Filamentous fungi are excellent organisms as cell factories for production of a variety of products. They are robust and naturally produce efficient enzymes for the decomposition and conversion of biological material. They also produce different compounds, many of which can have interesting commercial applications. Filamentous fungi are present almost everywhere in all kinds of habitats, where their heterogenic lifestyle requires access to organic carbon. Fungi uptake inorganic material and from this, they can synthesize the biomolecules they need, including all amino acids. The inorganic material can be divided into macronutrients such as oxygen, hydrogen, nitrogen, phosphorus, potassium, sulfur, and magnesium, and micronutrients, i.e., manganese, iron, zinc, copper, and molybdenum, which are essential for fungal growth
[1]. The organic carbon sources are derived from a large range of sources, ranging from single monomer sugars to complex polymers. Many fungi are saprophytes, where they play an important role in the environment as decomposers of dead organic material and as such are crucial for the conversion and mineralization of organic material
[2]. They have developed an efficient biomass degradation apparatus and secrete plant cell wall degrading enzymes to retrieve nutrients from complex material
[3][4][5][6]. Fungi have proven to be effective as industrial cell factories to produce a wide range of different products, due to their rapid growth, efficient utilization, and conversion of complex substrates into fermentable sugars. Among the most well-known ones are complex secondary metabolites such as antibiotics (e.g., penicillin), organic acids (e.g., citric acid) and various extracellular enzymes, including amylases and cellulases
[7][8][9].
2. Production of Native (Non-Recombinant) Proteins
2.1. Production of Enzymes for Plant Biomass Utilization
Fungi have adapted to very different environmental niches
[20]. Their adaptation is eased by a diversity of fungal enzymes that are secreted extracellularly, and synergistically used for deconstructing various insoluble plant and insect polymeric substrates into soluble sugar nutrients. The capability of fungi to use solid substrates at low water content results in high concentration of secreted enzymes and high biocatalytic effeciency. The fungi access the solid plant materials by hyphal extensions and secrete the enzymes from the hyphal tips
[21]. Fungi are of interest both as resources for the hunt for novel enzymes
[22][23], as well as for production of enzymes and enzyme cocktails
[5][24][25]. Among the enzymes are cellulases, amylases, pectinases, chitinases, proteases, and lipases that break down plant and insect biomass, e.g., cellulose, chitin, starch, pectin, proteins, and lipids
[16][26][27][28][29][30]. Due to their versatility, a wide variety of fungi can be isolated from very different environmental niches, such as soil, compost, decaying wood, decaying plant material, building materials and different foodstuffs
[20][22][23][29]. These niches include extreme temperatures and pH, although fungal growth is confined at temperatures beyond 65 °C
[31][32]. From different genome projects, it is obvious that filamentous fungi in general contain a great variety of plant biomass-degrading enzymes in their genomes
[8][33][34][35][36]. Furthermore, proteomic studies show that many of these enzymes are secreted when the fungi grow on lignocellulosic substrates
[25][37][38][39][40]. The secreted part of proteins is called the secretome but the secretome obviously changes according to the circumstances under which the fungi grow such as cultivation conditions and availability of nutrients. The secretome includes liberally released proteins and proteins, attached to the outer cell wall
[41]. Some fungi, especially within the genera
Trichoderma and
Aspergillus, which naturally secretes cellulases, amylases or other industrially relevant enzymes in large quantities, have been identified
[42][43][44][45].
The application of plant biomass for production of various bioproducts in biorefineries using the biochemical route, involves pretreatment of the biomass to open the recalcitrant material and make it accessible for subsequent enzymatic hydrolysis to produce fermentative sugars for further processing
[46][47]. The complete hydrolysis of lignocellulosic biomass requires an efficient cocktail of enzymes, which industrially most often are produced by filamentous fungi
[25][39]. Still, none of those fungi can naturally secrete efficient cocktails containing all the necessary enzymes with highest activities in an optimal ratio.
Trichoderma reesei is one of the most extensively used fungal species to produce cellulolytic enzymes in industry, due to its extraordinary high secretion capacity for enzymes, especially efficient cellulases
[16][34][48][49][50]. Still, it lacks sufficient β-glucosidase activity for efficient cellulose hydrolysis and there are numerous examples on supplementation of cellulases from
T. reesei with β-glucosidase preparations, especially from
Aspergillus niger or other
Aspergillus species
[4][24][51][52][53][54]. However, industrial production of cellobiohydrolases and β-glucosidases in separate organisms is expensive because of the requirement for double equipment. This issue can be solved by co-cultivation strategies if
T. reesei is cultivated together with
Aspergiilus species
[5][24][55] or by engineering filamentous fungi to produce multiple hydrolytic enzymes of desired top efficiencies in a single host, e.g., by introducing or up-regulating the demanded enzymes. The production of enzymes can be carried out on-site using cheap complex polymeric substrates e.g., containing agricultural sidestreams consisting of plant cell wall material for growth and enzyme production
[25][56]. Co-cultivation with various fungi for enzyme production, especially using solid state fermentation (SSF) with different solid agricultural sidestreams, where the fungi do not necessarily have direct contact but can thrive in micro-niches in the substrate, has been shown in several cases to enable production of efficient enzyme cocktails
[24][57], reviewed in
[54]. These cocktails are likely optimized for hydrolysis of the same substrate that was used to produce them, as their secretomes may be induced specifically by the composition of the polymers present in the substrates. Further investigation of molecular interactions between fungi in co-cultures is necessary to fully understand them and to further optimize their production of enzyme cocktails
[54].
2.2. Production of Mycoproteins for Food and Feed
Edible fungi have traditionally been part of the food system, especially mushrooms, where the fruiting body are used as food, or through food fermentation, including their widespread use in cheese production, e.g., blue, and white cheeses
[58]. Fungal fermented non-animal foods are an integrated part of the diet in many countries, especially in East and South Asia and certain African regions. The fermented foods are divided into high-protein meat alternatives from legumes or cereals, such as Tempeh and Oncom, and into salty amino acid sauce and paste, e.g., shoyu and miso
[58][59].
Mushrooms are the beyond ground fruiting bodies of macroscopic saprophytic fungi, most of which belong to the Basidiomycetes. Many of them are edible and have been used for food since ancient times, but only a smaller number are currently cultivated commercially
[60][61]. The global mushroom market has grown considerably in recent years, and among the most important cultivated mushrooms are
Agaricus bisporus (common mushroom),
Pleurotus ostreatus (oyster mushroom),
Lentinula edodes (shiitake),
Flammulina velutipes (enoki mushroom), and
Volvariella volvacea (paddy straw mushroom)
[60][61]. Mushrooms are known for their umami taste and for their nutritional properties; they are low in fat, high in protein, and high in dietary fibers. In addition, they contain a range of vitamins (including vitamin B), minerals, antioxidants, and nutraceuticals
[62][63][64]. Mushrooms are cultivated commercially on agricultural residues, enabling these waste materials to be converted into a valuable human food source
[60]. They are traditionally eaten fresh but are increasingly used as dried powder (flour) to fortify various food types with nutrients and especially proteins as they contain up to 20–25% protein
[61]. Such dried fungal products are called “mycoprotein”. As an example,
Pleurotus albidus mycoprotein flour was used to replace wheat flour to produce cookies. The mycoprotein flour significantly increased the nutritional value of the cookies due to the contents of protein, dietary fiber, and phenolic compounds. Furthermore, the mycoprotein increased the hardness and altered the color of the cookies
[65].
Besides these Basidiomycetes mushrooms, certain species of Ascomycetes and Zygomycetes microfungi have traditionally been used for food fermentation into meat-like products such as Tempeh (
Rhizopus oligosporus)
[66][67] and Oncom (
Neurospora spp.)
[68][69][70] in Asia. These microfungi have been found to contain higher protein in their mycelium than most Basidiomycetes mushrooms, and they are for this reason promising as alternative protein sources. For example,
Neurospora sitophila mycelium contains 39–45% protein, 28–30% carbohydrates, 10–12% crude fats, 5% minerals and vitamins, and 3% fibers
[68]. Traditionally, the base for production is SSF of particular soybeans but also other substrates such as chickpea, lupines, and various cereals are used
[66]. A growing interest in these Asian food types are seen both in the US and in Europe, where several novel companies have started production of “Tempeh” style food types using local products, such as lupines and peas, especially using
R. oligosporus (
Table 1).
Table 1. Examples of startup mycofood companies.
| Company |
Description |
| Beyond Coffee (DK) |
Beyond Coffee collects coffee grounds and other types of biomass sidestreams to grow oyster mushrooms (fruit bodies), which are sold to restaurants. They sell mycelium and rent out ‘minifarm’ to canteens for harvest in the canteen. http://www.beyondcoffee.eu/ (accessed on 8 February 2022) |
| Contempehrary (DK) |
Contempehrary produces and sales Nordic Tempeh (different types: fermented on oats, barley, rye, hemp, peas, or beans. Tempeh is made through SSF. https://contempehrary.com/ (accessed on 8 February 2022) |
| Enough Food (UK) |
Enough Food produces fungal mycelium products using SmF and uses the trading name Abunda. They are a B2B company and expect to launch products in 2022. https://www.enough-food.com/ (accessed on 8 February 2022) |
| InnomyLabs |
InnomyLabs works with the turn of mycelium into meat-analog products. They do not have products on the market. http://innomylabs.com/#!/-inicio/ (accessed on 8 February 2022) |
| Kernel MycoFood (USA) |
Kernel MycoFood makes fungal food ingredients made by SmF of Fusarium venenatum (like quorn) https://www.kernel.bio/ (accessed on 8 February 2022) |
| Leep Foods |
Leep Foods produces oyster mushrooms and blended products containing mushroom and meat. https://www.leepfoods.com/ (accessed on 8 February 2022) |
| Libre Foods (ES) |
LibreFoods works with mycelium-based food products. Products not yet on the market. https://www.librefoods.co/ (accessed on 8 February 2022) |
| Meati (USA) |
Meati produces whole cut mycelium-based products using SmF. They are in process with scaling their production. https://meati.com/ (accessed on 8 February 2022) |
| Mushlabs (DE) |
Mushlabs uses fungi to up-cycle nutrients in sidestreams from agro- and food industries. Products not yet on the market. https://www.mushlabs.com/ (accessed on 8 February 2022) |
| Myco Foods (UK) |
Myco Foods produces meat substitute products for the Food Industry https://www.mycofoods.co.uk/ (accessed on 8 February 2022) |
| MycoRena (S) |
Mycorena produces Fungi-based alternative protein for the food industry using SmF. Promyc® is a fungi-based natural ingredient to be used as meat replacement or dairy alternative. https://mycorena.com/ (accessed on 8 February 2022) |
| MycoTechnology (USA) |
MycoTechnology makes mycoprotein-rich food ingredients based on fungal fermentation. https://www.mycoiq.com/ (accessed on 8 February 2022) |
| MyForest Foods (USA) |
MyForestFoods is evolved from EcoVative, which produces various mycelium products. MyForestFoods have developed meat-free bacon. https://myforestfoods.com/home (accessed on 8 February 2022) |
| Mycovation (SGP) |
Mycovation claims to be the first Asian start up to produce mycelium based food products. They do not have products on the market. https://www.mycovation.asia/ (accessed on 8 February 2022) |
| Tempty Foods (DK) |
Tempty Foods is an early startup that produces Tempeh-like food products using SFF. They do not have products on the market yet. https://www.tempty-foods.com/ (accessed on 8 February 2022) |
| Quorn Foods (UK) * |
Quorn Foods has been on the market for a long time. They produce and sell quorn and quorn products based on mycelium made by fermentation of Fusarium venenatum worldwide. https://www.quorn.co.uk/ (accessed on 8 February 2022) |
The production of mycoprotein using solid-state fermentation has recently been expanded to other substrates than the already eatable substrates, e.g., using low value sidestreams from the food industry such as brewers spent grain
[69][71][72], pulp from sugar beets, potato starch production, coffee production or bran from flour production
[73]. The protein content in many of these plant-based sidestreams is low, and they often exhibit low quality, poor digestibility, and a low-quality profile of amino acids, with a low amount of some of the essential amino acids, especially lysine, methionine, cysteine, and tryptophan. Some of the edible Ascomycetes fungi can break down plant proteins and non-digestible fibers, thereby making the fermented combined fungus-plant products higher in protein content, improve the amino acid profile, and increase the digestibility. They can provide new food properties, in terms of texture, flavor, and increased solubility
[58][69]. In addition, fermentation can benefit the overall nutritional composition, as many of these fungi naturally synthesize vitamins such as B12, D6, and vitamin E, often lacking in other alternative protein products and in many of the plant-based sidestreams. Some of the plant-based sidestreams may contain anti-nutritional factors (ANFs), such as phytates and saponins, which may be reduced through the fermentation process, due to the action of phytases or other enzymatic activities. The ANFs can form insoluble complexes with valuable minerals such as Ca
2+, Mg
2+, Fe
2+ and Zn
2+, and thereby decreasing the bioavailability of the minerals.
For these purposes, there is an interest for identifying new and better-adapted strains, and for starter cultures for fermentation of such sidestreams. These strains should possess a GRAS (generally regarded as safe) or Qualified Presumed of Safety (QPS) status
[74][75], and if they do not, they will require an approval from EFSA for consumption in Europe. However, it is anticipated that there will be opportunities to expand the list of current strains with new strains, but the process of achieving GRAS or QPS status is a challenge.
Instead of the traditional use of microfungi for SSF of various food products (or solid sidestreams), there has been an increasingly interest to grow fungal mycelium in bioreactors, using submerged fermentation (SmF) in sugar-rich substrates
[10][13][76]. An advantage of SmF is that fungi can assimilate inorganic N-sources and can synthesize all amino acids, thereby producing protein from protein-free feedstocks supplemented with an N-source, in contrast to other alternative protein sources, e.g., insects. Several startups have entered this area of producing mycelium-based food, especially using SmF (
Table 1). The most well-known mycoprotein product on the market is called Quorn
TM (
Table 1), which has been produced since 1985
[76]. Prior to commercialization, there has been 20 years of research and development, including a major screening process involving over 3000 fungal species
[77].
Fusarium venenatum met all requirements of the screening, which included fast growth, filamentous morphology, lack of pigments, odors and toxins, and a protein content of over 45%. It was later approved for sale as a food protein source by the United Kingdom Ministry of Agriculture, Fisheries and Food
[78], and is now sold in 17 countries
[77]. Since the branched nature of fungal mycelium resembles muscle fibers, the mycoprotein can be used to achieve a meat-like texture in food products. Thus, the mycoprotein from
F. venenatum is used as an ingredient in various products such as alternative chicken patties, sausages, and burgers. On a dry basis,
F. venenatum can produce mycoprotein products with more than 60% protein
[10].
Mycoproteins from
F. venenatum and other mycelium fungi are promising sources of essential amino acids with an overall protein digestibility-corrected amino acid score of 0.996, indicating that they can be considered as high-quality protein
[76]. Recent research has investigated the health benefits of mycoprotein products and found that they have a higher weight-percentage protein content than other common plant or fungal sources of protein. Furthermore, the fibers found in the cell walls of mycoprotein is comprised of two-thirds beta-glucan and one-third chitin, creating a “fibrous chitin–glucan matrix”, which is largely insoluble in the small intestine
[76]. Review of 16 studies supports the role of mycoprotein in reducing overall cholesterol levels and short-term energy intake
[76]. Compared to other protein food sources, mycoprotein products also contain reasonable amounts of vitamin B9 (folate), vitamin B12, calcium, phosphorous, magnesium, and zinc.
Besides their nutritional benefits, mycoproteins together with other microbial “single cell proteins” produced through fermentation can be good alternatives to support future food production. This is especially important for climate-friendly production since they do not require large area of land and the production has in general a low water consumption, in particular in the case of SSF. Furthermore, the production can be made independently of seasonal and climatic variations throughout the year, and with a lower greenhouse gas emission compared to plant protein sources
[79].
3. Recombinant Production
3.1. Production of Recombinant Enzymes for Plant Biomass Utilization
Agriculture primarily produces plants that can be used as food or as feed for livestock. Only part of the plants is used directly for food or feed (for mainly monogastric animals), and traditionally leave a large amount of plant biomass. Biorefining using these plant sidestreams is gaining increasing interest, to produce fuels, biochemicals or as nutrition for microbes (food or feed production). The sidestreams contain lignocellulosic plant cell walls, which pose significant technical and economic challenges, and require substantial pre-use processing. In most cases, biomass conversion is carried out in several steps: pretreatment, enzymatic hydrolysis of the plant polymers to monomeric sugars, followed by fermentation of monomeric sugars to the desired product, which finally must be recovered
[5][80].
The enzymatic hydrolysis of plant biomass is performed using lignocellulosic enzymes, in particular cellulases. Efficient cellulolytic enzymes and other plant cell wall degrading enzymes are primarily produced by fungi, especially using
T. reesei and various
Aspergillus sp. as workhorses at an industrial level. These fungi and their enzyme systems have been central for many research programs, and the studies have greatly advanced the knowledge of production, secretion, and regulation of the relevant enzymes
[3][9][81]. Since cocktails composed of quite a few enzymes are needed for efficient hydrolysis of plant material, several studies have focused on selection of microorganisms capable of secreting a high and diversified number of enzymes
[22][23][27]. Studies have also focused on increasing the production efficiency of cellulolytic enzymes by optimizing the production and composition of the cellulolytic cocktail, including adding booster enzymes. Industrial strains of
T. reesei and
Aspergillus sp. have been developed into high performing cell factories for enzyme production, and the work has included trimming of the production hosts to produce the targeted (recombinant) products by optimizing and upregulating the enzymes
[9][17][42][43][44][82][83][84].
Some of the specific challenges to produce endogenous enzymes are related to the tight regulation of glucose by the gene expression in filamentous fungi. In the presence of glucose in the fermentation medium, the expression of the endogenous enzymes is carbon catabolite repressed, which limits the production. There are two ways to overcome this, either by using inducing media, e.g., based on complex carbohydrates, or preferably to genetically modify the production strain by manipulating the carbon catabolite repression system, which in
Aspergillus is encoded by CreA and in
Trichoderma by Cre1
[84][85]. The famous
T. reesei strain C-RUT30, derived from a large screening of mutants and selected for improved cellulase production, was later found to have deletions in the carbon catabolite regulator Cre1 gene
[84].
Zhang et al.
[85] review several strategies to increase the production of endogenous and recombinant lignocellulosic enzymes. These strategies include downregulation or deletion of genes involved in the PKA pathway, upregulation of the AMPK pathway, and overexpressing activators, which often have been found to be effective in increasing expression. At the same time, they point out that some of these changes may also cause deficiencies in strain growth and metabolism, and that a given strategy must be balanced between efficient protein production without impairing cell growth.
The industrial trimmed strains based on
T. reesei and different
Aspergillus sp. are also utilized as cell factories for production of a range of other relevant enzyme products, which are exploited by a broad range of industries, including food and feed, detergent, pulp and paper, and pharmaceutical
[9][13].
3.2. Production of Animal-Derived Food Proteins
Cellular food production (both non-GMO and GMO) has the potential to supplement or even substitute animal-based food production, due to decreasing costs and radically less climate impact compared to conventional egg, milk, and meat production
[15][86][87]. Recombinant tools make it easier to manipulate genetic material from e.g., cattle in microbial cells to produce molecules with precise properties, commonly known as “precision fermentation”. The food industry can therefore in future design, produce and purify animal-derived proteins on a commercial scale
[15]. Compared to plant proteins, the animal proteins generally have a higher nutritional value, and many of them, such as the whey proteins, are used as versatile ingredients in the food industry with many applications, due to their techno-functionalities as e.g., a gelling, foaming, or emulsifying agent. A major challenge in replacing animal products with animal proteins produced in microbes on a commercial scale is to ensure that the processes can be scaled up in a profitable way, as the food proteins have less commercial value compared to enzymes and pharmaceutical products in the fermentation industry. To keep production costs low, production could be carried out in biorefineries with lignocellulosic plant material, as shown by Wang et al.
[88], who produced recombinant bovine and human αS1-casein using a hydrolysate from wheat straw lignocellulose.
Several different microbial hosts can be used for precision fermentation, and filamentous fungi are among the choices. When producing animal proteins in fungi, the challenges are related to similar issues as for expression of other recombinant proteins such as addition of a host signal peptide, optimization of the nucleotide sequence for the gene of interest, and that the production hosts can create glycosylation patterns that are different from the native protein. Thus, the recombinant proteins may differ in the physiochemical and functional properties
[87].
The production of animal proteins in microbes, including filamentous fungi, is particular focused on milk and egg proteins. Among milk proteins, the whey protein b-lactoglobulin (BLG) is particularly interesting in this respect, as it is the main protein in whey. BLG has been shown to be an important component in many food products due to its versatile functional properties, as a gelling, foaming and emulsifying agent
[87][89]. Moreover, non-denatured BLG has been identified to be able to modulate human immune response and increase human cell proliferation
[90], which is important for human health. Other relevant milk proteins are the caseins, which are the most abundant protein components of milk (up to 80%) and are an important component of cheese. Additionally, caseins are used as food additives. Caseins are related phosphoproteins of different types, α-S1-, α-S2-, β-, and κ-casein, of which α-S1- and β-casein are the two most common caseins. Furthermore, β-casein comes in two genetic variants, A1 and A2, where A2 is preferable from a nutritional point of view, as the A1 variant has been linked to various chronic diseases
[91], which although has not been confirmed by EFSA
[92]. As BLG also is a key model protein in structural biology, it was recombinantly produced in yeast more than 20 years ago
[93].
The field of expressing dairy proteins is rapidly increasing globally, and some small startups have already begun to commercialize animal free milk proteins to produce novel food products, e.g., Perfect Day with products on the market. From “first movers” such as universities, the field is expanding into leading global food companies, including the Finnish dairy company, Valio. The main drivers still seem to be the small startups, which are increasingly attracting amounts of venture capital. In addition to Perfect Day, some of the leading startups are: Change Food, ReMilk, FORMO, Those Vegan Cowboys, and New Culture. The Good Food Institute estimates that 50 companies are currently working on launching fermentation produced animal proteins. Several commercial companies already produce dairy proteins using yeast as their production platform, but the products may exhibit non-optimal post-translational modifications (PTMs). Keppler et al.
[87] suggest using
E. coli as a production host, and to produce the BLG intracellularly to obtain recombinant proteins without undesirable modifications. However, this requires troublesome extraction procedures from the cells.
3.3. Production of Other Recombinant Proteins
3.3.1. Production of Hydrophobins
Hydrophobins (HFBs) are low molecular weight (primarily less than 20 kDA) secreted proteins about 100 amino acids and are unique to the fungal kingdom except Yeast
[94][95][96]. HFBs are found as multigene families with a low degree of sequence conservation, and they are characterized by the content of eight cysteine residues that form four disulfide bonds and contain 1α-helix and 2β-hairpins
[97]. The HFBs form spontaneously amphipathic monolayers at hydrophobic-hydrophilic interfaces and are therefore important for the fungi in their biological cycle and their interference with the surrounding environment. Due to their surface-activity including biosurfactant and emulsifying properties, and antifoaming activity, they are considered to have many biotechnological applications
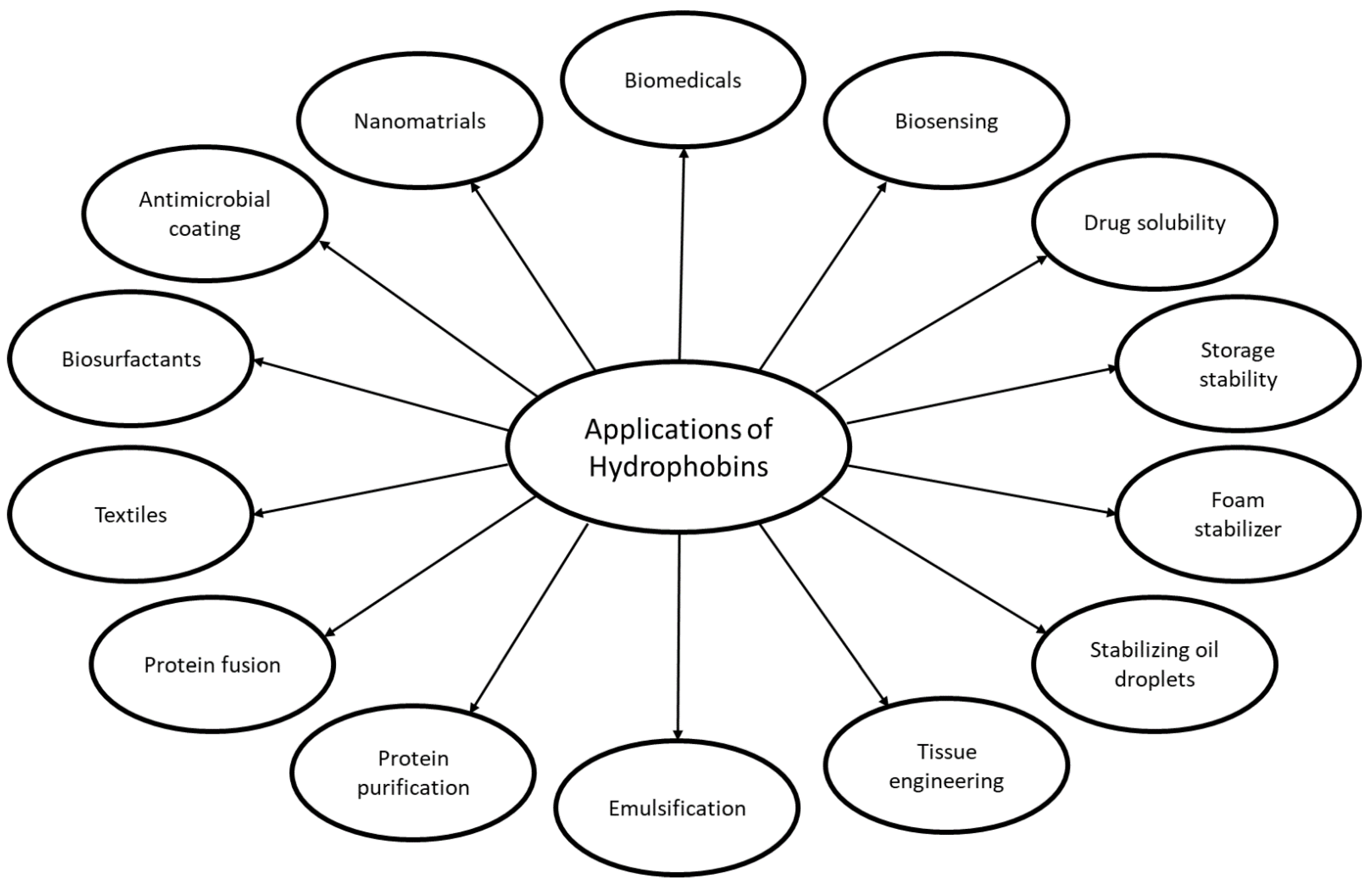
[96][97][98] (
Figure 1). The HFBs appear to be adapted to specific roles and are divided in 2 groups, Class I and Class II. Class I HFBs are less water soluble than Class II and have high variation among the proteins based on an inter-Cys-spacing construction, in contrast to HFBs from Class II, which are more conserved in sequence and inter-Cys-spacing
[95][96][97]. Some HFBs appear to be intermediate and do not clearly belonging to one of the 2 classes
[94]. Class I HFBs are involved in production of fibrillar structures (rodlets) that help fungal conidia bind to surfaces resulting in better resistance to the environment
[95]. Class II HFBs have a role in signaling the moisture conditions on the spore surface, which has been described for
Trichoderma [99]. If the water concentration decreases, the HFB concentration increases, which signals that the germination is not favorable, whereas the HFBs in wet conditions are low, which signals favorable germination conditions
[99].
Figure 1. An overview of the industrial and commercial applications of hydrophobins.
HFBs are produced in the cells, transported to the periplasm in lipid-enriched HFB vacuoles, and released to the exterior through the cell wall
[99]. In connection to conidiation, HFBs are highly expressed so that HFBs can coat the spores, thereby supporting the attachment to different surfaces and protecting the fungi from stress conditions
[99].
Another group of low molecular secreted proteins, the carbohydrate-binding proteins cerato-platanins (CPs), has been identified in various fungi
[98]. Like HFBs, they are surface-active and share some functional and structural characteristics with HFBs, such as hydrophobicity, and that they form aggregate layers at hydrophobic/hydrophilic interfaces. CPs from some of these fungi show biosurfactant and emulsifying applicability.
Due to the diversity and specificity of some of the HFBs, the variety of activities and lack of toxicity, there is a great interest in using HFBs for many different applications in the biotechnological, food, and pharmaceutical industry
[96][100] (
Figure 1). The proposed and tested applications include several drug-formulations including HFB fusion proteins, biosensor applications e.g., electrochemical biosensing, biomineralization, antimicrobial coatings, protein purification e.g., cellulases, immobilization of proteins and cells, fusion of anti-microbial peptides (AMPs) with HFBs for resistance to bacteria, foam stability in food for e.g., improved storage stability, emulsion of food products, gushing inducer in beer, stabilizing oil droplets, and many more are found
[97][100][101][102][103]. Therefore, this has created an interest in development of cell factories in various organisms, including yeasts and filamentous fungi, for recombinant production of the different HFBs or engineered versions of the proteins
[100]. Production yields are currently generally low and, therefore, optimization is needed to realize the industrial and commercial potential
[96][100].