Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariko Omatsu-Kanbe | -- | 2463 | 2022-07-08 07:10:01 | | | |
| 2 | Dean Liu | -7 word(s) | 2456 | 2022-07-11 04:03:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Omatsu-Kanbe, M.; Fukunaga, R.; Mi, X.; Matsuura, H. Atypically Shaped Cardiomyocytes. Encyclopedia. Available online: https://encyclopedia.pub/entry/24928 (accessed on 07 February 2026).
Omatsu-Kanbe M, Fukunaga R, Mi X, Matsuura H. Atypically Shaped Cardiomyocytes. Encyclopedia. Available at: https://encyclopedia.pub/entry/24928. Accessed February 07, 2026.
Omatsu-Kanbe, Mariko, Ryo Fukunaga, Xinya Mi, Hiroshi Matsuura. "Atypically Shaped Cardiomyocytes" Encyclopedia, https://encyclopedia.pub/entry/24928 (accessed February 07, 2026).
Omatsu-Kanbe, M., Fukunaga, R., Mi, X., & Matsuura, H. (2022, July 08). Atypically Shaped Cardiomyocytes. In Encyclopedia. https://encyclopedia.pub/entry/24928
Omatsu-Kanbe, Mariko, et al. "Atypically Shaped Cardiomyocytes." Encyclopedia. Web. 08 July, 2022.
Copy Citation
Atypically shaped cardiomyocytes (ACMs) are found in cultures of the cardiomyocyte-removed fraction obtained from cardiac ventricles from neonatal to aged mice. ACMs are thought to be a subpopulation of cardiomyocytes or immature cardiomyocytes, most closely resembling cardiomyocytes due to their spontaneous beating, well-organized sarcomere and the expression of cardiac-specific proteins, including some fetal cardiac gene proteins.
atypically shaped cardiomyocytes
ACMs
subpopulation of cardiomyocytes
spontaneous beating
ischemic resistance
cell fusion
1. Introduction
The mammalian heart is one of the organs with a low regeneration capacity after birth [1][2]. In the development stage, embryonic cardiomyocytes arise from the early cardiac progenitors or the proliferation of pre-existing cardiomyocytes [3]. Neonatal cardiomyocytes undergo additional rounds of DNA synthesis without cytokinesis, resulting in the binucleation or multinucleation shortly after birth [4], and have transient regeneration potential during this period [5]. In the adult heart, a number of studies have reported evidence to support the notion that the pre-existing cardiomyocytes are capable of re-entering the cell cycle in both the human and mouse heart [6][7][8][9][10], the rate and degree of cardiomyocyte renewal under both physiological and pathophysiological conditions are far too small; thus, dead cells are not substantially replaced by renewed cells [11][12]. In the mouse heart, the turnover of cells through the proliferation of resident cardiomyocytes is estimated to occur at a rate of approximately 1.3–4% per year, whereas in the human heart, the annual turnover rate of cardiomyocytes is observed to gradually decrease from 1% at 25 years of age to 0.45% at 75 years of age; thus, the rate of cardiomyocyte exchange in adulthood is <1% per year [6][13].
Following myocardial infarction, the heart loses approximately 25% of the cardiomyocytes within a few hours [14]; however, it is estimated that <0.1% of cardiomyocytes re-enter the cell cycle [15]. At present, the “proliferative activity” of the postnatal mammalian heart is thought to be limited to the process of multinucleation and polyploidization, which occurs in the early postnatal period and in the failing heart [12]. Under pathophysiological conditions, active cardiomyocyte proliferation to cardiac hypertrophy has been reported based on an autopsy of patients with LEOPARS syndrome [16]. One of the most effective tools for understanding the mechanism of binucleation, polyploidization and cell-cycle arrest of cardiomyocytes is now thought to be a model system using induced pluripotent stem cells (iPSCs) that reproduces the essential factors in the heart [17]. In addition, the upregulation of resident or bone marrow-derived progenitor cells has been reported to give rise to cardiomyocytes for repairing the heart [18][19][20][21][22][23][24][25].
As the regulation of cardiomyocyte regeneration has been one of the most important themes in clinical research, a number of studies have attempted to manipulate endogenous progenitor cells to induce differentiation into functional cardiomyocytes. The first adult resident cardiac stem cells were identified by the expression of stem cell receptor kinase (c-kit) but not blood lineage markers (Lin), c-kit+/Lin− cells, in 2003 [26]. Thereafter, the identification of cardiac stem or progenitor cells based on characteristics such as the expression of stem cell antigen-1 (Sca-1) [27][28] and LIM-homeobox transcription factor (islet 1) [29] and the ability to exclude Hoechst 33342 dye [30], has been reported. During this period, these cells were demonstrated to differentiate into cardiomyocytes in vitro in response to hormones or chemicals, such as 5′-azacytidine [27][28], oxytocin [28][30] and trichostatin A [30]. Adult cardiac stem cells isolated by c-kit+/Lin− selection have been shown to more systematically give rise to cardiomyocytes cultured in leukemia inhibitory factor (LIF)-deprived basic differentiation medium supplemented with several chemicals in sequence, including oxytocin and activin A [31]. Negative views have also been reported, with one review reporting that no reliable data have shown the existence of cardiomyocyte-differentiable stem cells in the adult heart that might be used in practical therapeutics to repair the injured myocardium [32]. Although there are still some limitations that hinder cell therapy using cardiac stem or progenitor cells, the methodology is constantly being innovated, with methods such as proteomic analyses of cardiac progenitor cells, including cells isolated based on surface-marker selection, differentiated human embryonic stem cells (ESCs) and iPSCs [33], and the 3D structure applications composed of cardiac and endothelial progenitor cells [34]. These methods have been accelerated with the aim of translation into cell therapy [1][2].
2. Atypically Shaped Cardiomyocytes (ACMs)
The isolation of cardiomyocytes is performed via coronary perfusion of the whole heart with enzymes using either the retrograde [35] or antegrade [36][37] perfusion method, and the cardiomyocyte-depleted fraction including interstitial heart cells of various cell lineages can then be cultured in semi-solid culture medium. After tightly adhering to the bottom of the appropriate dish, the cells grow over several days and start spontaneously beating. Given its peculiar morphology (Figure 1), researchers named these cells ACMs [38]. ACMs are also observed in the cell culture with a commonly used liquid culture medium [38], but the number of ACMs, especially beating cells, is extremely low. These beating cells have occasionally been observed as distorted or unusual cells present in culture dishes of isolated cardiomyocytes, where they have not received particular attention [36][39]. Observations indicate that 3D culture is a better condition for the development of these cells in comparison to two-dimensional (2D) culture with a liquid culture medium, which is considered one reason why these cells have not received much attention thus far.
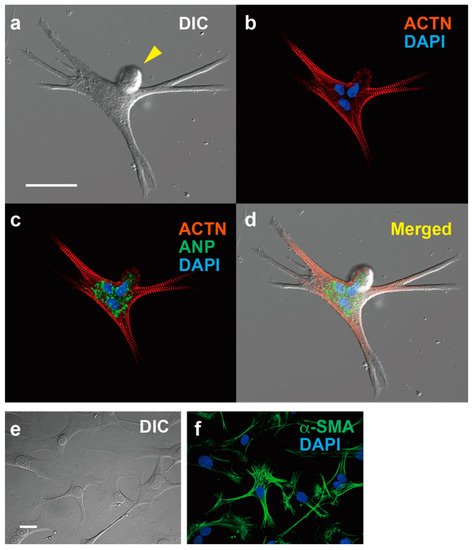
Figure 1. Immunofluorescent microscopy of ACM and cardiac fibroblasts. expressing ACTN and ANP, and cardiac fibroblasts expressing α-SMA. (a–d) ACM, bar, 50 µm. (a) Differential interference contrast image (DIC). (b) Immunostaining for α-actinin (ACTN, red) and DAPI staining for nuclei (blue). (c) Merged image for ACTN, DAPI and atrial natriuretic peptide (ANP, green). (d) Merged images for (a–c). (e,f) Cardiac fibroblasts, bar, 50 µm. (e) DIC. (f) Immunostaining for α-smooth muscle actin (α-SMA) and DAPI staining.
ACMs can be detected in cultures prepared from neonatal to aged mice without showing appreciable proliferation during long-term culturing [40]. The data thus indicate that ACMs survive in the heart for a life-long period but lack a self-renewing or clonogenic ability. A few days after plating, ACMs already show the automaticity and express characteristic proteins for ventricular and atrial myocytes, SA nodal pacemaker cells and fetal cardiac cells, indicating that the cells undergo the differentiation process to become cardiomyocytes as opposed to having multipotency. Therefore—at least based on the present data—it is unlikely that these would be classified as progenitor cells; rather, they would be classified as a subpopulation of cardiomyocytes or immature cardiomyocytes.
3. Characteristics of ACMs
3.1. Peculiar Morphology
One of the most obvious characteristics of ACMs, in addition to their spontaneous contracting activity, is that the cell shapes are markedly different from those of normal rod-shaped cardiac ventricular myocytes (Figure 1). ACMs possess a spherical shape at the start of culture, but after a few days of culture, they show peculiar shapes with many branches and/or protrusions, and most possess multiple nuclei with bulge(s) on the cell surface (Figure 1). However, while the cell shapes are complicated, the sarcomere structures are well organized and regularly arranged up to the branched tips [36][38][39]. The protrusions of ACMs not only come out horizontally, touching the bottom of the culture dish, but also grow three-dimensionally; thus, these cells are thicker than the proliferating fibroblast-like cells observed in the same culture dish, indicating that the cytoplasm is enriched in the contractile proteins. As the tight adherence of the plasma membrane to the culture dish may be a limitation to forming cells, the cell shapes in the heart tissues are not clear.
3.2. Automaticity
In most beating ACMs, the rhythmic action potentials are recorded that are reversibly suppressed by acetylcholine [38], while isoproterenol also shortens the peak intervals of the spontaneous Ca2+ transient in ACMs [41]. These findings are similar to the cell responses detected in sino-atrial (SA) node pacemaker cells [42][43], showing that the receptors and signal induction molecules for autonomic nervous systems function properly in ACMs. Unlike SA node pacemaker cells, ACMs often exhibit abnormal electrical automaticity caused by the repeated events of marked hyperpolarization to some extent and the subsequent depolarization. These arrhythmic events occur naturally, and the frequency varies from cell to cell.
It would be interesting to learn whether or not ACMs residing in the heart show automaticity under both physiological and pathophysiological conditions. The heart possesses a highly specialized system for generating rhythmic impulses to cause rhythmic contraction of the cardiomyocytes and for conducting these impulses rapidly throughout the heart. The electrical coupling within cardiomyocytes in ventricles is facilitated by gap junctions mainly composed of connexin 43 (Cx43) localized at the intercalated discs, whereas SA nodal cells do not express this type of gap junction proteins [44][45]. The expression of Cx43 in ACMs is low and particularly detected in the peri-nuclear area at the beginning of the culture before gradually increasing and spreading towards the cell periphery from five to eight days of culture along with the morphological maturation, confirmed by the expression of contractile protein [46]. Similar observations have been reported in which changes in the distribution of Cx43 in cardiomyocytes are observed in both cultured cells [47] and also in the myocardium of individuals with compensated and decompensated cardiac hypertrophy [48]. In addition, ACMs show an average maximal diastolic potential of around −65 mV [38], while the resting membrane potential in the isolated ventricular myocytes obtained from adult mice using the preparation method is approximately −74 mV on average [49], suggesting that the ACMs are not likely to electrically stimulate neighboring cardiomyocytes. These data suggest that the small native ACMs resident in the interstitial spaces have no—or very small—effects on the ventricular myocytes, at least in a healthy heart.
3.3. Protein Expression
While ACMs are small cells, with a diameter of approximately 10 µm immediately after isolation from the adult cardiac ventricular tissues, the large amounts of contractile proteins, such as α-actinin (ACTN) (Figure 1) and cardiac troponin T (cTnT), are synthesized within several days to help them increase their length more than 10-fold. Interestingly, ACMs, isolated from cardiac ventricular tissues, typically express hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4), T-type voltage-gated Ca2+ channel (CaV3.2), and atrial natriuretic peptide (ANP). It should be noted that these proteins are not functionally expressed in normal adult ventricles and are referred to as fetal cardiac gene proteins.
Adult cardiac stem cells, which were originally found in the c-kit+/Lin− cardiac cells subpopulation, possessing the characteristics of self-renewal, clonogenicity and multipotency, can regenerate functional myocardium in vivo [26]. Eliminating cells expressing endothelial and hematopoietic markers, such as CD31 and CD45, has been imperative for the isolation of cardiac stem cells from the heart [31][50]. ACMs do not show appreciable proliferation during long-term culturing and simultaneously express characteristic proteins of ventricular and atrial myocytes, SA nodal pacemaker cells and fetal cardiac cells, suggesting that these cells have different properties from cardiac stem cells or stem cells of other types. As expected, typical stem cell surface markers, Sca-1, c-kit, hematopoietic stem cell marker (CD34), platelet-endothelial adhesion molecule-1 (CD31) and vascular endothelial cell growth factor receptor 2 (Flk-1), are absent in ACMs. The evidence suggests that ACMs should not be classified as stem or progenitor cells that can undergo the differentiation process.
3.4. Ischemic Tolerance
Since ACMs are cultured in a methylcellulose-based semi-solid medium, it is more difficult to perform medium exchange than with a liquid medium, so a small droplet of the liquid medium is added from time to time during long-term culture. However, the proliferation of fibroblasts renders the culture condition an acidic one of chronic malnutrition. Surprisingly, ACMs in a medium that has turned a yellowish-orange color continue contracting rhythmically despite the harsh culture environment for over one month. Furthermore, another important feature of ACMs during long-term culture is that they fuse with each other while maintaining spontaneous contraction.
Cardiomyocytes are highly sensitive to hypoxia; for example, in the human heart, ventricular myocytes become irreversibly damaged approximately 30 min after blood flow stops [51][52]. Following the reestablishment of the blood flow, the reperfusion of the coronary artery paradoxically causes further—occasionally lethal—damage to the heart and as well as a wide range of organs [53]. ACMs can survive these lethal ischemic conditions by blocking airflow by covering the cell suspension with oil [54], with half of the cells subsequently able to develop into beating cells, while approximately 85% of ventricular myocytes die within 90 min [41]. ACMs have thus been shown to possess ischemic resistance in addition to resistance to malnutrition and acidic conditions, suggesting that these cells may survive after cardiomyocytes die in injured hearts. The finding that ACMs (Prp+/cTnT+ cells) survived in the peripheral area of infarction in pathological heart tissue specimens obtained from the patients who had a myocardial infarction [46] supports this view.
3.5. Constitutively Active Autophagy
Autophagy is an evolutionally conserved process for the degradation of long-lived and/or damaged proteins and organelles occurring in cells throughout the body [55][56][57] and during the neonatal period in particular, thus providing a necessary source of energy in various tissues [58]. Under physiological conditions, the autophagy activity in the heart remains low, aiding in the maintenance of the cell components, and is rapidly activated under pathophysiological conditions to support cardio-protection [59][60][61][62][63][64]. However, during the only early neonatal starvation period, autophagy in the heart is known to be activated [58].
In contrast, autophagy is constitutively activated in ACMs, not only to support cellular functions, but it also plays an essential role in the development of beating cells in the culture [41]. Constitutively active autophagy is thought to enable ACMs to rapidly synthesize proteins to grow into large beating cells and to continue beating in harsh environments by providing a source of energy.
3.6. Multinucleation and Cell Fusion
The most obvious characteristic of ACMs is that approximately 76% of these cells have multiple nuclei (Figure 1), sometimes more than four [65]. In mice, the majority of cardiomyocytes undergo additional DNA synthesis without cytokinesis within approximately 14 days after birth, resulting in multinucleation [3][12]. However, researchers have not observed such nuclear fission in ACMs during culturing; thus, one possible explanation for the multiple nuclei of ACMs is that cells fuse with each other, whether it occurs in vivo or after culturing.
“Native ACMs” that are resident in the heart tissues have often been observed as clusters [65], potentially resulting in the formation of fused cells with plural nuclei through the cell preparation procedure. Among the various interstitial cells that exist in the culture dish, ACMs are only able to fuse with the same type of cell [65]. When other types of cells are attached, ACMs pull the cell membrane along while beating, but they do not fuse with any cells other than ACMs [41]. These phenomena indicate that ACMs can only fuse with cells that have a cell membrane with the same membrane components, leading to the hypothesis that cardiomyocytes are indeed candidates for fusion with ACMs. Unfortunately, the coculture of ACMs and ventricular myocytes has not succeeded because isolated ventricular myocytes cannot survive in culture while ACMs settle at the bottom of the culture dish and grow. Some ACMs are observed to closely contact with ventricular myocytes but not attach, and then ventricular myocytes shrink and die over time. Therefore, the behavior of native ACMs should be explored in the future.
References
- Alonaizan, R.; Carr, C. Cardiac regeneration following myocardial infarction: The need for regeneration and a review of cardiac stromal cell populations used for transplantation. Biochem. Soc. Trans. 2022, 50, 269–281.
- Mehanna, R.A.; Essawy, M.M.; Barkat, M.A.; Awaad, A.K.; Thabet, E.H.; Hamed, H.A.; Elkafrawy, H.; Khalil, N.A.; Sallam, A.; Kholief, M.A.; et al. Cardiac stem cells: Current knowledge and future prospects. World J. Stem Cells 2022, 14, 1–40.
- Zhao, M.T.; Ye, S.; Su, J.; Garg, V. Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration. Front. Cell Dev. Biol. 2020, 8, 594226.
- Bishop, S.P.; Zhou, Y.; Nakada, Y.; Zhang, J. Changes in Cardiomyocyte Cell Cycle and Hypertrophic Growth During Fetal to Adult in Mammals. J. Am. Heart Asoc. 2021, 10, e017839.
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 25, 1078–1080.
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102.
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451.
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436.
- Ali, S.R.; Hippenmeyer, S.; Saadat, L.V.; Luo, L.; Weissman, I.L.; Ardehali, R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 8850–8855.
- Kimura, W.; Xiao, F.; Canseco, D.C.; Muralidhar, S.; Thet, S.; Zhang, H.M.; Abderrahman, Y.; Chen, R.; Garcia, J.A.; Shelton, J.M.; et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015, 523, 226–230.
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335.
- Ponnusamy, M.; Li, P.F.; Wang, K. Understanding cardiomyocyte proliferation: An insight into cell cycle activity. Cell. Mol. Life Sci. 2017, 74, 1019–1034.
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575.
- Murry, C.E.; Reinecke, H.; Pabon, L.M. Regeneration gaps: Observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006, 47, 1777–1785.
- Beltrami, A.P.; Urbanek, K.; Kajstura, J.; Yan, S.M.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 2001, 344, 1750–1757.
- Nakagama, Y.; Inuzuka, R.; Ichimura, K.; Hinata, M.; Takehara, H.; Takeda, N.; Kakiuchi, S.; Shiraga, K.; Asakai, H.; Shindo, T.; et al. Accelerated Cardiomyocyte Proliferation in the Heart of a Neonate with LEOPARD Syndrome-Associated Fatal Cardiomyopathy. Circ. Heart Fail. 2018, 11, e004660.
- Milliron, H.Y.; Weiland, M.J.; Kort, E.J.; Jovinge, S. Isolation of Cardiomyocytes Undergoing Mitosis With Complete Cytokinesis. Circ. Res. 2019, 125, 1070–1086.
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 103, 697–705.
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705.
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98.
- Muller, P.; Kazakov, A.; Semenov, A.; Bohm, M.; Laufs, U. Pressure-induced cardiac overload induces upregulation of endothelial and myocardial progenitor cells. Cardiovasc. Res. 2008, 77, 151–159.
- Kubo, H.; Jaleel, N.; Kumarapeli, A.; Berretta, R.M.; Bratinov, G.; Shan, X.; Wang, H.; Houser, S.R.; Margulies, K.B. Increased cardiac myocyte progenitors in failing human hearts. Circulation 2008, 118, 649–657.
- Lutz, M.; Rosenberg, M.; Kiessling, F.; Eckstein, V.; Heger, T.; Krebs, J.; Ho, A.D.; Katus, H.A.; Frey, N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit+ bone marrow-derived stem cells. Cardiovasc. Res. 2008, 77, 143–150.
- Waring, C.D.; Vicinanza, C.; Papalamprou, A.; Smith, A.J.; Purushothaman, S.; Goldspink, D.F.; Nadal-Ginard, B.; Torella, D.; Ellison, G.M. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur. Heart J. 2014, 35, 2722–2731.
- Marketou, M.E.; Parthenakis, F.; Vardas, P.E. Pathological Left Ventricular Hypertrophy and Stem Cells: Current Evidence and New Perspectives. Stem Cells Int. 2016, 2016, 5720758.
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776.
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318.
- Matsuura, K.; Nagai, T.; Nishigaki, N.; Oyama, T.; Nishi, J.; Wada, H.; Sano, M.; Toko, H.; Akazawa, H.; Sato, T.; et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004, 279, 11384–11391.
- Laugwitz, K.L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.Z.; Cai, C.L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653.
- Oyama, T.; Nagai, T.; Wada, H.; Naito, A.T.; Matsuura, K.; Iwanaga, K.; Takahashi, T.; Goto, M.; Mikami, Y.; Yasuda, N.; et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J. Cell Biol. 2007, 176, 329–341.
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116.
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020, 142, 275–291.
- Sebastião, M.J.; Marcos-Silva, L.; Gomes-Alves, P.; Alves, P.M. Proteomic and Glyco(proteo)mic tools in the profiling of cardiac progenitors and pluripotent stem cell derived cardiomyocytes: Accelerating translation into therapy. Biotechnol. Adv. 2021, 49, 107755.
- Monsanto, M.M.; Wang, B.J.; Ehrenberg, Z.R.; Echeagaray, O.; White, K.S.; Alvarez, R., Jr.; Fisher, K.; Sengphanith, S.; Muliono, A.; Gude, N.A.; et al. Enhancing myocardial repair with CardioClusters. Nat. Commun. 2020, 11, 3955.
- Shioya, T. A simple technique for isolating healthy heart cells from mouse models. J. Physiol. Sci. 2007, 57, 327–335.
- Ackers-Johnson, M.; Li, P.Y.; Holmes, A.P.; O’Brien, S.M.; Pavlovic, D.; Foo, R.S. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ. Res. 2016, 119, 909–920.
- Omatsu-Kanbe, M.; Yoshioka, K.; Fukunaga, R.; Sagawa, H.; Matsuura, H. A simple antegrade perfusion method for isolating viable single cardiomyocytes from neonatal to aged mice. Physiol. Rep. 2018, 6, e13688.
- Omatsu-Kanbe, M.; Matsuura, H. A novel type of self-beating cardiomyocytes in adult mouse ventricles. Biochem. Biophys. Res. Commun. 2009, 381, 361–366.
- Haftbaradaran Esfahani, P.; ElBeck, Z.; Sagasser, S.; Li, X.; Hossain, M.B.; Talukdar, H.A.; Sandberg, R.; Knoll, R. Cell shape determines gene expression: Cardiomyocyte morphotypic transcriptomes. Basic Res. Cardiol. 2019, 115, 7.
- Omatsu-Kanbe, M.; Yamamoto, T.; Mori, Y.; Matsuura, H. Self-beating atypically shaped cardiomyocytes survive a long-term postnatal development while preserving the expression of fetal cardiac genes in mice. J. Histochem. Cytochem. 2010, 58, 543–551.
- Omatsu-Kanbe, M.; Matsuura, H. Ischemic survival and constitutively active autophagy in self-beating atypically-shaped cardiomyocytes (ACMs): Characterization of a new subpopulation of heart cells. J. Physiol. Sci. 2013, 63, 17–29.
- Honjo, H.; Boyett, M.R.; Kodama, I.; Toyama, J. Correlation between electrical activity and the size of rabbit sino-atrial nodal cells. J. Physiol. 1996, 496, 795–808.
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227.
- Davis, L.M.; Kanter, H.L.; Beyer, E.C.; Saffitz, J.E. Distinct gap junction protein phenotypes in cardiac tissues with disparate conduction properties. J. Am. Coll. Cardiol. 1994, 24, 1124–1132.
- Verheijck, E.E.; van Kempen, M.J.; Veereschild, M.; Lurvink, J.; Jongsma, H.J.; Bouman, L.N. Electrophysiological featuRes. of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc. Res. 2001, 52, 40–50.
- Omatsu-Kanbe, M.; Nozuchi, N.; Nishino, Y.; Mukaisho, K.I.; Sugihara, H.; Matsuura, H. Identification of cardiac progenitors that survive in the ischemic human heart after ventricular myocyte death. Sci. Rep. 2017, 7, 41318.
- Formigli, L.; Francini, F.; Nistri, S.; Margheri, M.; Luciani, G.; Naro, F.; Silvertown, J.D.; Orlandini, S.Z.; Meacci, E.; Bani, D. Skeletal myoblasts overexpressing relaxin improve differentiation and communication of primary murine cardiomyocyte cell cultures. J. Mol. Cell. Cardiol. 2009, 47, 335–345.
- Kostin, S.; Dammer, S.; Hein, S.; Klovekorn, W.P.; Bauer, E.P.; Schaper, J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 2004, 62, 426–436.
- Hoshino, S.; Omatsu-Kanbe, M.; Nakagawa, M.; Matsuura, H. Postnatal developmental decline in IK1 in mouse ventricular myocytes isolated by the Langendorff perfusion method: Comparison with the chunk method. Pflugers Arch. 2012, 463, 649–668.
- Cianflone, E.; Cappetta, D.; Mancuso, T.; Sabatino, J.; Marino, F.; Scalise, M.; Albanese, M.; Salatino, A.; Parrotta, E.I.; Cuda, G.; et al. Statins Stimulate New Myocyte Formation After Myocardial Infarction by Activating Growth and Differentiation of the Endogenous Cardiac Stem Cells. Int. J. Mol. Sci. 2020, 21, 7927.
- Sheppard, M.N. The coronary arteries—Atherosclerosis and ischaemic heart disease. In Practical Cardiovascular Pathology, 2nd ed.; Makepeace, C., Ed.; Hodder Arnold: London, UK, 2011; pp. 24–66.
- Schoen, F.J. The Heart. In Robins and Cotoran Pathologic Basis of Disease, 9th ed.; Kumar, V., Abbas, A.K., Aster, J.C., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 523–578.
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100.
- Diaz, R.J.; Losito, V.A.; Mao, G.D.; Ford, M.K.; Backx, P.H.; Wilson, G.J. Chloride channel inhibition blocks the protection of ischemic preconditioning and hypo-osmotic stress in rabbit ventricular myocardium. Circ. Res. 1999, 84, 763–775.
- Yamaguchi, O. Autophagy in the Heart. Circ. J. 2019, 83, 697–704.
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721.
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477.
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036.
- Decker, R.S.; Wildenthal, K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am. J. Pathol. 1980, 98, 425–444.
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 2006, 281, 29776–29787.
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and BeClin.1 in mediating autophagy. Circ. Res. 2007, 100, 914–922.
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007, 13, 619–624.
- Kuma, A.; Komatsu, M.; Mizushima, N. Autophagy-monitoring and autophagy-deficient mice. Autophagy 2017, 13, 1619–1628.
- Ikeda, S.; Zablocki, D.; Sadoshima, J. The role of autophagy in death of cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 165, 1–8.
- Omatsu-Kanbe, M.; Nishino, Y.; Nozuchi, N.; Sugihara, H.; Matsuura, H. Prion protein- and cardiac troponin T-marked interstitial cells from the adult myocardium spontaneously develop into beating cardiomyocytes. Sci. Rep. 2014, 4, 7301.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
11 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




