Per- and Polyfluoroalkyl Substances (PFAS) are anthropogenic chemicals consisting of thousands of individual species. PFAS consists of a fully or partly fluorinated carbon–fluorine bond, which is hard to break and requires a high amount of energy (536 kJ/mole). Resulting from their unique hydrophobic/oleophobic nature and their chemical and mechanical stability, they are highly resistant to thermal, chemical, and biological degradation. To date, membrane technology is one of the effective process, which can remove PFAS from wastewater. Moreover, there are very few novel membrane approaches have been reported effective in removing and destroying PFAS.
- PFAS
- Nanofiltration
- Reverse Osmosis
- Novel Membranes
- Surface Modification
- Wastewater
- PFAS Rejection and Removal
- Membranes
- Wastewater treatment
1. Background on Per- and Polyfluoroalkyl Substances (PFAS)
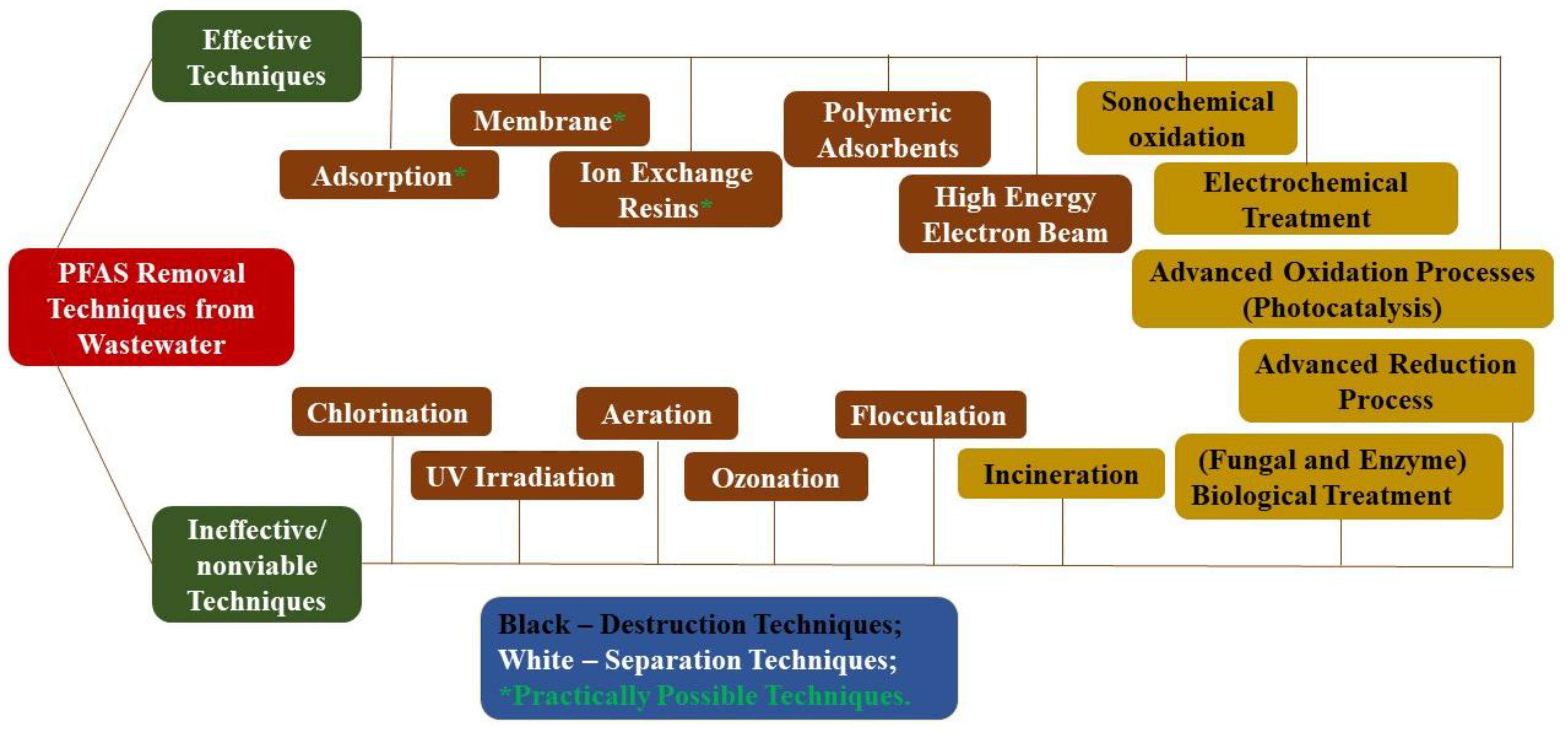 Figure 1. Techniques for removing Per- and Polyfluoroalkyl Substances (PFAS) from wastewater.
Figure 1. Techniques for removing Per- and Polyfluoroalkyl Substances (PFAS) from wastewater.2. Factors Controlling PFAS Separation by Membranes
3. Novel Membranes for PFAS Rejection and Removal
3.1. Polymeric Membranes
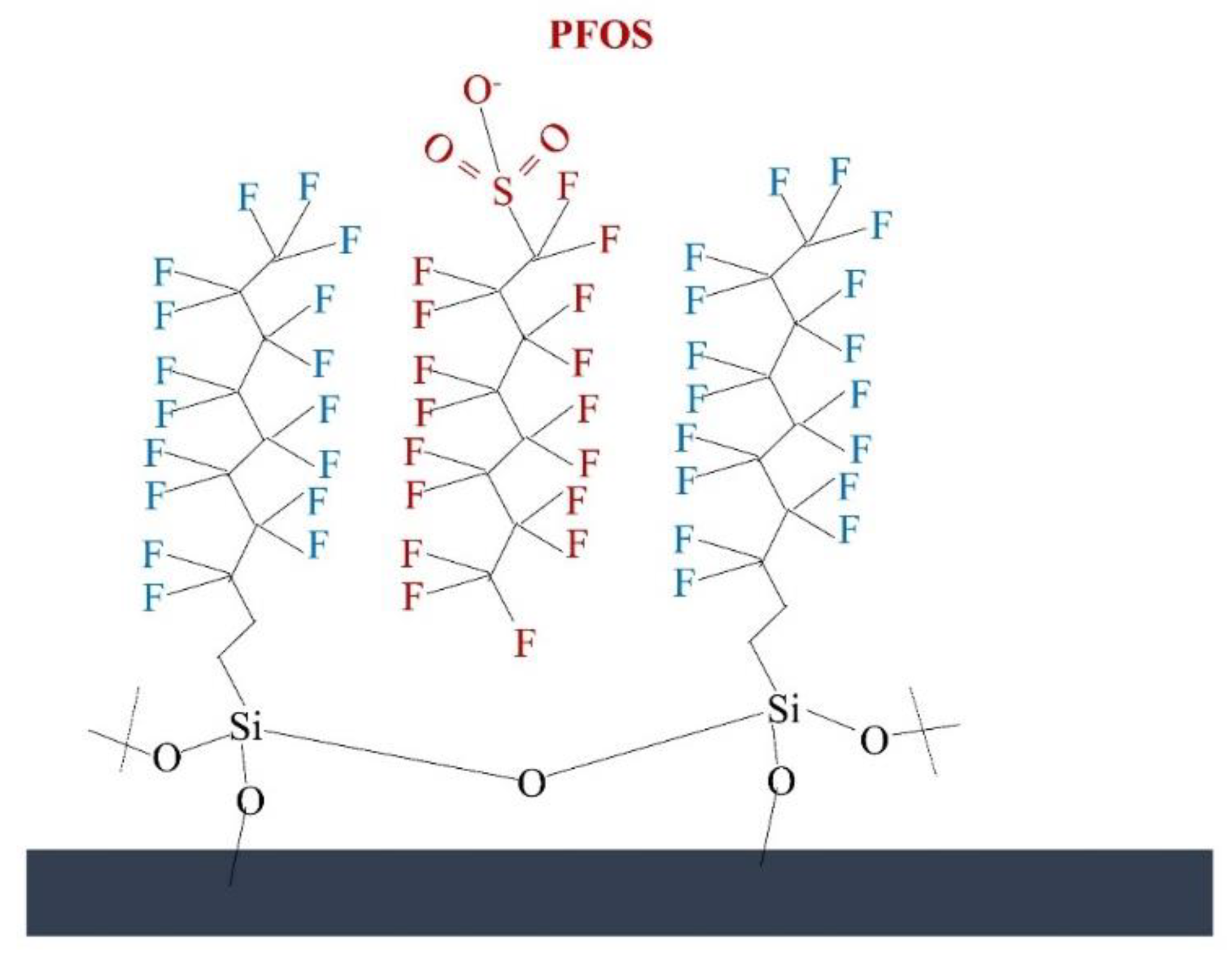 Figure 2. PFAS binding in the modified surface.
Figure 2. PFAS binding in the modified surface.3.2. Ceramic Membranes
3.3. Polyamide-Modified Thin Film Composite Membranes
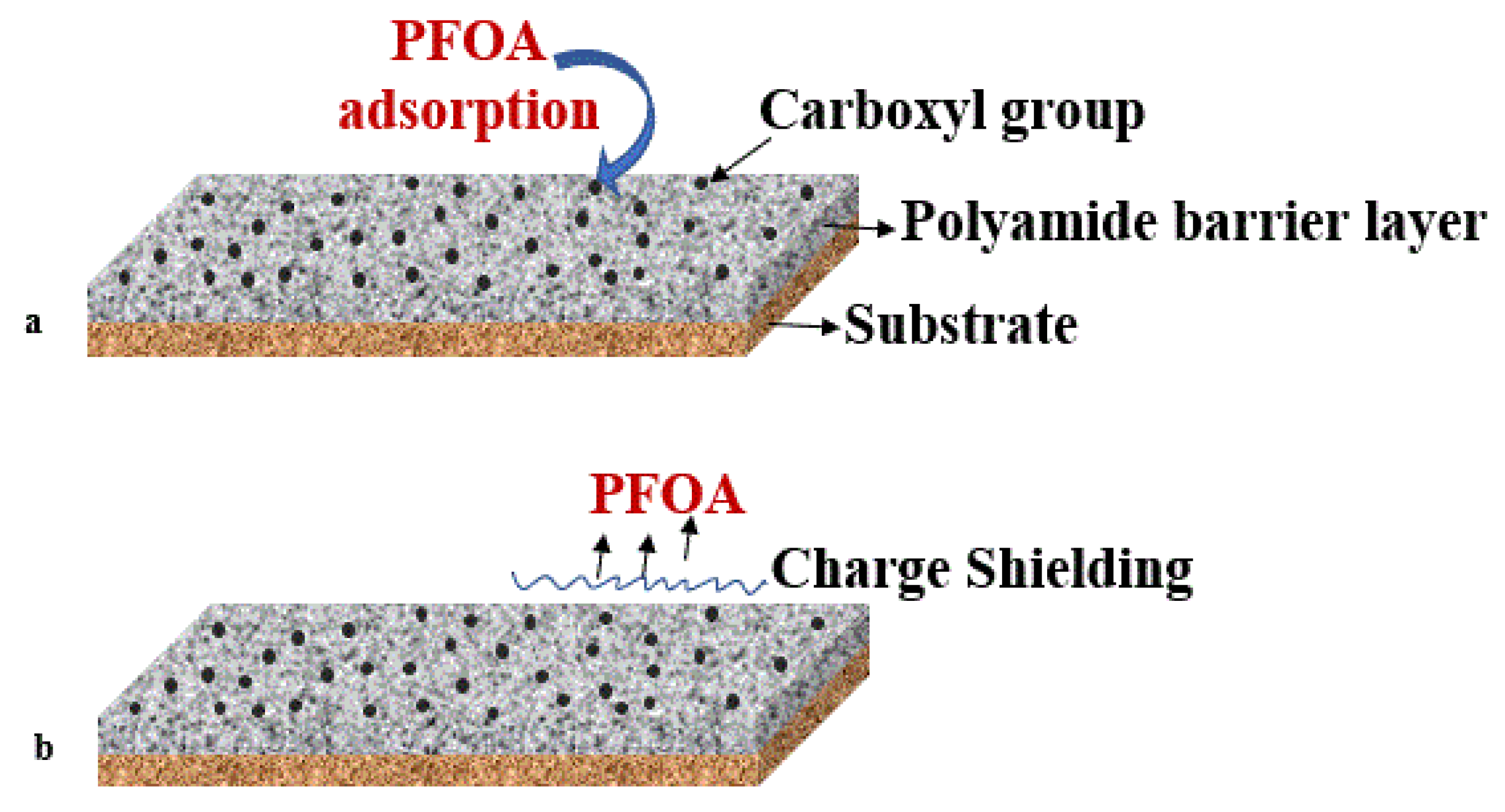 Figure 3. Polyamide barrier layer on membrane surface with (a) PFOA adsorption and (b) charge-shielding by carboxyl groups preventing PFAS transport to the membrane surface.
Figure 3. Polyamide barrier layer on membrane surface with (a) PFOA adsorption and (b) charge-shielding by carboxyl groups preventing PFAS transport to the membrane surface.3.4. Modified Silica Membrane
3.5. Graphene Oxide (GO)-Nanofiltration-Membranes
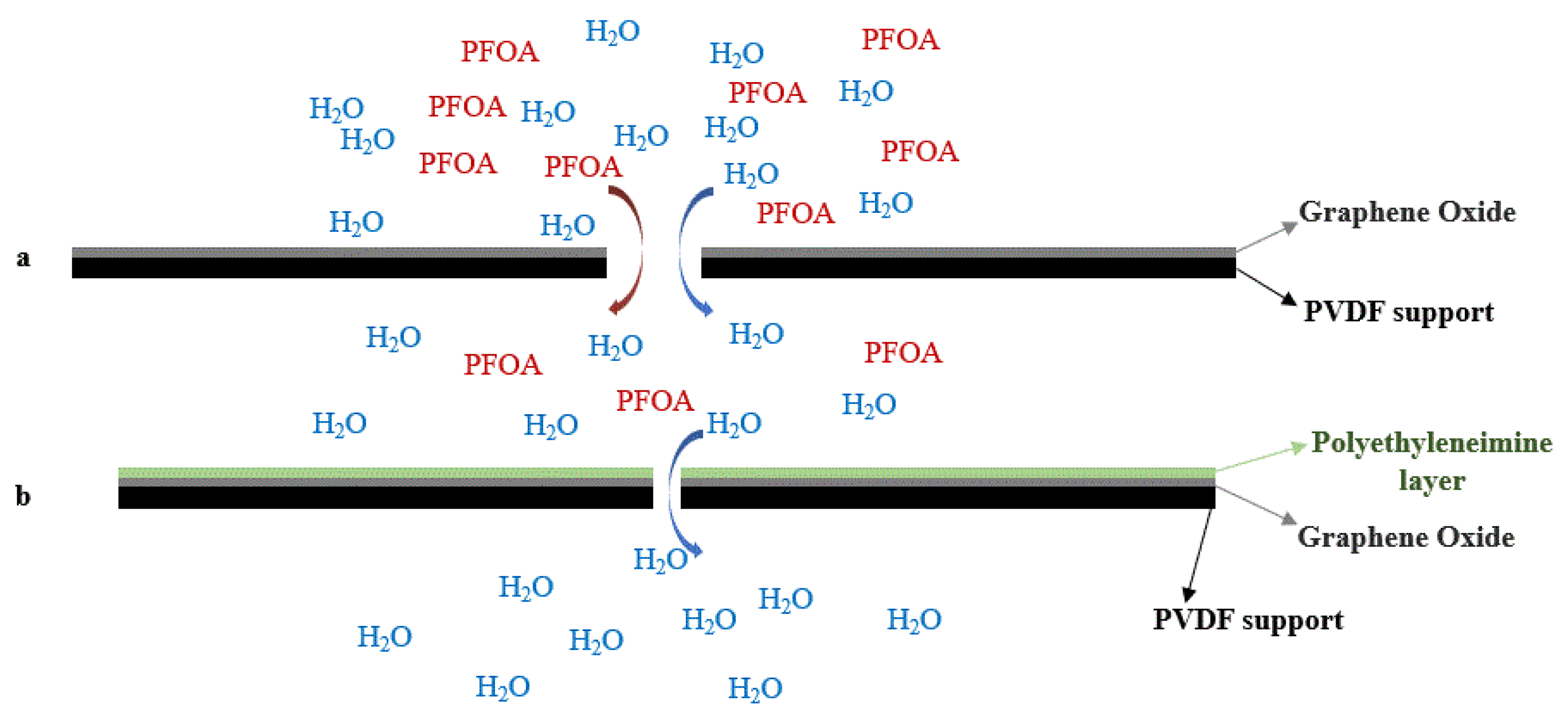 Figure 4. Schematic diagram of water permeance and retention changes for Graphene Oxide (GO) and GO-PEI membranes. (a) GO layer on top of PVDF support. (b) PEI layer on top of GO layer (interlayer space reduction).
Figure 4. Schematic diagram of water permeance and retention changes for Graphene Oxide (GO) and GO-PEI membranes. (a) GO layer on top of PVDF support. (b) PEI layer on top of GO layer (interlayer space reduction).This entry is adapted from the peer-reviewed paper 10.3390/membranes12070662
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373.
- Son, H.; Kim, T.; Yoom, H.; Zhao, D.; An, B. The Adsorption Selectivity of Short and Long Per- and Polyfluoroalkyl Substances (PFASs) from Surface Water Using Powder-Activated Carbon. Water 2020, 12, 3287.
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; Dewitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543.
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per-and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795.
- Domazet, S.L.; Jensen, T.K.; Wedderkopp, N.; Nielsen, F.; Andersen, L.B.; Grøntved, A. Exposure to perfluoroalkylated substances (PFAS) in relation to fitness, physical activity, and adipokine levels in childhood: The european youth heart study. Environ. Res. 2020, 191, 110110.
- Blake, B.E.; Fenton, S.E. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565.
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657.
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes. Chemosphere 2021, 271, 129530.
- Pétré, M.A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.U.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- And Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856.
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 2021, 751, 141622.
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583.
- Kostianoy, A.G.; De Boer, J.; Garrigues, P.; Gu, J.; Jones, K.C.; Knepper, T.P.; Newton, A.; Sparks, D.L. The Handbook of Environmental Chemistry: Polyfluorinated Chemicals and Transformation Products; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642218712.
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171.
- Cáñez, T.T.; Guo, B.; McIntosh, J.C.; Brusseau, M.L. Perfluoroalkyl and Polyfluoroalkyl substances (PFAS) in Groundwater at a Reclaimed Water Recharge Facility. Sci. Total Environ. 2021, 791, 147906.
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003.
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369.
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183.
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126.
- Krug, J.D.; Lemieux, P.M.; Lee, C.-W.; Ryan, J.V.; Kariher, P.H.; Shields, E.P.; Wickersham, L.C.; Denison, M.K.; Davis, K.A.; Swensen, D.A.; et al. Combustion of C 1 and C 2 PFAS: Kinetic modeling and experiments. J. Air Waste Manag. Assoc. 2022, 72, 1–15.
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957.
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303.
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738.
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochem. 2020, 69, 105245.
- Chen, X.; Vanangamudi, A.; Wang, J.; Jegatheesan, J.; Mishra, V.; Sharma, R.; Gray, S.R.; Kujawa, J.; Kujawski, W.; Wicaksana, F.; et al. Direct contact membrane distillation for effective concentration of perfluoroalkyl substances—Impact of surface fouling and material stability. Water Res. 2020, 182, 116010.
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168.
- Soriano, Á.; Gorri, D.; Biegler, L.T.; Urtiaga, A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019, 164, 114954.
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619.
- Jin, T.; Peydayesh, M.; Joerss, H.; Zhou, J.; Bolisetty, S.; Mezzenga, R. Amyloid fibril-based membranes for PFAS removal from water. Environ. Sci. Water Res. Technol. 2021, 7, 1873–1884.
- Ko, J.S.; Le, N.Q.; Schlesinger, D.R.; Johnson, J.K.; Xia, Z. Novel niobium-doped titanium oxide towards electrochemical destruction of forever chemicals. Sci. Rep. 2021, 11, 18020.
- Shrestha, B.; Ezazi, M.; Ajayan, S.; Kwon, G. Reversible adsorption and desorption of PFAS on inexpensive graphite adsorbents: Via alternating electric field. RSC Adv. 2021, 11, 34652–34659.
- Woodard, S.; Berry, J.; Newman, B. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation 2017, 27, 19–27.
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Effect of Membrane Pore Size on the pH-Sensitivity of Polyethersulfone Hollow Fiber Ultrafiltration Membrane. J. Appl. Polym. Sci. 2010, 116, 2658–2667.
- Wang, W.; Zhang, Y.; Esparra-Alvarado, M.; Wang, X.; Yang, H.; Xie, Y. Effects of pH and temperature on forward osmosis membrane flux using rainwater as the makeup for cooling water dilution. Desalination 2014, 351, 70–76.
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Fujii, S. Impact of feed water pH and membrane material on nanofiltration of perfluorohexanoic acid in aqueous solution. Chemosphere 2017, 183, 599–604.
- Pensini, E.; Dinardo, A.; Lamont, K.; Longstaffe, J.; Elsayed, A.; Singh, A. Effect of salts and pH on the removal of perfluorooctanoic acid (PFOA) from aqueous solutions through precipitation and electroflocculation. Can. J. Civ. Eng. 2019, 46, 881–886.
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975.
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per- And polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843.
- Jin, T.; Peydayesh, M.; Mezzenga, R. Membrane-based technologies for per- and poly-fluoroalkyl substances (PFASs) removal from water: Removal mechanisms, applications, challenges and perspectives. Environ. Int. 2021, 157, 106876.
- Zhao, P.; Xia, X.; Dong, J.; Xia, N.; Jiang, X.; Li, Y.; Zhu, Y. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Sci. Total Environ. 2016, 568, 57–65.
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797.
- Lath, S.; Knight, E.R.; Navarro, D.A.; Kookana, R.S.; McLaughlin, M.J. Sorption of PFOA onto different laboratory materials: Filter membranes and centrifuge tubes. Chemosphere 2019, 222, 671–678.
- Shih, K.; Wang, F. Adsorption Behavior of Perfluorochemicals (PFCs) on Boehmite: Influence of Solution Chemistry. Procedia Environ. Sci. 2013, 18, 106–113.
- Liu, C.J.; Strathmann, T.J.; Bellona, C. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Res. 2021, 188, 116546.
- Le, T.; Jamshidi, E.; Beidaghi, M.; Esfahani, M.R. Functionalized-MXene Thin-Film Nanocomposite Hollow Fiber Membranes for Enhanced PFAS Removal from Water. ACS Appl. Mater. Interfaces 2022, 14, 25397–25408.
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical. Water 2021, 13, 1327.
- Eke, J.; Banks, L.; Mottaleb, M.A.; Morris, A.J.; Tsyusko, O.V.; Escobar, I.C. Dual-functional phosphorene nanocomposite membranes for the treatment of perfluorinated water: An investigation of perfluorooctanoic acid removal via filtration combined with ultraviolet irradiation or oxygenation. Membranes 2021, 11, 18.
- Lee, T.; Speth, T.F.; Nadagouda, M.N. High-pressure membrane filtration processes for separation of Per- and polyfluoroalkyl substances (PFAS). Chem. Eng. J. 2022, 431, 134023.
- Johnson, J.K.; Hoffman, C.M.; Smith, D.A.; Xia, Z. Advanced Filtration Membranes for the Removal of Perfluoroalkyl Species from Water. ACS Omega 2019, 4, 8001–8006.
- Franke, V.; Ullberg, M.; McCleaf, P.; Wålinder, M.; Köhler, S.J.; Ahrens, L. The Price of Really Clean Water: Combining Nanofiltration with Granular Activated Carbon and Anion Exchange Resins for the Removal of Per- And Polyfluoralkyl Substances (PFASs) in Drinking Water Production. ACS ES&T Water 2021, 1, 782–795.
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Lee, M.; Li, K. Microstructured Ceramic Hollow Fiber Membranes and Their Applications; Elsevier B.V.: London, UK, 2017.
- Bagnato, G.; Sanna, A. Membrane Considerations and Plant Design for Pre-Combustion CO2 Capture; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Sonawane, S.; Thakur, P.; Sonawane, S.H.; Bhanvase, B.A. Nanomaterials for Membrane Synthesis: Introduction, Mechanism, and Challenges for Wastewater Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2021.
- Hsieh, H.P.; Liu, P.K.T.; Dillman, T.R. Microporous ceramic membranes. Polym. J. 1991, 23, 407–415.
- Nadagouda, M.N.; Lee, T. Cross-Flow Treatment of PFAS in Water: Materials Challenges and Potential Solutions. Accounts Mater. Res. 2021, 2, 129–133.
- Zhou, Y.; He, Z.; Tao, Y.; Xiao, Y.; Zhou, T.; Jing, T.; Zhou, Y.; Mei, S. Preparation of a functional silica membrane coated on Fe3O4 nanoparticle for rapid and selective removal of perfluorinated compounds from surface water sample. Chem. Eng. J. 2016, 303, 156–166.
- El Meragawi, S.; Akbari, A.; Hernandez, S.; Mirshekarloo, M.S.; Bhattacharyya, D.; Tanksale, A.; Majumder, M. Enhanced permselective separation of per-fluorooctanoic acid in graphene oxide membranes by a simple PEI modification. J. Mater. Chem. A 2020, 8, 24800–24811.
- Appleman, T.D.; Dickenson, E.R.V.; Bellona, C.; Higgins, C.P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 2013, 260, 740–746.
