
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rakeeb Ahmad Mir | -- | 4350 | 2022-07-11 16:17:50 | | | |
| 2 | Peter Tang | Meta information modification | 4350 | 2022-07-12 05:20:51 | | |
Video Upload Options
Nematodes are non-segmented roundworms evenly distributed with various habitats ranging to approximately every ecological extremity. These are the least studied organisms despite being the most diversified group. Nematodes are the most critical equilibrium-maintaining factors, having implications on the yield and health of plants as well as well-being of animals. As a result of the lack of precise taxonomic features, nematode taxonomy remains uncertain. Morphology-based identification has proved inefficacious in identifying and exploring the diversity of nematodes, as there are insufficient morphological variations. Different molecular and new evolving methodologies have been employed to augment morphology-based approaches and bypass these difficulties with varying effectiveness. These identification techniques vary from molecular-based targeting DNA or protein-based targeting amino acid sequences to methods for image processing. High-throughput approaches such as next-generation sequencing have also been added to this league. These alternative approaches have helped to classify nematodes and enhanced the base for increased diversity and phylogeny of nematodes, thus helping to formulate increasingly more nematode bases for use as model organisms to study different hot topics about human well-being.
1. Introduction
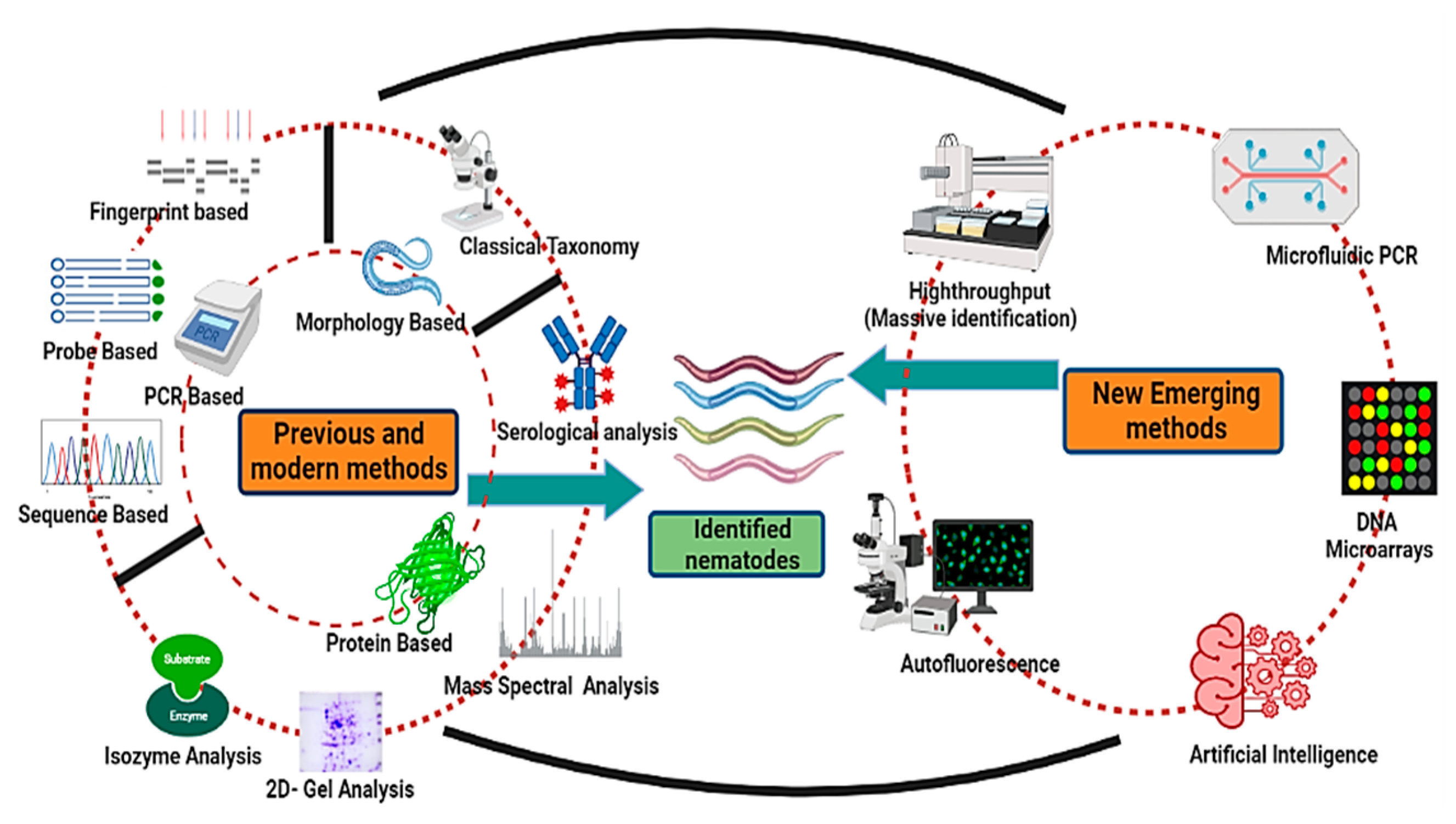
2. Conventional or Morphometric Method of Identification
2.1. Morphological Methods of Identification
3. Molecular Identification Methods

3.1. PCR-Based Methods

3.2. Fingerprint-Based Methods
3.2.1. RFLP (Restriction Fragment Length Polymorphism)
3.2.2. Random Amplified Polymorphic DNA (RAPD)
3.2.3. Amplified Fragment Length Polymorphism (AFLP)
3.3. Probe-Based Detection Methods
3.4. Sequence-Based Detection Method
4. Metabarcoding
5. Biochemical- and Protein-Based Methods of Identification
5.1. Analysis Based on Isozymes
5.2. Use of Two-Dimensional Gel (2-DGE) Analysis
5.3. Serological Analysis or Use of Antibodies
5.4. Use of Mass Spectrometry Analysis
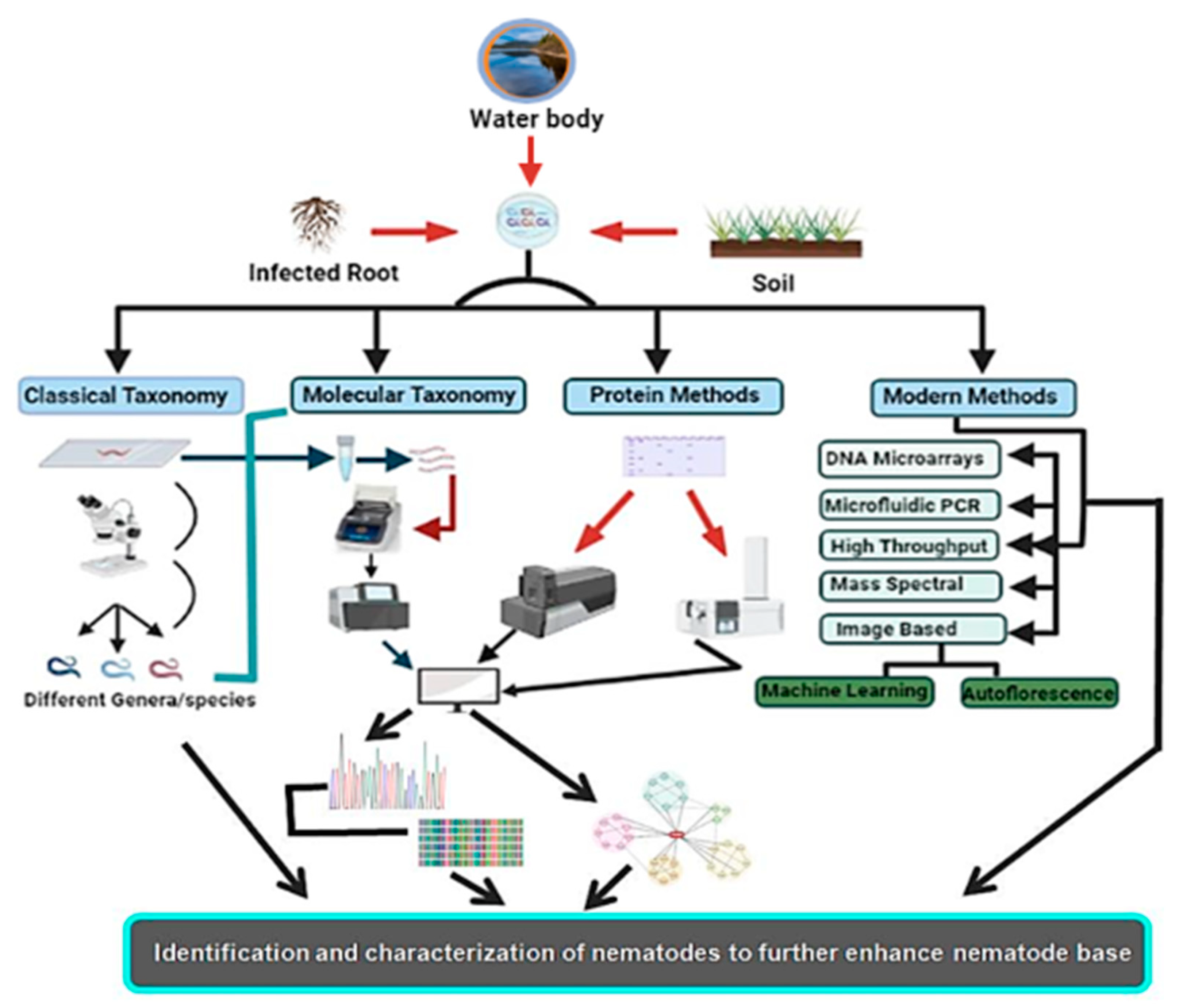
6. Emerging Methods of Nematode Identification
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915.
- Abebe, E.; Mekete, T.; Thomas, W.K. A critique of current methods in nematode taxonomy. Afr. J. Biotechnol. 2011, 10, 312–323.
- Sibert, J.R. Intertidal hyperbenthic populations in the Nanaimo Estuary. Mar. Biol. 1981, 64, 259–265.
- Roeber, F.; Jex, A.R.; Gasser, R.B. Next-generation molecular-diagnostic tools for gastrointestinal nematodes of livestock, with an emphasis on small ruminants: A turning point? Adv. Parasitol. 2013, 83, 267–333.
- De Oliveira, C.M.G.; Monteiro, A.R.; Blok, V.C. Morphological and molecular diagnostics for plant-parasitic nematodes: Working together to get the Identification done. Trop. Plant Pathol. 2011, 36, 65–73.
- Blaxter, M.L.; De Lay, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75.
- Meneely, P.M.; Dahlberg, C.L.; Rose, J.K. Working with worms: Caenorhabditis elegans as a model organism. Curr. Protoc. Essent. Lab. Tech. 2019, 19, e35.
- Ferri, E.; Barbuto, M.; Bain, O.; Galimberti, A.; Uni, S.; Guerrero, R.; Ferté, H.; Bandi, C.; Martin, C.; Casiraghi, M. Integrated taxonomy: Traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda). Front. Zool. 2009, 6, 1.
- Hunt, D.; Zafar, H. Taxonomy, Identification, and principal species. Root-Knot Nematodes 2009, 1, 55–97.
- Roeber, F.; Kahn, L. The specific diagnosis of gastrointestinal nematode infections in livestock: Larval culture technique, its limitations, and alternative DNA-based approaches. Vet. Parasitol. 2014, 205, 619–628.
- Gasser, R.B. Molecular tools—Advances, opportunities, and prospects. Vet. Parasitol. 2006, 136, 69–89.
- Huette, R.N.; Golden, A.M. Nathan augustus COBB: The Father of Nematology in the United States. Annu. Rev. Phytopathol. 1991, 29, 15–26.
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–415.
- Floyd, R.; Abebe, E.; Papert, A.; Blaxter, M. Molecular barcodes for soil nematode identification. Mol. Ecol. 2002, 11, 839–850.
- Hunt, D.J.; Palomares-Rius, J.; Manzanilla-López, R.H. Identification, Morphology and Biology of Plant Parasitic Nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Boston, MA, USA, 2018; Volume 10, pp. 20–61.
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992.
- Evangelina, G.L.; Sánchez-Puerta, M.V. Characterization of a Root-Knot Nematode Population of Meloidogyne arenaria from Tupungato (Mendoza, Argentina). J. Nematol. 2012, 3, 291–301.
- Mir, R.A.; Bhat, K.A.; Rashid, G.; Ebinezer, L.B.; Masi, A.; Rakwal, R.; Shah, A.A.; Zargar, S.M. DNA barcoding: A way forward to obtain deep insights about the realistic diversity of living organisms. Nucleus 2021, 2, 157–165.
- Ahmed, M.; Sapp, M.; Prior, T.; Karssen, G.; Back, M. Nematode taxonomy: From morphology to metabarcoding. Soil Discuss. 2015, 2, 1175–1220.
- Blok, V.C. Achievements in and future prospects for molecular diagnostics of plant-parasitic nematodes. Can. J. Plant Path. 2005, 2, 176–185.
- Reslova, N.; Skorpikova, L.; Kyrianova, I.A.; Vadlejch, J.; Höglund, J.; Skuce, P.; Kasny, M. The identification and semi-quantitative assessment of gastrointestinal nematodes in faecal samples using multiplex real-time PCR assays. Parasit. Vectors 2021, 9, 391.
- Ibrahim, I.; Handoo, Z.A.; Basyony, A.B. The cyst nematodes Heterodera and Globodera species in Egypt. Pak. J. Nematol. 2017, 2, 151–154.
- Madani, M.; Subbotin, S.A.; Moens, M. Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using Real-Time PCR with SYBR green I dye. Mol. Cell Probes 2005, 2, 81–86.
- Shah, A.A.; Mir, R.A. Role of DNA-based markers in nematode taxonomy: A review. Int. J. Nematol. 2015, 2, 208–214.
- Ndao, M. Diagnosis of parasitic diseases: Old and new approaches. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 278246.
- Handoo, Z.A.; Carta, L.K.; Skantar, A.M. Taxonomy, Morphology, and Phylogenetics of Coffee-Associated Root-Lesion Nematodes, Pratylenchus spp. In Plant-Parasitic Nematodes of Coffee; Souza, R.M., Ed.; Springer: Dordrecht, The Netherlands, 2008.
- Gasser, R.B.; Bott, N.J.; Chilton, N.B.; Hunt, P.; Beveridge, I. Toward practical, DNA based diagnostic methods for parasitic nematodes of livestock—Bionomic and biotechnological implications. Biotechnol. Adv. 2008, 26, 325–334.
- Mattiucci, S.; Nascetti, G. Chapter 2: Advances and Trends in the Molecular Systematics of Anisakid Nematodes, with Implications for their Evolutionary Ecology and Host-Parasite Co-Evolutionary Processes. Adv. Parasitol. 2008, 66, 47–148.
- Thevenoux, R.; Folcher, L.; Esquibet, M.; Fouville, D.; Montarry, J.; Grenier, E. The hidden diversity of the potato cyst nematode Globodera pallida in the south of Peru. Evol. Appl. 2020, 13, 727–737.
- Carneiro, R.M.D.G.; Correa, V.R.; Almeida, M.R.A.; Gomes, A.C.M.; Deimi, A.M.; Castagnone-Sereno, P.; Karssen, G. Meloidogyne lucin. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing different crops in Brazil, Chile, and Iran. Nematology 2004, 16, 289–301.
- Seesao, Y.; Audebert, C.; Verrez-Bagnis, V.; Merlin, S.; Jérôme, M.; Viscogliosi, E.; Dei-Cas, E.; Aliouat-Denis, C.M.; Gay, M. Monitoring of four DNA extraction methods upstream of high-throughput sequencing of Anisakidae nematodes. J. Microbiol. Methods 2014, 102, 69–72.
- Dawkins, H.J.S.; Spencer, T.L. The isolation of nucleic acid from nematodes requires an understanding of the parasite and its cuticular structure. Parasitol. Today 1989, 5, 73–76.
- Karanastasi, E.; Decraemer, W.; Zheng, J.; De Almeida, M.T.M.; Brown, D.J. Interspecific differences in the fine structure of the body cuticle of Trichodoridae Thorne, 1935 (Nematoda: Diphtherophorina) and review of anchoring structures of the epidermis. Nematology 2001, 3, 525–533.
- Castagnone-Sereno, P. Molecular tools for diagnosis. In Genomics and Molecular Genetics of Plant Nematode Interactions, 1st ed.; Jones, J., Gheisen, G., Fenoll, C., Eds.; Springer: New York, NY, USA, 2011; pp. 443–464.
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; C.A.B.I. U.S.D.A.: Wallingford, UK, 2000; Volume 8.
- Bogale, M.; Baniya, A.; Di Gennaro, P. Nematode Identification Techniques, and Recent Advances. Plants 2020, 24, 1260.
- Seesao, Y.; Gay, M.; Merlin, S.; Viscogliosi, E.; Aliouat-Denis, C.M.; Audebert, C.A. review of methods for nematode identification. J. Microbiol. Methods 2017, 138, 37–49.
- Holterman, M.M.; Oggenfuss, M.; Frey, J.E.; Kiewnik, S. Evaluation of high-resolution melting curve analysis as a new tool for root-knot nematode diagnostics. Phytopathology 2012, 160, 59–66.
- Blok, V.C.; Powers, T.O. Biochemical and Molecular Identification; Root-Knot Nematodes by R.N. Perry, M. Moens, and J. L. Starr; C.A.B.I. Flanders Research Institute for agriculture, Fisheries and Food: Ghent, Belgium, 2009; pp. 98–118.
- Subbotin, S.A.; Halford, P.D.; Warry, A.; Perry, R.N. Variations in ribosomal DNA sequences and phylogeny of Globodera parasitizing solanaceous plantas. Nematology 2000, 2, 591–604.
- Mullis, K.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific Enzymatic Amplification of DNA in vitro: The Polymerase Chain Reaction. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 263–273.
- Pontes, T.; D’Amelio, S.; Costa, G.; Paggi. Molecular characterization of larval anisakid nematodes from marine fishes of Madeira by a PCR-based approach, with evidence for a new species. J. Parasitol. 2005, 91, 1430–1434.
- Pineda, O.; Bonierbale, M.W.; Plaisted, R.L.; Brodie, B.B.; Tanksley, S.D. Identification of RFLP markers linked to the H1 gene conferring resistance to the potato cyst nematode Globodera rostochiensis. Genome 1993, 36, 152–156.
- Cameron, M.; Levy, P.; Nutman, T.; Vanamala, C.; Narayanan, P.; Rajan, T. Use of restriction fragment length polymorphisms (RFLPs) to distinguish between nematodes of pathogenic significance. Parasitology 1988, 96, 381–390.
- Sedlák, P.; Melounová, M.; Skupinová, S.; Vejl, P.; Domkářová, J. Study of European and Czech populations of potato cyst nematodes (Globodera rostochiensis and G. pallida) by RAPD method. Plant Soil Environ. 2004, 50, 10.
- Correa, V.R.; Mattos, V.S.; Almeida, M.R.A.; Santos, M.F.A.; Tigano, M.S.; Castagnone-Sereno, P.; Carneiro, R.M.D.G. Genetic diversity of the root-knot nematode Meloidogyne ethiopica and development of a species-specific SCAR marker for its diagnosis. Plant Pathol. 2014, 63, 476–483.
- Castagnone-Sereno, P.; Vanlerberghe-Masutti, F.; Leroy, F. Genetic polymorphism between and within Meloidogyne species detected with RAPD markers. Genome Natl. Res. Counc. Can. Génome Cons. Natl. Rech. Can. 1995, 37, 904–909.
- Randig, O.; Leroy, F.; Castagnone-Sereno, P.R.A.P.D. Characterization of Single Females of the Root-knot Nematodes, Meloidogyne spp. Eur. J. Plant Pathol. 2001, 107, 639–643.
- Höglund, J.; Engström, A.; Morrison, D.; Mattsson, J. Genetic diversity assessed by amplified fragment length polymorphism analysis of the parasitic nematode Dictyocaulus viviparus the lungworm of cattle. Int. J. Parasitol. 2004, 34, 475–484.
- Li, Y.; Lawrence, G.W.; Lu, S.; Balbalian, C.; Klink, V.P. Quantitative field testing Heterodera glycines from metagenomic DNA samples isolated directly from soil under agronomic production. PLoS ONE 2014, 9, e89887.
- Marché, L.; Valette, S.; Grenier, E.; Mugniéry, D. Intra-species DNA polymorphism in the tobacco cyst-nematode complex (Globodera tabacum) using A.F.L.P. Genome 2001, 44, 941–946.
- Fang, W.; Xu, S.; Zhang, S.; Wang, Y.; Chen, X.; Luo, D. Multiple primer PCR for the Identification of anisakid nematodes from Taiwan Strait. Exp. Parasitol. 2010, 124, 197–201.
- Umehara, A.; Kawakami, Y.; Araki, J.; Uchida, A. Multiplex PCR for the Identification of Anisakis simplex Sensu stricto, Anisakis pegreffii, and the other anisakid nematodes. Parasitol. Int. 2008, 57, 49–53.
- Sint, D.; Raso, L.; Traugott, M. Advances in multiplex PCR: Balancing primer efficiencies and improving detection success. Methods Ecol Evol. 2012, 3, 898–905.
- Mossali, C.; Palermo, S.; Capra, E.; Piccolo, G.; Botti, S.; Bandi, C.; D’Amelio, S.; Giuffra, E. Sensitive detection and quantification of anisakid parasite residues in food products. Foodborne Pathog. Dis. 2010, 7, 391–397.
- Fang, W.; Liu, F.; Zhang, S.; Lin, J.; Xu, S.; Luo, D. Anisakis pegreffii: A quantitative fluorescence PCR assay for detection in situ. Exp. Parasitol. 2011, 127, 587–592.
- Stirling, G.; Griffin, D.; Ophel-Keller, K.; McKay, A.; Hartley, D.; Currar, J.; Stirling, A.; Monsour, C.; Winch, J.; Hardie, B. Combining an initial risk assessment process with DNA assays to improve prediction of soilborne diseases caused by root-knot nematode (Meloidogyne spp.) and Fusarium oxysporum f. sp. lycopersici in the Queensland tomato industry. Australas. Plant Pathol. 2004, 33, 285–293.
- Amiri, S.; Subottin, S.A.; Moens, M. Identification of the beet cyst nematode Heterodera schachtii by PCR. Eur. J. Plant Pathol. 2002, 108, 497–506.
- Sapkota, R.; Skantar, A.M.; Nicolaisen, M. A TaqMan real-time PCR assay for detection of Meloidogyne hapla in root galls and soil. Nematology 2016, 18, 147–154.
- Huang, D.; Yan, G.; Gudmestad, N.; Skantar, A. Quantification of Paratrichodorus allius in DNA extracted from soil using TaqMan Probe and S.Y.B.R. Green real-time PCR assays. Nematology 2017, 19, 987–1001.
- Toumi, F.; Waeyenberge, L.; Viaene, N.; Dababat, A.; Nicol, J.M.; Ogbonnaya, F.; Moens, M. Development of two species-specific primer sets to detect the cereal cyst nematodes Heterodera avenae and Heterodera filipjevi. Eur. J. Plant Pathol. 2013, 136, 613–624.
- Van Megen, H.; Van Den Elsen, S.; Holterman, M.; Karssen, G.; Mooyman, P.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 11, 927–950.
- Hadziavdic, K.; Lekang, K.; Lanzen, A.; Jonassen, I.; Thompson, E.M.; Troedsson, C. Characterization of the 18s rRNA gene for designing universal eukaryote specific primers. PLoS ONE 2014, 9, e87624.
- DeSalle, R.; Egan, M.G.; Siddall, M. The unholy trinity: Taxonomy, species delimitation, and DNA barcoding. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1905–1916.
- Bu, Y.; Niu, H.; Zhang, L. Phylogenetic analysis of the genus Cylicocyclus (Nematoda: Strongylidae) based on nuclear ribosomal sequence data. Acta Parasitol. 2013, 58, 167–173.
- Félix, M.A.; Braendle, C.; Cutter, A.D. A streamlined system for species diagnosis in Caenorhabditis (Nematoda: Rhabditidae) with name designations for 15 distinct biological species. PLoS ONE 2014, 9, e94723.
- Zajac, A.M. Gastrointestinal nematodes of small ruminants: Life cycle, anthelmintics, and diagnosis. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 529–541.
- McLeod, R.S. Costs of major parasites to the Australian livestock industries. Int. J. Parasitol. 1995, 25, 1363–1367.
- Zarlenga, D.S.; Hoberg, E.P.; Stringfellow, F.; Lichtenfels, J.R. Comparisons of two polymorphic species of Ostertagia and phylogenetic relationships within the Ostertagiinae (Nematoda: Trichostrongyloidea) inferred from ribosomal DNA repeat and mitochondrial DNA sequences. J. Parasitol. 2001, 84, 806–812.
- Chilton, N.B.; Newton, L.A.; Beveridge, I.; Gasser, R.B. Evolutionary relationships of trichostrongyloid nematodes (Strongylida) inferred from ribosomal DNA sequence data. Mol. Phylogenet. Evol. 2001, 19, 367–386.
- DellAnno, A.; Carugati, L.; Corinaldesi, C.; Riccioni, G.; Danovaro, R. Unveiling the Biodiversity of Deep-Sea Nematodes through Metabarcoding: Are We Ready to Bypass the Classical Taxonomy? PLoS ONE 2015, 10, e0144928.
- Pedram, M.; Pourjam, E.; Atighi, M.R.; Panahandeh, Y. Further studies on soil nematode fauna in northwestern Iran with the description of one new species. J. Nematol. 2015, 47, 148.
- Holovachov, O.; Haenel, Q.; Bourlat, S.J.; Jondelius, U. Taxonomy assignment approach determines the efficiency of identification of O.T.U.s in marine nematodes. R. Soc. Open Sci. 2017, 4, 170315.
- Valentini, A.; Mattiucci, S.; Bondanelli, P. Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial Cox2 sequences, and comparison with allozyme data. J. Parasitol. 2006, 92, 156–166.
- Navas, A.; López, J.A.; Espárrago, G.; Camafeita, E.; Albar, J.P. Protein variability in Meloidogyne spp. (Nematoda: Meloidogynidae) revealed by two-dimensional gel electrophoresis and mass spectrometry. J. Proteome Res. 2002, 1, 421–427.
- Millares, P.; LaCourse, E.J.; Perally, S.; Ward, D.A.; Prescott, M.C.; Hodgkinson, J.E.; Brophy, P.M.; Rees, H.H. Proteomic profiling and protein identification by MALDI-TOF mass spectrometry in unsequenced parasitic nematodes. PLoS ONE 2012, 7, e33590.
- Schots, A.; Hermsen, T.; Schouten, S.; Gommers, F.J.; Egberts, E. Serological differentiation of the potato-cyst nematodes Globdera pallida and G. rostochiensis: II. Preparation and characterization of species-specific monoclonal antibodies. Hybridoma 1989, 8, 401–413.
- Akintayo, A.; Tylka, G.L.; Singh, A.K.; Ganapathysubramanian, B.; Singh, A.; Sarkar, S. A deep learning framework to discern and count microscopic nematode eggs. Sci. Rep. 2018, 8, 9145.
- Hakim, A.; Mor, Y.; Toker, I.A.; Levine, A.; Neuhof, M.; Markovitz, Y.; Rechavi, O. WorMachine: Machine learning-based phenotypic analysis tool for worms. BMC Biol. 2018, 16, 8.
- Qazi, F.; Khalid, A.; Poddar, A.; Tetienne, J.P.; Nadarajah, A.; Aburto-Medina, A.; Shahsavari, E.; Shukla, R.; Prawer, S.; Ball, A.S.; et al. Real-time detection and Identification of nematode eggs genus and species through optical imaging. Sci. Rep. 2020, 10, 7219.
- Eves-van den Akker, S.; Lilley, C.J.; Reid, A.; Pickup, J.; Anderson, E.; Cock, P.J.; Blaxter, M.; Urwin, P.E.; Jones, J.T.; Blok, V.C. A metagenetic approach to determine the diversity and distribution of cyst nematodes at the level of the country, the field and the individual. Mol. Ecol. 2015, 24, 5842–5851.
- Golden, T.; Hubbard, A.; Melov, S. Microarray analysis of variation in individual aging C. elegans: Approaches and challenges. Exp. Gerontol. 2006, 41, 1040–1045.
- Esbenshade, P.R.; Triantaphyllou, A.C. Isozyme phenotypes for the Identification of Meloidogyne species. J. Nematol. 1990, 22, 10–15.
- Bird, A.F. Serological studies on the plant-parasitic nematode, Meloidogyne javanica. Exp. Parasitol. 1964, 15, 350–360.
- Lee, S.H. Attempts to use immunodiffusion for species identification of Meloidogyne (Abstr.). Nematologica 1965, 11, 41.
- Misaghi, I.; McClure, M.A. Antigenic Relationship of Meloidogyne incognita, M. javanica, and M. arenaria. Phytopathology 1974, 64, 698–701.
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497.
- Goldstein, L.D.; Chen, Y.J.J.; Wu, J.; Chaudhuri, S.; Hsiao, Y.C.; Schneider, K.; Hoi, K.H.; Lin, Z.; Guerrero, S.; Jaiswal, B.S.; et al. Massively parallel single-cell B-cell receptor sequencing enables rapid discovery of diverse antigen-reactive antibodies. Commun. Biol. 2019, 2, 304.
- Ahmad, F.; Babalola, O.O.; Tak, H.I. Potential of MALDI-ToF mass spectrometry as a rapid detection technique in plant pathology: Identification of plant-associated microorganisms. Anal. Bioanal. Chem. 2012, 404, 1247–1255.
- Perera, M.R.; Vanstone, V.A.; Jones, M.G.K. A novel approach to identify plant-parasitic nematodes using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1454–1460.
- Biron, D.G.; Joly, C.; Marché, L.; Galéotti, N.; Calcagno, V.; Schmidt-Rhaesa, A.; Renault, L.; Thomas, F. First analysis of the proteome in two nematomorph species, Paragordius tricuspidatus (Chordodidae) and Spinochordodes tellinii (Spinochordodidae). Infect. Genet. Evol. 2005, 5, 167–175.





