al. Evaluation of Iron Status in European Adolescents through Biochemical Iron Indicators: The HELENA Study. Eur. J. Clin. Nutr. 2011, 65, 340–349.
- Horton-French, K.; Dunlop, E.; Lucas, R.M.; Pereira, G.; Black, L.J. Prevalence and Predictors of Vitamin D Deficiency in a Nationally Representative Sample of Australian Adolescents and Young Adults. Eur. J. Clin. Nutr. 2021, 75, 1627–1636.
- Ritterhouse, L.L.; Lu, R.; Shah, H.B.; Robertson, J.M.; Fife, D.A.; Maecker, H.T.; Du, H.; Fathman, C.G.; Chakravarty, E.F.; Scofield, R.H.; et al. Vitamin D Deficiency in a Multiethnic Healthy Control Cohort and Altered Immune Response in Vitamin D Deficient European-American Healthy Controls. PLoS ONE 2014, 9, e94500.
- van der Merwe, L.F.; Eussen, S.R. Iron Status of Young Children in Europe. Am. J. Clin. Nutr. 2017, 106, 1663S–1671S.
- Constantini, N.W.; Eliakim, A.; Zigel, L.; Yaaron, M.; Falk, B. Iron Status of Highly Active Adolescents: Evidence of Depleted Iron Stores in Gymnasts. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 62–70.
- Malczewska, J.; Raczynski, G.; Stupnicki, R. Iron Status in Female Endurance Athletes and in Non-Athletes. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 260–276.
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernandez-Lázaro, D. Effects of Vitamin D Supplementation on Haematological Values and Muscle Recovery in Elite Male Traditional Rowers. Nutrients 2018, 10, 1968.
- Lee, J.A.; Hwang, J.S.; Hwang, I.T.; Kim, D.H.; Seo, J.-H.; Lim, J.S. Low Vitamin D Levels Are Associated with Both Iron Deficiency and Anemia in Children and Adolescents. Pediatr. Hematol. Oncol. 2015, 32, 99–108.
- Syed, S.; Michalski, E.S.; Tangpricha, V.; Chesdachai, S.; Kumar, A.; Prince, J.; Ziegler, T.R.; Suchdev, P.S.; Kugathasan, S. Vitamin D Status Is Associated with Hepcidin and Hemoglobin Concentrations in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1650–1658.
- Asp, M.L.; Richardson, J.R.; Collene, A.L.; Droll, K.R.; Belury, M.A. Dietary Protein and Beef Consumption Predict for Markers of Muscle Mass and Nutrition Status in Older Adults. J. Nutr. Health Aging 2012, 16, 784–790.
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-Enriched Diet, with the Use of Lean Red Meat, Combined with Progressive Resistance Training Enhances Lean Tissue Mass and Muscle Strength and Reduces Circulating IL-6 Concentrations in Elderly Women: A Cluster Randomized Controlled Trial. Am. J. Clin. Nutr. 2014, 99, 899–910.
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462.
- USDA Ground Beef Calculator: USDA ARS. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/ground-beef-calculator/ (accessed on 29 August 2019).
- Valenzuela, C.; de Romaña, D.L.; Olivares, M.; Morales, M.S.; Pizarro, F. Total Iron and Heme Iron Content and Their Distribution in Beef Meat and Viscera. Biol. Trace Elem. Res. 2009, 132, 103–111.
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma Concentrations of 25-Hydroxyvitamin D in Meat Eaters, Fish Eaters, Vegetarians and Vegans: Results from the EPIC-Oxford Study. Public Health Nutr. 2011, 14, 340–346.
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. J. Nutr. 2010, 140, 817–822.
- Office of the Surgeon General (US). Lifestyle Approaches to Promote Bone Health; Office of the Surgeon General: Bethesda, MD, USA, 2004.
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D Fortification in the United States and Canada: Current Status and Data Needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S.
- Yetley, E.A. Assessing the Vitamin D Status of the US Population. Am. J. Clin. Nutr. 2008, 88, 558S–564S.
- Dunlop, E.; James, A.P.; Cunningham, J.; Strobel, N.; Lucas, R.M.; Kiely, M.; Nowson, C.A.; Rangan, A.; Adorno, P.; Atyeo, P.; et al. Vitamin D Composition of Australian Foods. Food Chem. 2021, 358, 129836.
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of Vitamin D Fortified Foods on Bone Markers and Muscle Strength in Women of Pakistani and Danish Origin Living in Denmark: A Randomised Controlled Trial. Nutr. J. 2019, 18, 82.
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein after Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242.
- Hanach, N.I.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 59–69.
- Radavelli-Bagatini, S.; Zhu, K.; Lewis, J.R.; Dhaliwal, S.S.; Prince, R.L. Association of Dairy Intake with Body Composition and Physical Function in Older Community-Dwelling Women. J. Acad Nutr. Diet 2013, 113, 1669–1674.
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182.
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936.
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between Dietary Nutrient Intake and Sarcopenia in the SarcoPhAge Study. Aging Clin. Exp. Res. 2019, 31, 815–824.
- U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025, 9th ed. Available online: https://www.dietaryguidelines.gov/ (accessed on 15 February 2022).
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923.
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Diet Assoc. 2009, 109, 1582–1586.
- Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 88–94.
- Morris, M.S.; Jacques, P.F. Total Protein, Animal Protein and Physical Activity in Relation to Muscle Mass in Middle-Aged and Older Americans. Br. J. Nutr. 2013, 109, 1294–1303.
- Hawley, A.L.; Liang, X.; Børsheim, E.; Wolfe, R.R.; Salisbury, L.; Hendy, E.; Wu, H.; Walker, S.; Tacinelli, A.M.; Baum, J.I. The Potential Role of Beef and Nutrients Found in Beef on Outcomes of Wellbeing in Healthy Adults 50 Years of Age and Older: A Systematic Review of Randomized Controlled Trials. Meat Sci. 2022, 189, 108830.
- Kim, I.-Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein Intake Distribution Pattern Does Not Affect Anabolic Response, Lean Body Mass, Muscle Strength or Function over 8 Weeks in Older Adults: A Randomized-Controlled Trial. Clin. Nutr. 2018, 37, 488–493.
- Formica, M.B.; Gianoudis, J.; Nowson, C.A.; O’Connell, S.L.; Milte, C.; Ellis, K.A.; Daly, R.M. Effect of Lean Red Meat Combined with a Multicomponent Exercise Program on Muscle and Cognitive Function in Older Adults: A 6-Month Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 112, 113–128.
- Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1429.
- ten Haaf, D.S.M.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of Protein Supplementation on Lean Body Mass, Muscle Strength, and Physical Performance in Nonfrail Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059.
- Charlton, K.; Walton, K.; Batterham, M.; Brock, E.; Langford, K.; McMahon, A.; Roodenrys, S.; Koh, F.; Host, A.; Crowe, R.; et al. Pork and Chicken Meals Similarly Impact on Cognitive Function and Strength in Community-Living Older Adults: A Pilot Study. J. Nutr. Gerontol. Geriatr. 2016, 35, 124–145.
- Murphy, K.J.; Parker, B.; Dyer, K.A.; Davis, C.R.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. A Comparison of Regular Consumption of Fresh Lean Pork, Beef and Chicken on Body Composition: A Randomized Cross-over Trial. Nutrients 2014, 6, 682–696.
- Granic, A.; Hurst, C.; Dismore, L.; Aspray, T.; Stevenson, E.; Witham, M.D.; Sayer, A.A.; Robinson, S. Milk for Skeletal Muscle Health and Sarcopenia in Older Adults: A Narrative Review. Clin. Interv. Aging 2020, 15, 695–714.
- Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A.; et al. Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients 2021, 13, 948.
- Huschtscha, Z.; Parr, A.; Porter, J.; Costa, R.J.S. The Effects of a High-Protein Dairy Milk Beverage With or Without Progressive Resistance Training on Fat-Free Mass, Skeletal Muscle Strength and Power, and Functional Performance in Healthy Active Older Adults: A 12-Week Randomized Controlled Trial. Front. Nutr. 2021, 8, 644865.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
899
Revisions:
2 times
(View History)
Update Date:
12 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




