Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Avner Ronen | -- | 3411 | 2022-07-04 07:11:20 | | | |
| 2 | Camila Xu | -37 word(s) | 3374 | 2022-07-08 03:15:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Das, S.; Ronen, A. Removal of Per-and Polyfluoroalkyl Substances by Novel Membranes. Encyclopedia. Available online: https://encyclopedia.pub/entry/24895 (accessed on 07 February 2026).
Das S, Ronen A. Removal of Per-and Polyfluoroalkyl Substances by Novel Membranes. Encyclopedia. Available at: https://encyclopedia.pub/entry/24895. Accessed February 07, 2026.
Das, Suman, Avner Ronen. "Removal of Per-and Polyfluoroalkyl Substances by Novel Membranes" Encyclopedia, https://encyclopedia.pub/entry/24895 (accessed February 07, 2026).
Das, S., & Ronen, A. (2022, July 07). Removal of Per-and Polyfluoroalkyl Substances by Novel Membranes. In Encyclopedia. https://encyclopedia.pub/entry/24895
Das, Suman and Avner Ronen. "Removal of Per-and Polyfluoroalkyl Substances by Novel Membranes." Encyclopedia. Web. 07 July, 2022.
Copy Citation
Per- and Polyfluoroalkyl Substances (PFAS) are anthropogenic chemicals consisting of thousands of individual species. PFAS consists of a fully or partly fluorinated carbon–fluorine bond, which is hard to break and requires a high amount of energy (536 kJ/mole). Resulting from their unique hydrophobic/oleophobic nature and their chemical and mechanical stability, they are highly resistant to thermal, chemical, and biological degradation. To date, membrane technology is one of the effective process, which can remove PFAS from wastewater. Moreover, there are very few novel membrane approaches have been reported effective in removing and destroying PFAS.
PFAS
Nanofiltration
Reverse Osmosis
Novel Membranes
Surface Modification
Wastewater
PFAS Rejection and Removal
Membranes
Wastewater treatment
1. Background on Per- and Polyfluoroalkyl Substances (PFAS)
Per- and Polyfluoroalkyl Substances (PFAS) are anthropogenic chemicals consisting of thousands of individual species. Resulting from their hydrophobic and oleophobic nature and chemical and mechanical stability, PFAS have been used extensively worldwide since the 1940s as oil- and water-repellent products, mainly in non-stick household items, paints, food packaging, cosmetics, lubricants, electronics, and aviation film-forming foam (AFFF) for firefighting [1]. PFAS consist of a carbon chain with carbon–fluorine (C–F) bonds which require a high amount of energy to break (536 kJ/mole) [2], resulting in stable compounds that are difficult to degrade naturally; therefore, they remain present in the environment for long durations [3].
Exposure to PFAS may result in health issues such as hormonal imbalances, liver disfunction, a compromised immune system, cancer, fertility disorders, negative effects on fetal growth, and learning ability in children [4]. Exposure routes are by inhaling, ingesting, and direct skin contact [5]. Furthermore, ultra-short chain PFAS (C ≤ 2, e.g., C2F6, CHF3, CF4, etc.) are volatile as well as highly water-soluble, and can easily enter the human body when breathing or consuming food or drinking water [6][7]. The adverse health effects of PFAS are not only limited to humans; they could be equally harmful to animals and livestock [4].
One of the main exposure routes to PFAS is by wastewater effluents [1] from industries manufacturing them or from municipal wastewater impacted by PFAS-related products. As PFAS are not easily removed by conventional biological wastewater treatment processes such as activated sludge [8], effluents were shown to contain varying PFAS species which could contaminate the aquatic environment. In the US, wastewater effluents were shown to contain ƩPFAS29: 20–4773 ng/L (from 29 PFAS species) in North Carolina [9] and ƩPFAS17: 442–2234 ng/L in Nevada [10]. Similar data was obtained in Europe, where ƩPFAS8: 1.2–290 ng/L was obtained in the Netherlands [11][12], ƩPFAS8: 0.3–90.4 ng/L was found in Germany [12], and ƩPFAS8: 1.7–200 ng/L was found in Italy [12].
As the awareness of the presence and environmental and health impacts of PFAS has been growing, several countries have published guidelines and regulations addressing PFAS concentrations in drinking water [13]. The US Environmental Protection Agency has set the PFOA and PFOS (individually or combined) limit in drinking water to 70 ng/L [14], whereas for the UK, Germany, Italy, Netherlands, and Sweden, it is 10, 300, 30–500, 200–390, and 90 ng/L, respectively [15].
Resulting from their stability, PFAS were shown to have limited biodegradation in environmental conditions and low environmental concentrations [16]. Biodegradation was shown only in specific cases of co-metabolism [17][18]. Therefore, the destruction or removal of PFAS is mainly based on high-energy incineration [19] or advanced oxidation processes, e.g., electrochemical oxidation, microwave treatment, photocatalytic degradation [20][21], pyrolysis, plasma-based treatment [22], and sonochemical reactions [23] (Figure 1). As PFAS degradation techniques are energy extensive, they are relatively expensive, especially considering the large volume and high flow rate of wastewater and groundwater requiring treatment. Among the techniques mentioned, membrane-based treatment can concentrate low concentrations of PFAS present in the wastewater/groundwater and is therefore one of the most effective treatment techniques in terms of cost and efficiency [24][25][26].
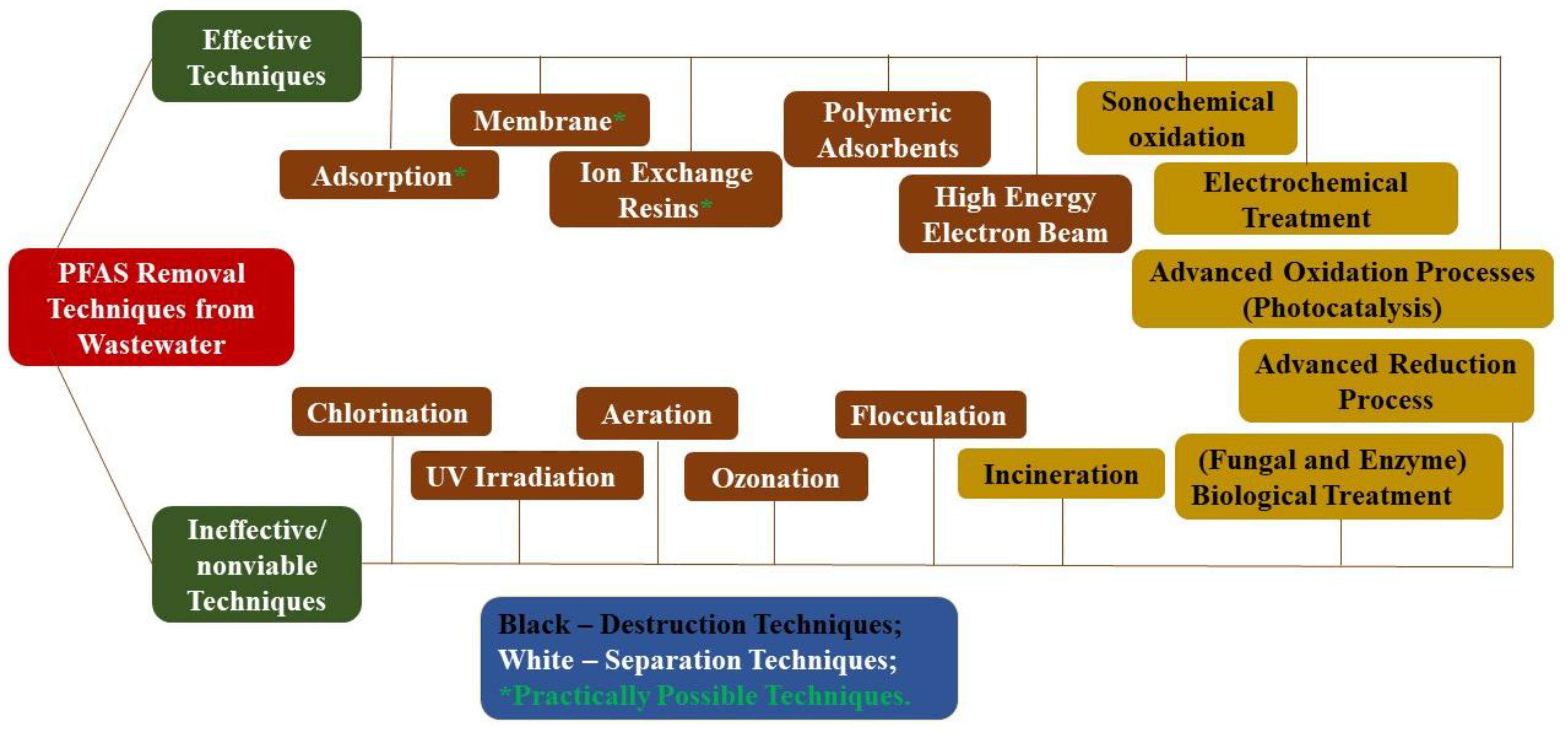 Figure 1. Techniques for removing Per- and Polyfluoroalkyl Substances (PFAS) from wastewater.
Figure 1. Techniques for removing Per- and Polyfluoroalkyl Substances (PFAS) from wastewater.In recent years, a growing number of studies have addressed the environmental occurrence, fate, and transport of PFAS in potable water and wastewater treatment plants, as well as varying treatment approaches [2][27][28][29][30][31]. Therefore, it is necessary to systematically review and critically analyze the state of knowledge and determine research gaps for suitable and economically viable technology leading to PFAS removal from water.
2. Factors Controlling PFAS Separation by Membranes
PFAS rejection by membrane technology can be impacted through several parameters. To enhance or optimize the process performance, it is necessary to understand the basic parameters that control and affect membrane treatment efficiency. Here, researchers present some of the crucial parameters.
Effect of organic matter: Organic matter present in the feed solution can influence PFAS treatment by coupling or reacting with the targeted PFAS compound, changing the membrane surface charge and leading to membrane fouling. Most of the reported work investigated the effect of fulvic acid and/or humic acid as an organic matter in simulated wastewater containing PFAS. However, the industrial wastewater matrix has a wide range of organic (proteins, amino acids, humic acids, fulvic acid, carboxylic acid, etc.) or inorganic (heavy metals, sulfur, phosphorous, etc.) compounds along with varying PFAS compounds. Organic matter was shown to bind to the membrane’s surface and lead to fouling. In consequence, the permeate flux decreases, and the rejection rate may increase. Moreover, fouling caused by organic matter deposition onto a composite membrane can impact the membrane’s surface charge, turning it more hydrophilic, which may not favor the removal of large chain PFAS.
Effect of pH: Changes in the solution’s pH may impact the membrane’s surface charge according to the isoelectric point of the compounds and the relevant groups at the membrane’s surface. Furthermore, in some cases, the membrane’s pore size, flux, and rejection rate can be manipulated by changing the pH [32][33]. Depending on the functional groups at the membrane’s surface and their pKa values, the membrane’s surface may be positively charged, which attracts anionic PFAS (negatively charged), resulting in a decrease in rejection. On the other hand, if the membrane surface is negatively charged, the electrostatic interaction between anionic PFAS and negatively charged membrane can enhance PFAS separation efficiency [28]. In some cases, the membrane’s pore size can be manipulated by controlling the solution’s pH, which affects PFAS removal [34]. The electrostatic repulsion force inside the membrane pores may be reduced when the pH is lowered, which leads to membrane pore size shrinkage and results in enhanced rejection rate [35].
Effect of ions and ionic strength: The interaction between different ions (present in water and on the membrane surface) and PFAS is a critical factor. Due to electrostatic interaction between PFAS and ions present in the water, ions can bind PFAS to form larger clusters which may even lead to partial pore-blocking [36]. This is expected to reduce the transport of short and long-chained PFAS through the membrane, resulting in a better PFAS removal. In addition, the increase of valance ions present in the water matrix can improve the electrostatic interaction between PFAS and ions, which promotes the PFAS clustering. For instance, the presence of PO43− resulted in better removal efficiency in comparison with Cl− or SO42− when assessing the removal of positively charged compounds. This phenomenon is not only limited to anions, and the presence of divalent and trivalent cations (Ca2+, Pb2+, Fe3+) can also enhance PFAS rejection.
Effect of chain length and hydrophobicity: PFAS are widely used for their hydrophobic and oleophobic nature. Long-chain PFAS are considered more hydrophobic than short-chain PFAS due to the presence of a longer hydrophobic ‘tail’. During membrane filtration of PFAS containing wastewater, PFAS are prone to interacting with solid surfaces (e.g., membranes) present in the system. Therefore, hydrophobic membranes may have an added value when used to remove PFAS by coupling filtration and adsorption [37][38][39].
Effect of membrane zeta potential: The zeta potential of a membrane is a function of surface-attached groups (e.g., carboxyl, amine groups) and of the ionic strength. Increasing the ionic strength typically results in a reduction in membrane surface charge as a result of ‘shrinkage’ of the electrostatic double layer and masking of surface charge. As a result, lower membrane surface charge leads to weak electrostatic repulsive forces between similar charged PFAS and membranes and resulting in a reduced rejection rate [40]. Accordingly, maximum rejections by electrostatic forces may be achieved by increasing the charge density at the membrane surface, thus leading to higher electrostatic repulsion forces [38].
Effect of membrane surface properties: Among different NF membranes, organic membranes have an advantage over inorganic membranes, resulting from easier processing, appropriate robustness, and low cost [38]. The active layer of the membrane is a key component in quantifying the PFAS removal performance. The NF membrane surface is usually negatively charged and hydrophilic, along with having a low molecular weight cutoff, which affects the membrane performance. During the separation process, PFAS concentrate (micelles or hemi-micelles) can form on the membrane surface due to concentration polarization. PFAS micelles are formed at relatively high concentrations [41]. A critical micelles concentration of PFOA and PFOS ranges between 25–38 mM and 8 mM, respectively [42].
The formation of micelles was shown to reduce the PFAS rejection caused by fouling enhanced concentration polarization (CP), where the solute concentration at the membrane surface is remarkably higher than in the bulk solution [43].
In the case of PFAS removal via an adsorption-based process coupled with membranes, the membrane’s surface is coated with adsorbent materials and the process is controlled by the characteristics of adsorbents, such as adsorbent particle size, surface area, pore-volume, etc. In such cases, the electrostatic force between the membrane surface and the PFAS mostly depends on the adsorbent material and not the membrane’s properties [44].
The initial concentration of PFAS: The initial concentration of the PFAS in the feed affects both permeate flux and removal efficiency.
Several reported studies [45][46] addressed the removal efficiency of PFAS when treating a high feed concentration (100 ppm). A high concentration of PFAS in the feed may lead to micelle formation when concentrations reach the critical micelle concentration. While in typical wastewater and groundwater streams, it is very unlikely that the critical micelle concentration will be reached; this should be noted when treating concentrated PFAS streams such as industrial effluents.
Since membrane technology for PFAS removal has several benefits over other available techniques (adsorption, ion exchange resins, etc.), researchers will review and discuss aspects and modern techniques related to membrane technology in the next section.
3. Novel Membranes for PFAS Rejection and Removal
The use of NF and RO membranes can efficiently remove PFAS from water but may be impacted by fouling or require high pressure/energy to achieve appropriate separation [47]. Various modifications have been suggested to improve the membrane’s performance and longevity, including the development of novel membranes and surface modification of commercial membranes, which play a critical role in PFAS rejection [46]. Novel membranes developed for PFAS removal and treatment are described below:
3.1. Polymeric Membranes
Linear fluorinated silane-functionalized aluminum oxide hydroxide-modified membranes with an effective pore size of 1 µm were fabricated by Johnson et al. [48]. The removal mechanism of PFOA (0.39 ng/L) and PFOS (0.86 ng/L) in this work was based on the hypothesis that the perfluorinated side chains present on the prepared surface would have a favorable fluorophilic (C−F···F−C) interaction with PFAS (Figure 2). The fabricated membrane (containing 13–17 fluorine atoms) efficiently removed >90% (unmodified membrane showed ~80% removal) of the PFOA and PFOS at a high flux rate (1223 LMH, pressure drop 0.0413–0.317 bar, pH 7.5, filtration time 30 min). This work shows the advantage of using a hydrophobic surface to remove PFAS by adsorption to the membrane’s surface. As the fluorophilic interaction is strong, the membrane will eventually reach maximum capacity and rejection will be impacted; furthermore, such a mechanism requires regeneration when reaching adsorption capacity. These have not been addressed in the presented work. Furthermore, hydrophobic membranes have a higher fouling tendency in comparison to hydrophobic membranes, but fouling of the membrane is not addressed. However, due to the hydrophobic surface, a high-pressure drop (0.317 bar) was observed across the membrane thickness during the operation. To overcome or reduce the back pressure drop, the membrane surface was further modified with hydrophilic poly(ethylene glycol) (PEG) units. The introduction of further PEG modification successfully reduced the pressure drop (~0.0413 bar), and 99.9% PFAS removal was achieved. Furthermore, a detailed analysis of membrane-fouling and regeneration/lifespan of the modified membrane needs to be done to understand the process in detail [48].
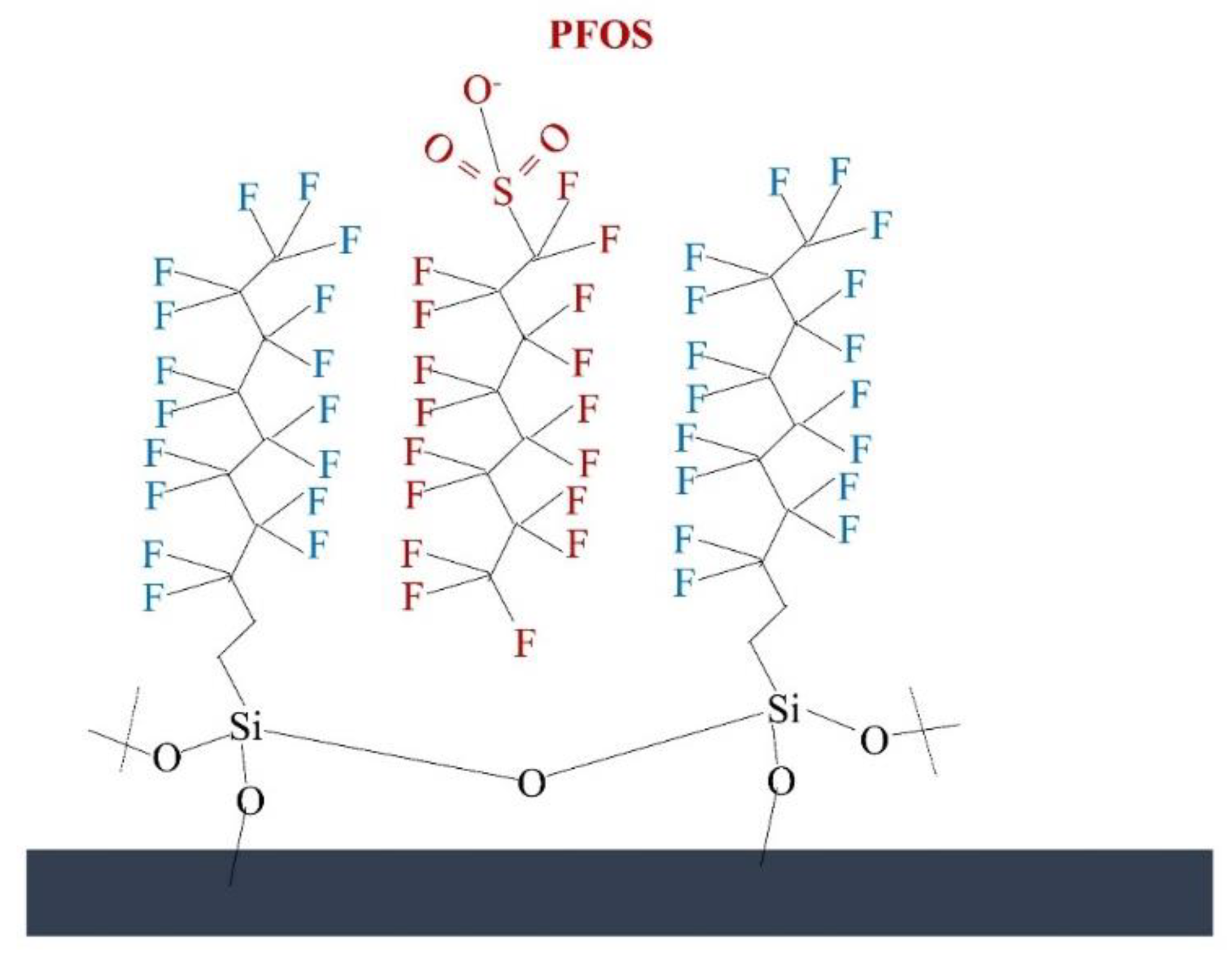 Figure 2. PFAS binding in the modified surface.
Figure 2. PFAS binding in the modified surface.In addition, amyloid fibril-based membranes were used for the removal of 16 different PFAS (~400 ng/L) from wastewater [28]. The membranes were able to efficiently remove 99% of long- and medium-chain PFAS (Molecular weight 214–714 Da). Solution pH was shown to impact the removal efficiency, as at low pH values (about pH of 2) the amyloid fibril membrane becomes more positively charged and attracts negatively charged PFAS (due to electrostatic interactions). Furthermore, the significant role of hydrophobic interaction between PFAS and amyloid surface was also shown with long-chain PFAS, which were adsorbed to the hydrophobic surface. Finally, it was suggested that the amyloid-carbon hybrid membrane (permeability ~1739 LMH/bar) shows better performance in short-chain PFAS removal (>96%) compared to a pristine NF membrane (20–90%), resulting from a highly adsorbent amyloid-carbon surface. The operating cost of this membrane ($ 0.042/m3, energy requirement 0.2 kWh/m3) is moderate compared to the NF membrane ($ 0.016–0.16/m3 [49], energy requirement 0.528 kWh/m3), and it is made up of by-products from the dairy industry (prepared in the water phase and biodegradable), making it suitable, appropriate, and convenient for such an application. While results are encouraging in terms of an economical and sustainable treatment approach that can be implemented to remove PFAS efficiently from wastewater, further consideration regarding scaling up, fouling, and operational conditions is necessary [28].
3.2. Ceramic Membranes
In addition to polymeric membranes, ceramic membranes have been used to remove PFAS. The principal advantages of inorganic ceramic membranes over polymeric membranes include high thermal stability, mechanical strength, and chemical stability. Ceramic membranes can withstand a broad range of temperatures and harsh pH environments. Furthermore, they typically do not exhibit irreversible changes in structure that may affect their operational performance [50][51][52]. Commonly used materials to develop ceramic membranes are microporous glasses, titania, silica, alumina, zeolites, and zirconia [53]. Methods used to fabricate inorganic ceramic membranes mainly involve sol-gel, solid-state sintering, chemical extraction, phase-separation, and chemical vapor deposition [50]. The pore size of commercially available ceramic membranes ranges from approximately 4 nm to 10 µm, which is similar to MF, UF, and NF membranes [54]. Therefore, ceramic membranes are less effective in rejecting and removing most PFAS, especially when addressing short-chain PFAS.
While some ceramic membranes are not able to directly remove PFAS, they can be used for coupled processes, which include adsorption and filtration, mainly used to separate the adsorbing material from the liquid phase; for example, Murray et al. (2019) [25] used a commercial ceramic membrane along with a superfine powder activated carbon adsorbent to remove a mixture of 12 different types of PFAS collected from a firefighting training area, with a concentration ranging between 1.18–55.7 ng/L. In addition to removing the activated carbon (20 L adsorption tank, 100–500 mg/L), the ceramic membrane (60–65 LMH, crossflow velocity 0.19 m/s, permeate flow 43–48 mL/min, experiment duration 42–200 h) was able to mainly remove long-chain PFAS. The adsorption of PFAS was achieved by using different adsorbents (granular activated carbon (GAC) and super fine powder activated carbon (SPAC)), and the parameters affecting the adsorption capacity (SPAC > 480 × GAC) were adsorbent particle size (SPAC—0.88 µm, GAC—650 µm), adsorbent pore size (SPAC—2.70 nm, GAC—2.58 nm), adsorbent specific surface area (SPAC—927 m2/g, GAC—784 m2/g), and chain length of PFAS (C4–C8).
Resulting from adsorption followed by ceramic nanofiltration, the permeate sample contains substituted perfluoroalkane derivatives and sulfonamide precursor substances (such as long-chain (3.3% FPeSA (C5) and 0.7% FOSA (C8)) and short-chain(43% FBSA (C4) and 53% FPrSA (C3)), which indicates the inefficient outcome of the system. The pressure drop during the operation (reflected in specific flux calculation) confirms membrane fouling, mainly by the formation of a cake layer (after ~150 L of wastewater treatment). During the activated carbon separation process, the carbon particles can build up on the membrane surface and serve as a narrow protective layer that inhibits the foulants from reaching the membrane surface. It is possible that the foulants are getting adsorbed and removed by the protective layer of carbon formed on the membrane surface, which leads to a long-term operation of the membrane with minimum fouling. For the long-term operation, the researchers have also used back-pulses for 2 s in every 5 min of interval to prevent fouling. Further effort is needed to reduce the operating cost and remove short-chain PFAS [25].
3.3. Polyamide-Modified Thin Film Composite Membranes
Another approach to modifying membranes to enhance PFAS rejection was suggested by Nadagouda and Lee (2021); they suggested modifying the NF/RO membrane’s surface charge using nano-porous polyamide. This modification increased the negative charge at the membrane’s surface. A negative charge was estimated to enhance the rejection of anionic PFAS molecules by electrostatic repulsion between the negatively charged membrane and anionic PFAS (Figure 3a,b), which can also help prevent fouling. Moreover, the researchers implied that elevated pH would improve the rejection, as carboxyl groups at the membrane’s surface may be deprotonated, and therefore, the membrane’s negative charge will increase [55].
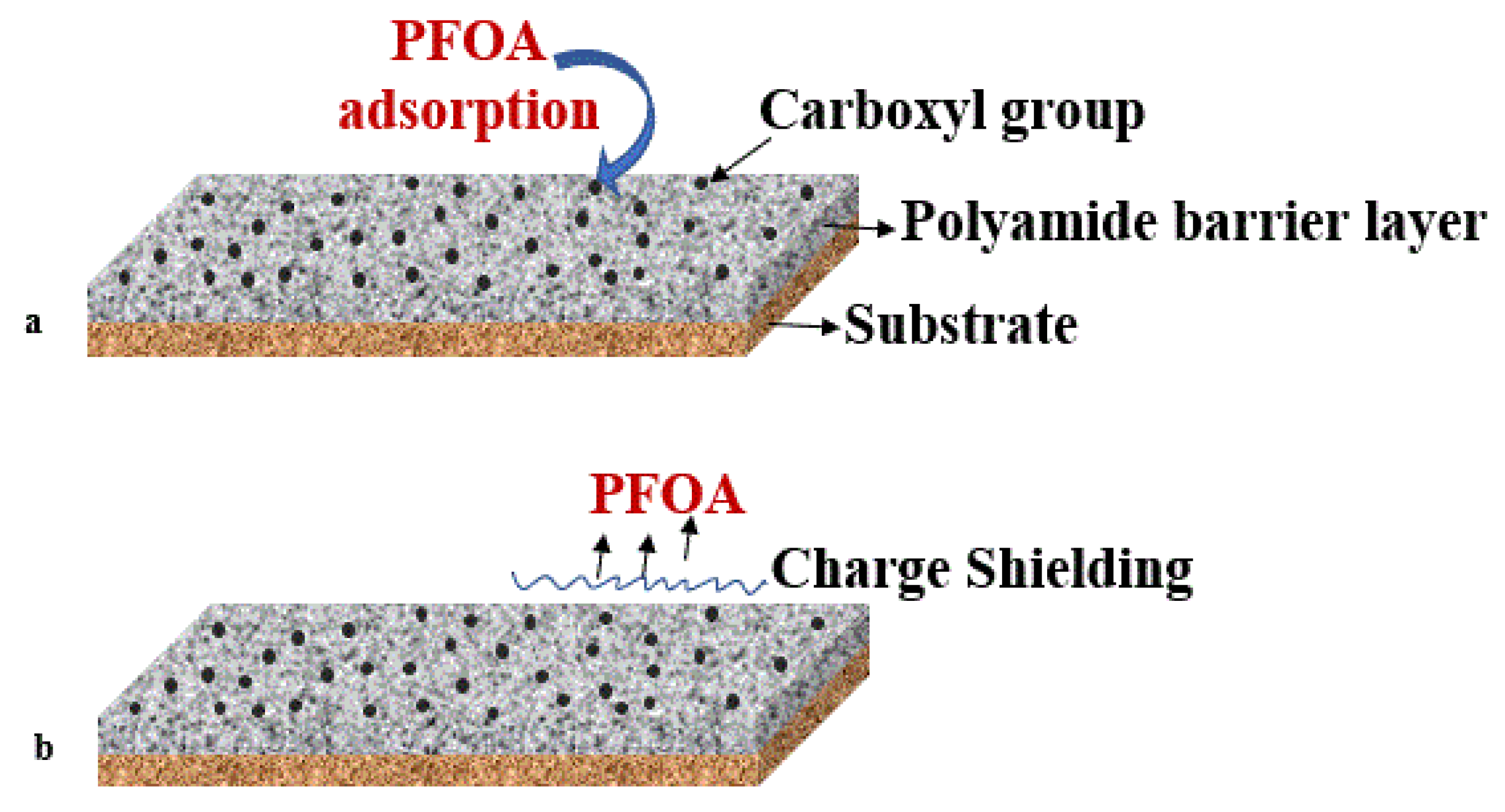 Figure 3. Polyamide barrier layer on membrane surface with (a) PFOA adsorption and (b) charge-shielding by carboxyl groups preventing PFAS transport to the membrane surface.
Figure 3. Polyamide barrier layer on membrane surface with (a) PFOA adsorption and (b) charge-shielding by carboxyl groups preventing PFAS transport to the membrane surface.3.4. Modified Silica Membrane
Additional research by Zhou et al. (2016) [56] shows how PFAS (PFHxS, PFOS, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, and PFTA, concentration 0.5–50 ng/L, pH 3, volume 1 L) removal efficiency can be enhanced by a novel Fe3O4 nanoparticle-coated silica membrane with fluorinated groups (Fe3O4@SiO2-NH2&F13). PFAS could be adsorbed by the magnetic Fe3O4 particles due to electrostatic and F-F interaction (range of adsorption capacity is 13.2–111.14 mg/g, at room temperature for 24 h). Compared to activated carbon (58.61% adsorption), the magnetic material (86.29% adsorption) showed better adsorption capacity. The researchers confirm the stability and reusability (5 cycles of adsorption and regeneration, by applying an external magnetic field and washing with acetonitrile: ammonia-methanol (7 mol/L) (6:4 v/v) and ethanol: water (5:5 v/v) solution three times, respectively) of the material, and showed no significant reduction (<5%) in efficiency even after the fifth run. Furthermore, the addition of organic matter, i.e., humic acid, did not show a significant influence on rejection; however, additional information is needed on fouling and fouling mitigation to understand the applicability of such membranes [56].
3.5. Graphene Oxide (GO)-Nanofiltration-Membranes
GO-nanofiltration-membranes were developed by Meragawi et al. (2020) [57] to remove PFAS from wastewater, and they exhibited inferior performance (74.3% efficiency for 50 ppm PFOA, transmembrane pressure 1 bar, permeate flow rate 10 ± 2.1 LMH/bar) compared to normal NF membranes [58]. GO had an extended interlayer spacing in aqueous media because of water molecules clustering around the oxidized functional groups. This expanded interlayer spacing allows water transport but prohibits PFOA molecules from passing the membrane due to size exclusion.
Further surface modification of the GO-membranes by solution casting of polyethyleneimine (PEI) improved its permeance, selectivity, and mechanical stability. The electron-rich polyethyleneimine deoxygenates GO, leading to a reduction in interlayered spacing and improving the hydrophobicity of the surface layer. Furthermore, the retention of GO-PEI-modified membranes showed improved performance, resulting from the enhanced steric exclusion derived from the decreased interlayer spacing (Figure 4a,b). The introduction of polyethyleneimine underwent reduction and cross-linking reactions, and demonstrated a performance improvement (96.5% removal, permeate flowrate 15.9 ± 1.3 LMH/bar, which is an improvement of >22%) for the same concentration of PFOA. Furthermore, antifouling property and better abrasion resistance were observed due to the hydrophilic surface (contact angle 24.7° of compared to GO membrane with a contact angle of 54.8°). Antifouling ability was evaluated while filtering a solution containing bovine serum albumin (BSA, 30 mg/L, for 2 h). The fouled GO-PEI membranes showed promising results with sodium hydroxide (at pH 9 with 50 mL for 15 min, followed by DI water wash for 2 h) wash instead of ethanol (at pH 9 with 50 mL for 15 min, followed by DI water wash for 2 h) wash. This work demonstrates that the hydrophilic GO-PEI surface improves water permeance and shows that incorporating a hydrophilic PEI layer on the top of the GO layer lowers the requirement of energy for water permeance into hydrophobic pores, leading to further improvement while also improving PFOA rejection as a result of membrane pore size [57].
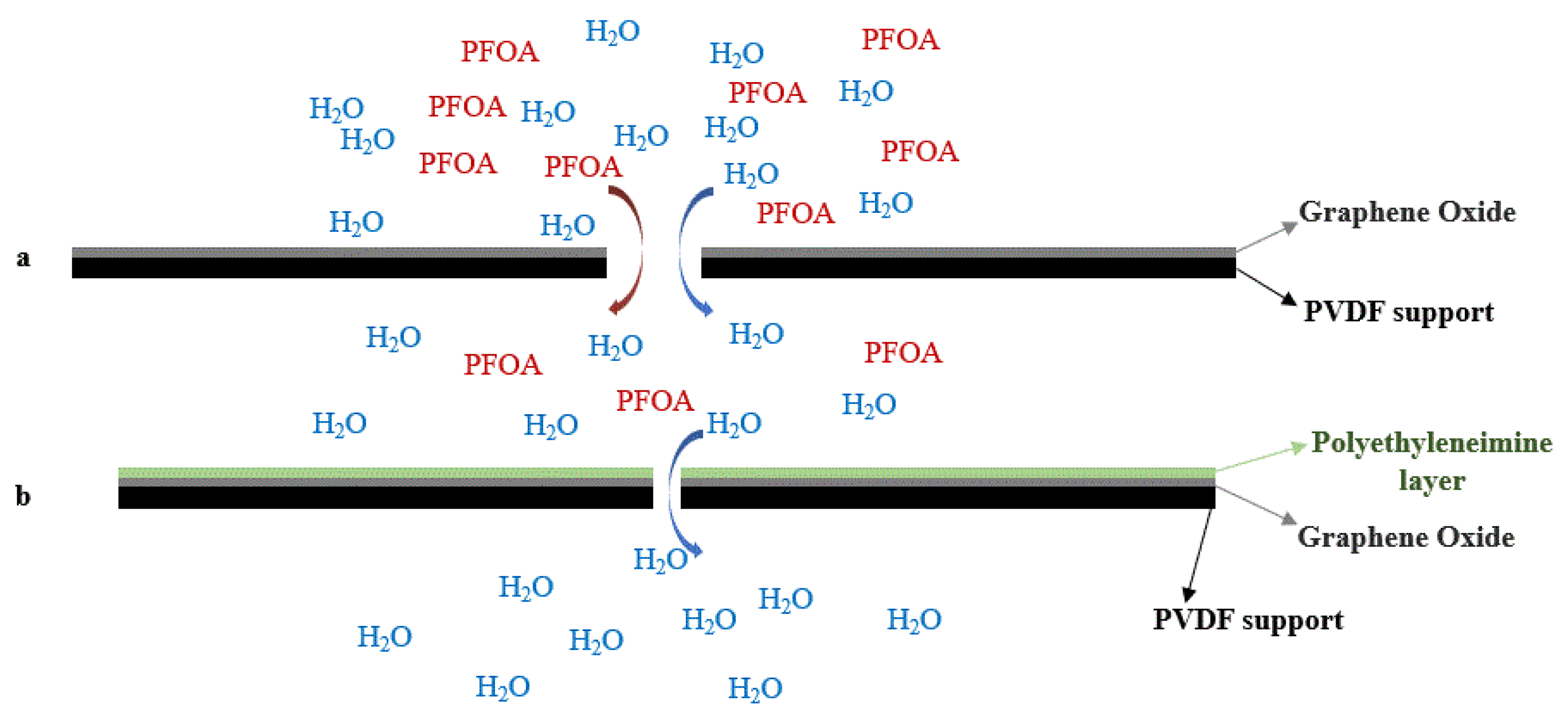 Figure 4. Schematic diagram of water permeance and retention changes for Graphene Oxide (GO) and GO-PEI membranes. (a) GO layer on top of PVDF support. (b) PEI layer on top of GO layer (interlayer space reduction).
Figure 4. Schematic diagram of water permeance and retention changes for Graphene Oxide (GO) and GO-PEI membranes. (a) GO layer on top of PVDF support. (b) PEI layer on top of GO layer (interlayer space reduction).References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373.
- Son, H.; Kim, T.; Yoom, H.; Zhao, D.; An, B. The Adsorption Selectivity of Short and Long Per- and Polyfluoroalkyl Substances (PFASs) from Surface Water Using Powder-Activated Carbon. Water 2020, 12, 3287.
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; Dewitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543.
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per-and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795.
- Domazet, S.L.; Jensen, T.K.; Wedderkopp, N.; Nielsen, F.; Andersen, L.B.; Grøntved, A. Exposure to perfluoroalkylated substances (PFAS) in relation to fitness, physical activity, and adipokine levels in childhood: The european youth heart study. Environ. Res. 2020, 191, 110110.
- Blake, B.E.; Fenton, S.E. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565.
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657.
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes. Chemosphere 2021, 271, 129530.
- Pétré, M.A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.U.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- And Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856.
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 2021, 751, 141622.
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583.
- Kostianoy, A.G.; De Boer, J.; Garrigues, P.; Gu, J.; Jones, K.C.; Knepper, T.P.; Newton, A.; Sparks, D.L. The Handbook of Environmental Chemistry: Polyfluorinated Chemicals and Transformation Products; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642218712.
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171.
- Cáñez, T.T.; Guo, B.; McIntosh, J.C.; Brusseau, M.L. Perfluoroalkyl and Polyfluoroalkyl substances (PFAS) in Groundwater at a Reclaimed Water Recharge Facility. Sci. Total Environ. 2021, 791, 147906.
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003.
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369.
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183.
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126.
- Krug, J.D.; Lemieux, P.M.; Lee, C.-W.; Ryan, J.V.; Kariher, P.H.; Shields, E.P.; Wickersham, L.C.; Denison, M.K.; Davis, K.A.; Swensen, D.A.; et al. Combustion of C 1 and C 2 PFAS: Kinetic modeling and experiments. J. Air Waste Manag. Assoc. 2022, 72, 1–15.
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957.
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303.
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738.
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochem. 2020, 69, 105245.
- Chen, X.; Vanangamudi, A.; Wang, J.; Jegatheesan, J.; Mishra, V.; Sharma, R.; Gray, S.R.; Kujawa, J.; Kujawski, W.; Wicaksana, F.; et al. Direct contact membrane distillation for effective concentration of perfluoroalkyl substances—Impact of surface fouling and material stability. Water Res. 2020, 182, 116010.
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168.
- Soriano, Á.; Gorri, D.; Biegler, L.T.; Urtiaga, A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019, 164, 114954.
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619.
- Jin, T.; Peydayesh, M.; Joerss, H.; Zhou, J.; Bolisetty, S.; Mezzenga, R. Amyloid fibril-based membranes for PFAS removal from water. Environ. Sci. Water Res. Technol. 2021, 7, 1873–1884.
- Ko, J.S.; Le, N.Q.; Schlesinger, D.R.; Johnson, J.K.; Xia, Z. Novel niobium-doped titanium oxide towards electrochemical destruction of forever chemicals. Sci. Rep. 2021, 11, 18020.
- Shrestha, B.; Ezazi, M.; Ajayan, S.; Kwon, G. Reversible adsorption and desorption of PFAS on inexpensive graphite adsorbents: Via alternating electric field. RSC Adv. 2021, 11, 34652–34659.
- Woodard, S.; Berry, J.; Newman, B. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation 2017, 27, 19–27.
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Effect of Membrane Pore Size on the pH-Sensitivity of Polyethersulfone Hollow Fiber Ultrafiltration Membrane. J. Appl. Polym. Sci. 2010, 116, 2658–2667.
- Wang, W.; Zhang, Y.; Esparra-Alvarado, M.; Wang, X.; Yang, H.; Xie, Y. Effects of pH and temperature on forward osmosis membrane flux using rainwater as the makeup for cooling water dilution. Desalination 2014, 351, 70–76.
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Fujii, S. Impact of feed water pH and membrane material on nanofiltration of perfluorohexanoic acid in aqueous solution. Chemosphere 2017, 183, 599–604.
- Pensini, E.; Dinardo, A.; Lamont, K.; Longstaffe, J.; Elsayed, A.; Singh, A. Effect of salts and pH on the removal of perfluorooctanoic acid (PFOA) from aqueous solutions through precipitation and electroflocculation. Can. J. Civ. Eng. 2019, 46, 881–886.
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975.
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per- And polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843.
- Jin, T.; Peydayesh, M.; Mezzenga, R. Membrane-based technologies for per- and poly-fluoroalkyl substances (PFASs) removal from water: Removal mechanisms, applications, challenges and perspectives. Environ. Int. 2021, 157, 106876.
- Zhao, P.; Xia, X.; Dong, J.; Xia, N.; Jiang, X.; Li, Y.; Zhu, Y. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Sci. Total Environ. 2016, 568, 57–65.
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797.
- Lath, S.; Knight, E.R.; Navarro, D.A.; Kookana, R.S.; McLaughlin, M.J. Sorption of PFOA onto different laboratory materials: Filter membranes and centrifuge tubes. Chemosphere 2019, 222, 671–678.
- Shih, K.; Wang, F. Adsorption Behavior of Perfluorochemicals (PFCs) on Boehmite: Influence of Solution Chemistry. Procedia Environ. Sci. 2013, 18, 106–113.
- Liu, C.J.; Strathmann, T.J.; Bellona, C. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Res. 2021, 188, 116546.
- Le, T.; Jamshidi, E.; Beidaghi, M.; Esfahani, M.R. Functionalized-MXene Thin-Film Nanocomposite Hollow Fiber Membranes for Enhanced PFAS Removal from Water. ACS Appl. Mater. Interfaces 2022, 14, 25397–25408.
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical. Water 2021, 13, 1327.
- Eke, J.; Banks, L.; Mottaleb, M.A.; Morris, A.J.; Tsyusko, O.V.; Escobar, I.C. Dual-functional phosphorene nanocomposite membranes for the treatment of perfluorinated water: An investigation of perfluorooctanoic acid removal via filtration combined with ultraviolet irradiation or oxygenation. Membranes 2021, 11, 18.
- Lee, T.; Speth, T.F.; Nadagouda, M.N. High-pressure membrane filtration processes for separation of Per- and polyfluoroalkyl substances (PFAS). Chem. Eng. J. 2022, 431, 134023.
- Johnson, J.K.; Hoffman, C.M.; Smith, D.A.; Xia, Z. Advanced Filtration Membranes for the Removal of Perfluoroalkyl Species from Water. ACS Omega 2019, 4, 8001–8006.
- Franke, V.; Ullberg, M.; McCleaf, P.; Wålinder, M.; Köhler, S.J.; Ahrens, L. The Price of Really Clean Water: Combining Nanofiltration with Granular Activated Carbon and Anion Exchange Resins for the Removal of Per- And Polyfluoralkyl Substances (PFASs) in Drinking Water Production. ACS ES&T Water 2021, 1, 782–795.
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Lee, M.; Li, K. Microstructured Ceramic Hollow Fiber Membranes and Their Applications; Elsevier B.V.: London, UK, 2017.
- Bagnato, G.; Sanna, A. Membrane Considerations and Plant Design for Pre-Combustion CO2 Capture; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Sonawane, S.; Thakur, P.; Sonawane, S.H.; Bhanvase, B.A. Nanomaterials for Membrane Synthesis: Introduction, Mechanism, and Challenges for Wastewater Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2021.
- Hsieh, H.P.; Liu, P.K.T.; Dillman, T.R. Microporous ceramic membranes. Polym. J. 1991, 23, 407–415.
- Nadagouda, M.N.; Lee, T. Cross-Flow Treatment of PFAS in Water: Materials Challenges and Potential Solutions. Accounts Mater. Res. 2021, 2, 129–133.
- Zhou, Y.; He, Z.; Tao, Y.; Xiao, Y.; Zhou, T.; Jing, T.; Zhou, Y.; Mei, S. Preparation of a functional silica membrane coated on Fe3O4 nanoparticle for rapid and selective removal of perfluorinated compounds from surface water sample. Chem. Eng. J. 2016, 303, 156–166.
- El Meragawi, S.; Akbari, A.; Hernandez, S.; Mirshekarloo, M.S.; Bhattacharyya, D.; Tanksale, A.; Majumder, M. Enhanced permselective separation of per-fluorooctanoic acid in graphene oxide membranes by a simple PEI modification. J. Mater. Chem. A 2020, 8, 24800–24811.
- Appleman, T.D.; Dickenson, E.R.V.; Bellona, C.; Higgins, C.P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 2013, 260, 740–746.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
08 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




