1. Vitamin D and Skeletal Muscle Health
1.1. Vitamin D and Skeletal Muscle Physiology
Vitamin D is a fat-soluble prohormone that provides key functions in multiple endocrine and autocrine processes throughout the body. Vitamin D can be obtained by both food sources and solar ultraviolet B (UVB) of 290–315 nm and in conjunction, is utilized to synthesize cholecalciferol, or vitamin D
3 [
61,
62]. This production of vitamin D is dependent upon the solar elevation in which vitamin D is predominately produced when the elevation angle is greater than 45 degrees [
63]. Once synthesized in the skin from sunlight or absorbed from food, vitamin D
3 binds to vitamin D-binding protein (VDBP) and is transferred to the liver where it is converted to 25-hydroxycholecalciferol (25(OH)D) and transported to the kidney for further processing. Within the kidney, 25(OH)D is activated to the form of 1,25-dihydroxycholecalciferol [1,25(OH)
2D], or calcitriol [
64,
65,
66]. This active form can then perform functions such as calcium and phosphate regulation through the binding of vitamin D receptor (VDR). Vitamin D receptor is present on many body tissues including skeletal muscle, intestines, myocardium, bone, nervous system, as well as immune cells, indicating inadequate vitamin D effects multiple tissues within the body, resulting in a potential link to multiple pathological diseases including cardiovascular disease, inflammatory conditions, and respiratory illness [
67,
68,
69,
70,
71].
The mechanism for the role vitamin D has on skeletal muscle involves VDR expression found in skeletal muscle cells [
72,
73,
74]. Expression of VDR in the nucleus of skeletal muscle cells is necessary for vitamin D uptake [
75], and reduced VDR concentrations have affected the contractility of muscle cells and may affect skeletal muscle repair and recovery [
76,
77]. Animal studies have demonstrated that mice without the VDR gene had smaller muscle fibers, lower body size and weight, and impaired movement compared to mice with VDR gene [
78]. Additionally, VDR concentrations have been shown to increase after supplementation of 1,25(OH)
2D
3 and 25(OH)D
3 in muscle cells, which was suggested to be linked to muscle cell regeneration [
79].
Further support for the importance of VDR in skeletal muscle health was demonstrated in human studies. Expression of VDR primarily has been found to be located on fast-twitch muscle fibers [
80], and interestingly, it has been identified that fast-twitch muscle fibers following vitamin D supplementation [
81]. Thus, this provides support for the importance of VDR concentration, related to vitamin D status, for improving skeletal muscle health in humans. Supplementation with vitamin D
3 in vitamin D insufficient females resulted increased VDR concentration [
82], indicating that adequate VDR and vitamin D concentrations can support muscle fiber growth. Additionally, vitamin D actions on skeletal muscle through VDR may also influence calcium regulation and muscle contraction, anabolic or growth pathways, oxidative phosphorylation and mitochondrial function, and muscle inflammation [
83].
1.2. Vitamin D Status and Skeletal Muscle Health
Vitamin D mechanistically appears to influence skeletal muscle health. Therefore, this effect on skeletal muscle health in conjunction with a high prevalence of vitamin D deficiency in certain populations such as youth, athletes, and older adults warrants an examination of the associations between vitamin D and measurements of muscle mass, muscle strength, and muscle function. Many studies in athletic populations reported associations between concentrations of 25(OH)D and measurements of performance [
84,
85,
86,
87,
88]; however, other studies reported no associations between vitamin D and performance outcomes [
89,
90]. Koundorakis et al. reported correlations between serum 25(OH)D concentrations and tests of anaerobic and aerobic performance. These included moderate to high positive associations between serum 25(OH)D and jumping ability and VO
2max (r = 0.394–0.740) and moderate negative associations between serum 25(OH)D and sprint times (r = −410–−0.649) during soccer pre- and post-season [
84]. However, in male hockey players, there were no associations between serum 25(OH)D and aerobic exercise variables determined during a graded exercise test [
89]. Forney et al. reported a positive association between serum 25(OH)D and VO
2max (r = 0.360) but not with tests of anaerobic performance or muscle strength [
90]. Additionally, submaximal aerobic performance and aerobic power was better in athletes with higher serum 25(OH)D levels compared to those with levels considered deficient (<35 ng·mL
−1 and <30 ng·mL
−1, respectively) [
87,
88]. Peak torque was found to be 12–17% higher in those with higher serum 25(OH)D levels [
85,
86]. These results suggest that while evidence supporting a positive association between vitamin D and athletic performance is inconclusive, many studies suggest that vitamin D levels are moderately related to performance. It is important to note that a majority of the studies were performed in young adult male athletes with varying tests of performance. Future studies inclusive of male and female athletes from a variety of athletic backgrounds is necessary.
In older adults, vitamin D showed consistent low to moderate relationships with muscle mass [
91,
92,
96], muscle strength [
4,
20,
91,
93,
94,
95] and muscle function [
4,
20,
92,
94,
95,
97], with only a few of the studies reviewed finding no associations with skeletal muscle health. For example, while Conzade et al. reported a positive relationship between serum 25(OH)D concentrations and changes in muscle mass and an inverse relationship with time to complete TUG, there were no relationships with handgrip strength and gait speed [
96]. Furthermore, Vaes et al. reported that older adults with serum 25(OH)D levels <50 nmol·L
−1 and 50–75 nmol·L
−1 had lower scores for tests of muscle function compared to those with levels >75 nmol·L
−1 but observed no relationships with tests of muscle strength [
97]. Similarly, in youth, positive relationships were consistently reported with tests of anaerobic (broad jump, vertical jump, sprints) performance and aerobic performance (estimated VO
2max) [
98,
99,
100,
102]. However, there were mixed results with tests of muscle strength and serum 25(OH)D concentrations [
101,
103,
104]. These results indicate that vitamin D levels are impactful on skeletal muscle mass and function across the lifespan, but the effects on muscle strength is inconclusive. In addition to these findings, it is reported that 25(OH)D metabolite accumulates in skeletal muscle cells, suggesting that maintaining muscle mass can also play a role in preserving vitamin D status in times when deficiency may become prevalent, suggesting that skeletal muscle health may have an influence on vitamin D levels [
105,
106].
1.3. Vitamin D Interventions and Skeletal Muscle Health
Multiple studies have examined the effects of vitamin D supplementation on muscle mass, strength, and function, typically with doses ranging from 1000 to 4000 IU·d
−1 over 4–12 weeks to over 60,000 IU·week
−1 for up to 4 months. Contrasting results of the effects vitamin D supplementation has on muscle health have been reported, potentially due to the large variety in dosage, type of vitamin D supplementation, duration of study, and target population [
107,
108]. In athletes, vitamin D supplementation has shown conflicting results with measurements of athletic performance. While some studies reported an increase in maximal strength performance for leg extensions, leg curls, and chin ups [
109,
110], other studies found no change in strength when compared to a control [
111,
112,
113,
114,
115]. Close et al. demonstrated an increase in anaerobic performance (vertical jump height and 10 m sprint) after 6 weeks of 5000 IU vitamin D supplementation [
112], while other studies showed no effect on anaerobic performance after supplementation [
111,
113,
115]. One study reported an increase in VO
2 max after 5000 IU·d
−1 for 8 weeks [
114], while another reported no changes in VO
2 max after 12 weeks of 3000 IU·d
−1 [
115]. No studies in athletes examined the effects of vitamin D supplementation on muscle mass.
In older adults, the effects of vitamin D supplementation on strength were overall positive. Hajj et al. reported a greater change in handgrip strength compared to a placebo (10.13 to 27.98 ng·mL
−1,
p < 0.001 versus 10.56 to 15.71 ng·mL
−1,
p < 0.001) [
122]. Bauer et al. observed an increase of 0.79 kg in handgrip strength over 13 weeks (
p = 0.005); however, this supplementation also included whey protein and leucine, which may have contributed to the increase in strength [
118]. Multiple studies have examined the effects of supplementation on tests of muscle function including the Short Physical Performance Battery (SPPB), chair stand, time-up and go, postural sways, and tests of walking speed. Increases in SPPB and chair stand performances were observed after 800–1000 IU of vitamin D compared to a placebo [
118,
119], but no differences were reported in other studies [
116,
117,
121,
123,
127]. Similarly, studies that examined the effects of vitamin D on muscle mass were generally positive. In those receiving vitamin D supplementation, appendicular muscle mass increased [
118,
122] and lean mass was maintained over 9 months while decreasing in the placebo group [
119]. Additionally, Ceglia et al. reported a 10.6% increase in muscle fiber cross-sectional area and 29.7% increase in VDR concentration, indicating a potential mechanistic influence of vitamin D on skeletal muscle [
82]. These results indicate that while vitamin D supplementation may have a potential positive influence on muscle strength and function in older adults, there is more evidence supporting a beneficial effect on muscle mass in this population. Additionally, in all populations reviewed, the dosage and duration of treatment varied, ensuing in inconclusive results.
Few studies were observed reporting the effects of vitamin D supplementation on skeletal muscle health in a youth population [
124,
125,
126]. These studies also varied in age, duration, and dosage of vitamin D supplementation. While each intervention resulted in an increase in serum 25(OH)D levels, there were no positive effects in muscle mass, strength, or function when compared to a placebo [
124,
125,
126]. This suggests that vitamin D supplementation may be more effective and necessary as an individual ages, potentially due to reduced dietary intake, reduced sun exposure and ability of the skin to produce vitamin D, and impaired absorption [
128,
129], as well as impairments in vitamin D actions on skeletal muscle through mitochondrial dysfunction and compromised anabolic pathways with age [
130]. Several studies indicate a relationship between serum 25(OH)D levels and muscle mass and strength, suggesting a mechanistic link between low vitamin D levels and declines in muscle mass, strength, and function. Vitamin D deficiency has been extensively researched in sarcopenic older adults, suggesting that while deficiency of vitamin D may lead to sarcopenia and related adverse outcomes such as higher risk of falls, muscle fiber atrophy, and disability during hospitalization [
131,
132], supplementation has shown conflicting results in improving sarcopenia [
133,
134] However, the conflicting results in intervention studies cannot confirm vitamin D supplementation has an effective way to increase performance or prevent and/or treat sarcopenia in older adults. It is more likely that protein sources including a variety of nutrients including vitamin D have a greater effect. For example, 12–13-week interventions of supplementation including vitamin D and leucine-enriched whey protein showed improvements in muscle mass and lower extremity strength and function in sarcopenic older adults [
8,
118,
135], specifically if adequate protein was consumed [
8,
118,
135].
Although many prospective cross-sectional and intervention studies have examined vitamin D and skeletal muscle health, conflicting results indicate that while there is potential for positive benefits of maintaining an adequate vitamin D status, there is no conclusive evidence that vitamin D levels beyond optimal range provide any enhancement to skeletal muscle health in a variety of populations. However, there appears to be a greater response in an older population, suggesting that maintaining levels through dietary intake and supplementation becomes more important with age. Additionally, the studies reviewed showed great diversity in participant characteristics, outcome measurements, and/or dosage amount and duration even within the separate population groups, which may have influenced the response or association between vitamin D and skeletal muscle health.
2. Iron and Skeletal Muscle Health
2.1. Iron and Skeletal Muscle Physiology
Iron is an essential mineral for multiple processes in the body that influence skeletal muscle performance such as oxygen transport, electron transport, and red blood cell production [
136,
137,
138]. There is approximately 3–4 g of iron within the human body, in which about 70% of the body’s iron is found within hemoglobin (Hb) in red blood cells and myoglobin (Mb) within skeletal muscle [
139]. Specifically, skeletal muscle contains about 10–15% of the iron in the body, mainly within oxidative fibers high in myoglobin [
140]. The iron within the body is meticulously recycled to replace iron losses that occur within the gastrointestinal tract, skin, hair, sweat, and menses [
141,
142]. However, despite this efficient regulatory process, iron deficiency remains the most common nutritional deficiency in the world [
139,
143,
144], typically from diminished iron absorption or increased iron loss, which is greater in females compared to males due to loss during menstruation [
145]. Additionally, a vegan or vegetarian diet can be a risk factor for developing anemia, suggesting that dietary choices can be impactful for maintaining optimal iron status [
146]. However, iron overload in thalassemia is an opposing challenge of iron deficiency that is influenced by iron regulation [
147,
148]. With the potential adverse health outcomes of iron overload, such as increased morbidity, maintaining an optimal iron status is necessary.
A number of biomarkers are utilized to reflect parts of the iron metabolism process, and therefore, are useful in isolation and in conjunction with one another. A common marker used to determine iron deficiency is ferritin. Ferritin is reflective of the body iron stores [
149,
150] and is used to determine the first stage of iron deficiency [
139,
151] defined as a lack of body iron stores. Ferritin levels <12–15 μg·L
−1 indicate depleted iron stores; however, cutoff criteria between 15 and 35 μg·L
−1 have often been utilized to diagnosis iron deficiency [
47,
152,
153]. It is important to note that ferritin is an acute phase protein and is elevated with the presence of inflammation, so diagnosis with ferritin alone warrants caution in those with inflammation [
154]. Correction for inflammation and measurement of inflammatory status are recommended when examining ferritin [
155,
156].
Decreased transferrin saturation or increased soluble transferrin receptor (sTfR) portrays the second stage of iron deficiency, reflecting reduced erythropoiesis. Transferrin saturation of <15–20% is considered indicative of iron deficiency [
139]. An elevated sTfR indicates tissue iron deficiency and shows an inverse relationship with iron deficiency severity [
157]. Together, the ratio of serum sTfR and serum ferritin can provide an index that reflects body iron. This measurement is effective for monitoring fluctuations in iron status [
158].
The last stage of iron deficiency results in iron deficiency anemia with the addition of Hb as a biomarker [
159]. Concentrations of Hb is a commonly measured parameter due to its affordability and accessibility; however, Hb is not specific to iron due to other potential contributors such as folate or vitamin B12 deficiency or anemia of chronic inflammation [
139,
160]. The inclusion of a second biomarker, such as ferritin or sTfR, simultaneously with Hb can confirm the diagnosis of iron deficiency anemia. Cutoff values range based on sex, age, and ethnicity [
161]. According to the World Health Organization [
159], diagnosis of anemia utilizing Hb concentrations have cutoff criteria of <115 g·L
−1 for ages 5 to 11 years, <120 g·L
−1 for ages 12–14 years, and <120 g·L
−1 and 130 g·L
−1 for females and males, respectively, 15 years or older [
159,
162].
Iron is essential for skeletal muscle function largely due to several pathways. While known for its necessary role for Hb production in red blood cells, iron is required for many processes for energy metabolism [
163,
164]. In particular, oxidative metabolism requires iron for adequate oxygen supply and the transfer of electrons during redox reactions [
165,
166]. Additionally, the majority of iron in skeletal muscle is within slow twitch muscle fibers that are abundant in myoglobin, in which oxidative metabolism occurs [
166,
167]. Enzymatic complexes within the electron transport chain rely on iron to function, indicating that an adequate supply of iron is essential for the oxidation of fuel sources for energy [
164,
165]. This indicates that the aerobic capacity of an individual greatly relies on the oxygen-carrying capacity of the blood as well as the muscle oxidative capacity [
140], both of which are heavily dependent upon iron.
2.2. Iron and Skeletal Muscle Health
Multiple studies have examined associations between iron status and parameters of skeletal muscle health. In youth populations, the association between iron biomarkers and performance seemed to largely depend on what performance metric were utilized. Wang et al. reported that iron deficiency was related to lower fat-free associated VO
2max within females, and both males and females with iron deficiencies had lower energy expenditure at leisure compared to adequate iron group [
168]. Arsenault et al. reported that females with low ferritin levels had lower levels of performance during a shuttle run test, whereas males with low ferritin levels had lower long jump scores compared to those of normal ferritin levels [
169]. Lastly, in a study conducted by Gracia-Marco et al. Hb concentrations were associated with estimated VO
2max results in male adolescents only [
99]. These varying outcomes provide insight into iron deficiency playing a role in oxygen transport directly influencing aerobic activities. The influence of iron status on anaerobic activities is further warranted to understand the role of iron on muscle strength, health, and function within youth populations.
Similar observations were observed in athletic populations where iron status seems to be more influential to the performance of aerobic-based activities. In a study conducted by DellaValle & Hass, female rower athletes that were categorized as iron depleted without anemia (IDNA) (serum ferritin < 20.0 μg·L
−1), had lower VO
2max and higher blood lactate concentrations during a 4 km rowing test [
46]. In addition, the authors suggested that iron status also likely played a role in training load of the athletes where those athletes categorized as IDNA had lower training times than the non-anemic group during a 4-week observation. In addition, Tsai et al. reported that mildly anemic males enlisted within the Taiwan Military were likely to be the worst 10% performers during a standard 3000 m run test but were likely to be the best 10% performers during an anaerobic test such as the 2 min push-up test [
170]. Shoemaker et al. also indicated within youth athletic population that performance during aerobic fitness tests such as vertical jump, broad jump, agility drill times, 20-yard dash time, power push up force, were related to Hb status in males and with sTfR and iron intake in females [
171]. Together these studies further support the notion that iron plays a role in aerobic metabolism and cardiorespiratory fitness; however, the role on iron status and anaerobic fitness tests require further investigation.
Within the older adult population, iron status seems to play a role in frailty. For example, multiple studies reported that lower Hb concentrations were associated with higher frailty scores or associated with a greater risk of frailty than non-frail individuals [
172,
174,
176,
177]. However, in older adults, iron status seemed to vary based on muscle strength metrics. For example, low Hb levels were reported to not be associated with grip strength in older adults in Brazil [
174]; however, in hospitalized older adults there was a positive association between Hb levels at baseline and at discharge within those with iron deficiency. Together, these associations suggest that low iron status is related to decreased aerobic and anaerobic performance. While few studies examined the associations between iron status and muscle mass, low Hb concentrations were related to lower muscle mass and the presence of frailty in older adults [
172,
173,
174,
175,
176]. However, there was no concluding evidence on muscle strength and function measurements.
2.3. Iron Interventions and Skeletal Muscle Health
Iron supplementation is a common method utilized to correct iron deficiency, particularly in athletic populations. Oral supplementation doses ranging from 40 to 400 mg·d
−1 for treatment durations of 6–24 weeks has been effective in improving iron status [
178,
179,
180,
181]. However, there are contrasting results regarding the effectiveness of iron supplementation for performance measurements reflective of skeletal muscle health. Multiple studies have examined the effects of iron supplementation on performance in athletes, yet there is a lack of studies examining if iron supplementation influences skeletal muscle mass or is effective in other healthy populations such as youth or older adults. In aerobic-based athletes (rowers, runners, and cyclists), iron supplementation over 6 weeks was effective in improving aerobic performance such as 4 km time trial and VO
2max for rowers and runners [
179,
180]. However, a shorter intervention of 80 mg·d
−1 showed no improvement in muscle recovery from cycling performance [
182]. One study examined muscle strength measurements in female volleyball players. Iron supplementation of 325 mg·d
−1 over 11 weeks improved strength in two power exercises and total strength over six different exercises [
181]. These studies indicate that improving iron status via supplementation may be effective in improving performance-based measurements of skeletal muscle health. Future research should examine the effects of improving iron status on skeletal muscle mass, strength, and function in other vulnerable populations such as youth and older adults.
3. Interrelationship between Vitamin D, Iron, and Chronic Inflammation
3.1. Anti-Inflammatory Role of Vitamin D
In addition to its role on skeletal muscle health, vitamin D is thought to have anti-inflammatory actions through activation and differentiation of inflammatory cells, leading to a reduction in risk of infection and inflammation [
12,
13,
183,
184]. Vitamin D has immunomodulatory benefits including the enhancement of antimicrobial peptides and defensins to improve cellular immunity and reduce cytokine storms linked to infection. Adequate levels of vitamin D are related to the decrease in production of pro-inflammatory cytokines and an increase in anti-inflammatory cytokines [
14,
15,
16,
17,
18]. Additionally, Shoemaker et al. recently reviewed the effects of vitamin D supplementation on reducing the risks of respiratory tract infections and viral infections including SARS-CoV-2 and indicate that sub-optimal vitamin D status increases risk for incidence, complication, and mortality due to infection and the presence of inflammation (manuscript in press). Therefore, vitamin D is a potential nutritional strategy that may reduce chronic inflammation.
The relationship between vitamin D status and inflammation has been studied previously in older adults [
185,
186]. Furthermore, vitamin D supplementation has demonstrated beneficial effects on chronic inflammation [
7,
14,
15,
16,
187]. For example, Liberman et al. reported that 13 weeks of vitamin D and protein supplementation was effective in preventing increases in inflammatory cytokines compared to a placebo in older adults [
7]. Similarly, Pereira et al. reported that 12 weeks of oral nutritional supplementation rich in vitamin D, HMB, and protein improved multiple biomarkers related to inflammation, immune function, and overall muscle health [
188].
3.2. Connection between Vitamin D and Iron Status
Vitamin D and iron are both essential nutrients for skeletal muscle health, suggesting that optimal status in both micronutrients may interactively benefit skeletal muscle health. Vitamin D is important for the regulation of iron metabolism; therefore, low vitamin D status may result in low iron status [
9,
11,
34]. Due to the relationship observed between vitamin D action on pro-inflammatory cytokines and mechanisms behind iron regulation [
9,
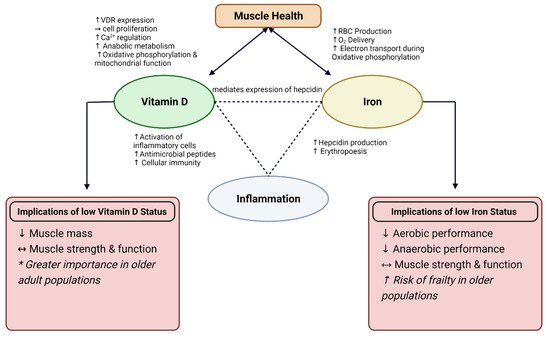
35], examining the physiological functions of vitamin D and iron status on skeletal muscle health and inflammation is an important next step in promoting health and performance. This theorized interaction is displayed in
Figure 1. The connection between vitamin D and iron status is thought to be associated with hepcidin, an antimicrobial peptide that is essential for the regulation of iron metabolism [
9,
35]. Iron absorption and excretion is a highly regulated process, in which iron absorption increases with deficiency and decreases when iron stores are full. Systemic iron status, erythropoiesis requirements, and presence of inflammation can all influence this regulatory process [
136]. The presence of high inflammation results in an increase in hepcidin production, causing iron to sequester and limit iron-supported erythropoiesis [
189]. This leads to diminished ability to absorb iron, thus leading to iron deficiency anemia.
Figure 1. Interaction between Vitamin D and Iron on muscle health and role for inflammation. Depicted implications of low Vitamin D and Iron status on muscle health and performance. VDR = Vitamin D Receptor; RBC = red blood cell, Ca2+ = calcium; O2 = oxygen; ↑ = increase; ↓ = decrease; ↔ = conflicting results. * More profound results observed in interventions with older adults.
Additionally, vitamin D can mediate the expression of hepcidin through the binding of VDR with a gene promotor called HAMP gene to downregulate hepcidin production [
35]. Furthermore, the role vitamin D has in decreasing expression of inflammatory cytokines that have a stimulating role on hepcidin production may indirectly contribute to this integration. Through in vitro studies, there is evidence that adequate levels of vitamin D are associated with reduced concentrations of hepcidin due to the suppression of the HAMP gene, as well as due to reduced concentrations of pro-inflammatory cytokines such as IL-1β and IL-6 [
11]. This suggests the potential for vitamin D levels to influence iron regulation through hepcidin, specifically in the presence of inflammation. Additionally, vitamin D supplementation has been found to decrease hepcidin, and thus may have benefits in altered iron status, particularly in those with chronic inflammation [
11]. While typically associated with anemia related to inflammation, it is possible that this mechanism may also be related to iron deficiency with or without anemia due to the reduction in iron necessary to support erythropoiesis. This suggests that those with chronic inflammation may have greater iron requirements to increase circulating iron concentrations and promote red blood cell production, indicating the need for nutritional support with both vitamin D and iron.
Relationships between low vitamin D status and low iron status are reported, providing further support for this nutritional interaction [
49,
50,
190]. Vitamin D deficiency and low iron status are prevalent in multiple populations including older adults [
22,
40,
44,
45,
51], children [
52,
53,
54,
55], and athletes [
47,
56,
57], emphasizing the potential influence these nutrients may have on skeletal muscle health throughout the lifespan. Additionally, associations between vitamin D deficiency and low iron status have been demonstrated. For example, Malczewska-Lenczowska et al. reported that female athletes with iron deficiency also had lower vitamin D concentrations. Female athletes with vitamin D deficiency also had lower ferritin and iron concentrations and higher total iron binding capacity and sTfR, indicating low iron status [
49]. Additionally, vitamin D supplementation (3000 IU·day
−1) was effective in preventing a decline in Hb and hematocrit and improve transferrin levels, as well as concentrations of vitamin D in elite male rowers [
191]. These findings support the association between vitamin D and iron status in athletes, although it is unclear which of the nutrients is the cause or the effect in the relationship. Further research is needed to examine if this nutrient interaction is influential to skeletal muscle health.
A retrospective study in children aged 10–20 years demonstrated an association between vitamin D deficiency and both anemia and iron deficiency when accounting for contributing factors [
192]. The relationship between Hb and vitamin D was more prominent in female children, compared to males, suggesting that those vulnerable to nutritional deficiencies may be most affected by this nutritional interaction with skeletal muscle health through the growth and development stage. Similarly, in a pediatric population of inflammatory bowel disease patients, children with vitamin D concentrations ≥ 30 ng mL
−1 had lower hepcidin and higher Hb concentrations when controlling for inflammation [
193]. In older adults, the prevalence of vitamin D deficiency was higher in those with anemia due to inflammation (56%) and nutritional deficiency (48%) [
51]. These findings suggest that children and older adults are at risk for compounding nutritional deficiencies, along with inflammation, that may be influential to the muscle growth and atrophy typically observed at each life stage. Therefore, adequate consumption of nutrients such as iron and vitamin D is essential for these populations.
4. Animal Food Sources
Adequate consumption of vitamin D and iron may be key in enhancing muscle mass, strength, and performance. Animal-source foods are abundant in vitamin D and iron, which may help individuals reach optimal, bioavailable intakes of these nutrients to support skeletal muscle health [
58,
59,
60]. Specifically, beef is rich in bioavailable heme iron that may reduce the risk of iron deficiency and anemia [
194,
195]. Heme iron is found only in animal-source foods and are better absorbed than plant sources containing non-heme iron [
195]. In particular, beef sources including ground beef, beef liver, and bottom round beef cuts are abundant sources of iron, containing 2–5 g of heme iron per 3 oz. serving. Previous studies have indicated the consumption of iron-rich red meat, along with resistance training, have shown beneficial effects on muscle mass, muscle strength, and reduce inflammatory markers [
58,
59], providing support for iron’s role in muscle health.
While vitamin D originates from sunlight exposure, dietary intake of vitamin D can be obtained from a variety of food sources in which approximately 60% of intake comes from animal-source foods such as fish, meat, and eggs [
196]. Dietary intake of vitamin D range from 3.8 to 7.2 µg·d
−1 in youth, 3.6 to 5.4 µg·d
−1 in adults, and 3.9 to 5.1 µg·d
−1 in older adults [
197], which is lower than the recommended intake between 10 and 15 µg·d
−1 (400–600 IU) [
198]. Fortified milk provides a majority of the vitamin D within the American diet [
199], with approximately 3 µg per cup [
200]. Additionally, other key sources of vitamin D include beef liver and other beef sources, fatty fish such as salmon, eggs, and chicken [
201]. Previous studies have observed positive results in vitamin D status after supplementing with vitamin D-fortified milk [
202], suggesting potential for increasing muscle health through food sources.
Adequate nutritional intake is essential for muscle growth, performance, and preventing of sarcopenia. In particular, nutrients abundant in animal food sources such as vitamin D, iron, and protein, have been related to athletic performance, functional performance, and muscle growth [
58,
59,
203,
204,
205]. Criticism of the current Recommended Daily Allowance (RDA) of 0.8 g·kg
−1·d
−1 warrants an increase specifically when protein anabolism is effected such as during the aging process and during exercise [
206,
207]. Sarcopenic older adults had lower intakes of protein, lipids, and micronutrients including iron and vitamin D [
208,
209]. Additionally, oral nutritional supplementation rich in protein and vitamin D resulted in improvement of markers of health, strength, and inflammation in malnourished, sarcopenic older adults [
188]. These results indicate that an animal-source food matrix may be optimal when trying to enhance skeletal muscle function and reduce the risk of chronic inflammation.
Dietary protein from animal sources has long been established as beneficial for skeletal muscle by increasing muscle protein synthesis due to the essential amino acid content [
210,
211]. Animal protein sources effectively improved skeletal muscle strength and mass in healthy young adults and older adults [
58,
59,
203,
212,
213], indicating functional benefits of including dietary animal-source foods. Red meat, such as beef, and dairy products are two groups of animal-source foods that show promise for improving skeletal muscle health, potentially due to the nutrient content of these foods.
Multiple studies have examined the effects of beef intake on skeletal muscle-based outcomes in older adults [
58,
59,
214,
215,
216]. Recent reviews have concluded that beef and/or the nutrients found within beef may improve muscle function [
214,
217]. Asp et al. investigated the relationship between beef intake and muscle mass in older adults ages 60–88 years, reporting that beef intake was positively related to mid-arm muscle area. Furthermore, regression analysis predicted that a 1 oz increase in beef consumption per day would result in a 2.23 cm
2 increase in mid-arm muscle area [
58]. In agreement, Morris & Jacques also predicted a linear increase in muscle mass (appendicular skeletal muscle index) in association with a 100 g per week increase in beef intake [
213]. Lean red meat enhanced the effects of resistance training on muscle mass and strength in older females [
59] and in 1 repetition maximum leg extension strength in older males [
215]. In contrast, when examining the effects of lean beef in addition to resistance training, no additional benefits were observed in older adults when beef was consumed twice a week for 24 weeks [
216]. Additionally, lean beef and protein supplementation had no positive effect on fat-free mass in older adults [
215,
218]. Meals containing pork, beef, or chicken showed similar impact on body composition and strength, indicating that high-quality protein sources, in general, have the same effect on skeletal muscle health [
219,
220]. Furthermore, consumption of beef protein isolate, chicken protein isolate, or whey protein all resulted in increases in lean body mass, regardless of source of protein [
203]. Higher intake of protein source foods including red meat, poultry, fish, dairy, soy, nuts, seeds, and legumes were positively associated with higher percent skeletal muscle mass over time in older adults, indicating that higher intake of animal-source foods can help maintain skeletal muscle with age [
212]. These studies indicate conflicting skeletal muscle health outcomes with animal protein consumption; however, adequate consumption of protein sources in general appear to be a beneficial method for preserving muscle mass and strength. Future random controlled trials are required to provide further support of animal-source foods such as beef and poultry as dietary sources to promote skeletal muscle health.
Fortified dairy products are also nutrient-dense foods with the potential to improve skeletal muscle health [
204,
205]. In addition to many dairy products being fortified with vitamin D, they are rich in protein and many micronutrients including vitamin B12, calcium, riboflavin, and zinc, that are important for muscle health and function [
221]. Consumption of dairy milk with additional protein improved fat-free mass, strength, and power in young males when consumed following resistance training [
222]. However, in older adults, consuming higher protein dairy milk did not further improve the benefits obtained from resistance training alone on fat-free mass, power, or physical performance, but did improve maximal strength measurements [
223].
This entry is adapted from the peer-reviewed paper 10.3390/nu14132717