
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cassio Luiz Coutinho Almeida da Silva | -- | 2748 | 2022-07-06 23:14:17 | | | |
| 2 | Dean Liu | Meta information modification | 2748 | 2022-07-08 03:42:33 | | | | |
| 3 | Dean Liu | -9 word(s) | 2739 | 2022-07-08 03:46:01 | | | | |
| 4 | Dean Liu | Meta information modification | 2739 | 2022-07-11 03:33:54 | | |
Video Upload Options
Boswellia trees, found throughout the Middle East and parts of Africa and Asia, are the source of frankincense oil. Since antiquity, frankincense has been traded as a precious commodity, but it has also been used for the treatment of chronic disease, inflammation, oral health, and microbial infection.
1. Introduction

2. Description of Frankincense Resins, Oils, and Boswellic Acids
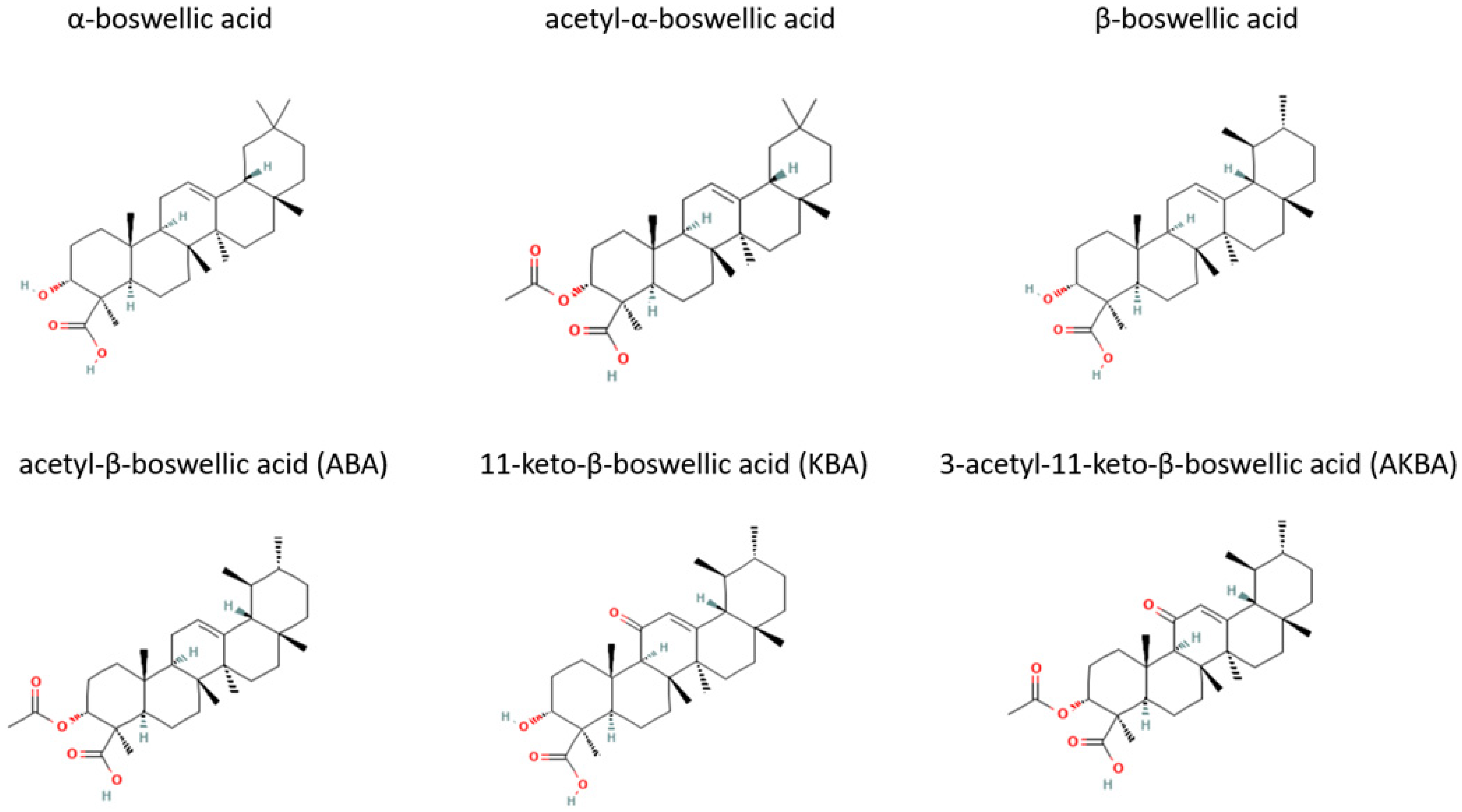
3. Modern Medicinal Uses of Frankincense
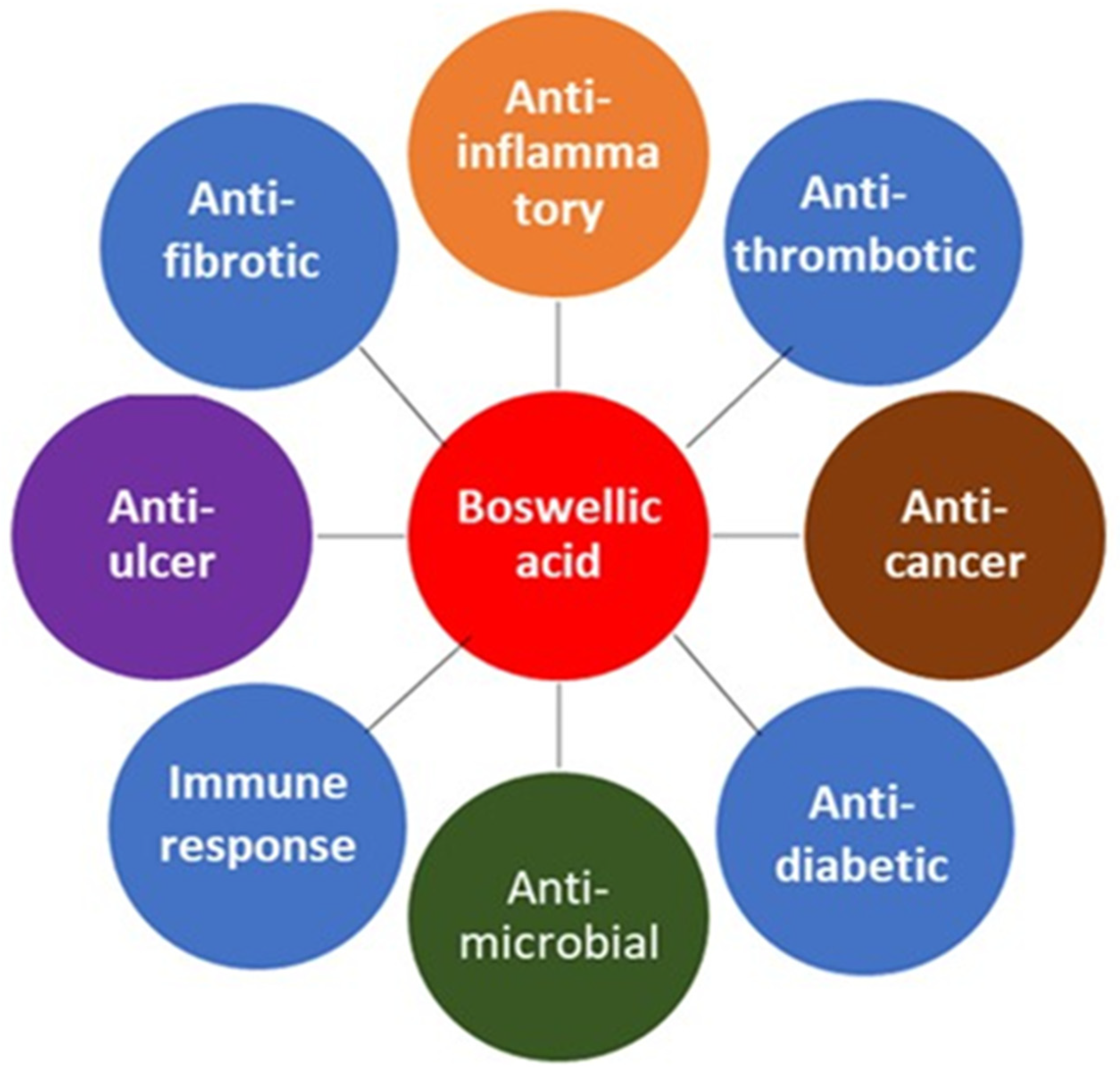
4. Antimicrobial Activity of Frankincense
The oleo gum resin essential oils from B. carterii (Somalia), B. papyrifera (Ethiopia), B. serrata (India), and B. rivae (Ethiopia) were individually tested against different fungi and Gram-positive and Gram-negative bacteria. The essential oils exhibited significant antifungal activity against both C. albicans and C. tropicalis, with the essential oils from B. carterii and B. papyrifera showing the best activity [50]. The MIC values for C. albicans and C. tropicalis were 12.86 and 12.86 μg/mL (B. serrata), 6.16 and 6.16 μg/mL (B. carterii), 6.09 and 6.09 μg/mL (B. papyrifera), and 2.65 and 27.38 μg/mL (B. rivae), respectively, compared with the MIC of 0.15 μg/mL for amphoterecin B. The essential oil of B. rivae resin exhibited the best activity against C. albicans [50]. Limonene present in the essential oils is thought to be responsible for the antifungal activity [51], since oleo gum resin essential oils without limonene lack antifungal activity. The oleo gum resins also showed significant antibacterial effects against Gram-positive S. aureus and S. epidermidis, with MIC values ranging from 3.52 to 107.20 μg/mL. These values can be compared with the MIC of 1 μg/mL for vancomycin and 2 μg/mL for amikacin used as controls in the experiments [50]. The frankincense compounds against Gram-negative E. coli and P. aeruginosa showed MIC ranging from 6.60 to 107.18 μg/mL, compared to the vancomycin MIC of 10 and 5 μg/mL for E. coli and P. aeruginosa, respectively. It is noteworthy that some of the frankincense compounds had antimicrobial effects at concentrations that were comparable or very close to concentrations of the standard antibiotics used in the in vitro experiments. Future studies might confirm and expand the results to in vivo and clinical models.
Dental caries and periodontitis remain two of the major oral diseases that affect more than half of the global population [52]. Periodontitis is often referred as a common inflammatory disease in humans. The etiology of these diseases has been linked to certain bacterial species, plaque and biofilm formation, and the resultant inflammatory states. Current therapies for treating periodontal disease place an emphasis on biofilm removal. This is exercised via a combination of mechanical (such as scaling and root planing) and antibiotic treatments. However, these treatment modalities are still unable to fully remove biofilms. This has led to an interest in incorporating herbal extracts into current treatment regimens.
Many studies have been performed using the resin of B. serrata. As noted above, this resin contains boswellic acid, which are the active components in its anti-inflammatory effects. In addition, both the boswellic acid and the essential oils of B. serrata exhibit antimicrobial properties. Of the four major boswellic acids, acetyl-11-keto-β-boswellic acid (AKBA), has consistently demonstrated the greatest antibacterial effects. In fact, several studies have determined that AKBA is the most active component in the resin of B. serrata and other Boswellia species [53].
In terms of antibacterial effectiveness, AKBA isolated from B. serrata demonstrated activity against Streptococcus mutans, Enterococcus faecalis, Enterococcus faecium, Actinomyces viscosus, Streptococcus sanguinis, Fusobacterium nucleatum, Prevotella intermedia, and Porphyromonas gingivalis. Furthermore, the post-antibiotic effects of AKBA were determined to be greater than those of ciprofloxacin, and AKBA was also shown to inhibit biofilms of the cariogenic bacteria S. mutans [53].
In a double-blinded, randomized clinical trial, the effectiveness of traditional scaling and root planing methods were compared to the use of B. serrata extract or powder [54]. The results indicated that the addition of B. serrata extract or powder to traditional scaling and root planing treatments leads to better gingival health as measured by gingival, plaque, and bleeding indices, as well as probed pocket depths [54]. The authors concluded that the anti-inflammatory properties of B. serrata extract had results comparable or even superior to traditional scaling and root planing treatments. Given its antibacterial, antifungal, and antioxidant properties, it is not surprising that frankincense also has promising beneficial effects on oral health.
5. Anti-Inflammatory Effects of Frankincense
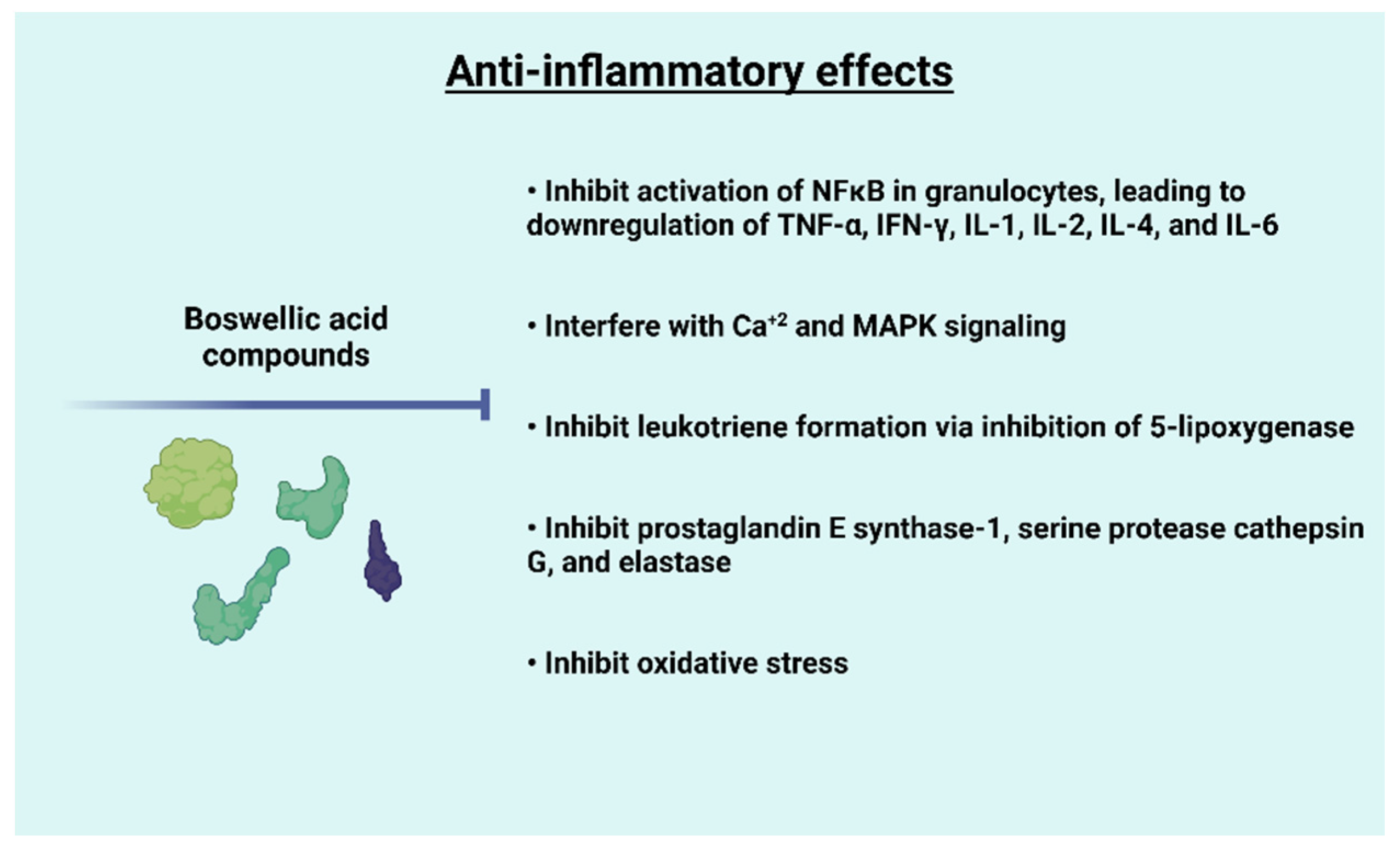
References
- Yu, D.; Ryu, K.; Zhi, S.; Otto, S.J.G.; Neumann, N.F. Naturalized Escherichia coli in Wastewater and the Co-evolution of Bacterial Resistance to Water Treatment and Antibiotics. Front. Microbiol. 2022, 13, 810312.
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283.
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense—Therapeutic properties. Postepy Hig. Med. Dosw. (Online) 2016, 70, 380–391.
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022.
- Van Wyk, B.E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015, 176, 118–134.
- Eslamieh, J. Cultivation of Boswellia, 2nd ed.; A Book’s Mind: Fort Collins, CO, USA, 2017.
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369.
- Britannica, The Editors of Encyclopaedia. Frankincense. Available online: https://www.britannica.com/topic/frankincense (accessed on 21 February 2022).
- Bokelmann, J.M. Medicinal Herbs in Primary Care: An Evidence-Guided Reference for Healthcare Providers. In Frankincense/Boswellia (Boswellia serrata/sacra/carterii): Bark Resin; Bokelmann, J.M., Ed.; Medicinal Herbs in Primary Case; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–360.
- Shah, S.A.; Rathod, I.S.; Suhagia, B.N.; Pandya, S.S.; Parmar, V.K. A simple high-performance liquid chromatographic method for the estimation of boswellic acids from the market formulations containing Boswellia serrata extract. J. Chromatogr. Sci. 2008, 46, 735–738.
- Chevrier, M.R.; Ryan, A.E.; Lee, D.Y.; Zhongze, M.; Wu-Yan, Z.; Via, C.S. Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro. Clin. Diagn. Lab. Immunol. 2005, 12, 575–580.
- Barnett, J.R.; Langenheim, J.H. Plant resins: Chemistry, evolution, ecology and ethnobotany. Ann. Bot. 2004, 93, 2.
- Hughes, L. The Funerals of the Russian Emperors and Empresses. In Monarchy and Religion: The Transformation of Royal Culture in Eighteenth-Century Europe; Schaich, M., Ed.; Oxford University Press: Oxford, UK, 2007; pp. 395–419.
- Berhanu, Y.; Vedeld, P.; Angassa, A.; Aune, J.B. The contribution of frankincense to the agro-pastoral household economy and its potential for commercialization—A case from Borana, southern Ethiopia. J. Arid Environ. 2020, 186, 104423.
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152.
- Jones, J.K.N.; Nunn, J.R. The Structure of Frankincense Gum. J. Am. Chem. Soc. 1955, 77, 5745–5746.
- Hosain, N.A.; Ghosh, R.; Bryant, D.L.; Arivett, B.A.; Farone, A.L.; Kline, P.C. Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. Int. J. Biol. Macromol. 2019, 133, 76–85.
- Amri, A.S.L.; Jesil, A.; Salim, A.; Saravanam, A.M. Extraction of Essential Oil from Frankincense Using Steam Distillation. Int. J. Trend Res. Dev. 2019, 6, 3.
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261.
- Wang, F.; Li, Z.L.; Cui, H.H.; Hua, H.M.; Jing, Y.K.; Liang, S.W. Two new triterpenoids from the resin of Boswellia carterii. J. Asian Nat. Prod. Res. 2011, 13, 193–197.
- Ren, P.; Ren, X.; Cheng, L.; Xu, L. Frankincense, pine needle and geranium essential oils suppress tumor progression through the regulation of the AMPK/mTOR pathway in breast cancer. Oncol. Rep. 2018, 39, 129–137.
- Shen, T.; Lou, H.X. Bioactive constituents of myrrh and frankincense, two simultaneously prescribed gum resins in chinese traditional medicine. Chem. Biodivers. 2008, 5, 540–553.
- Vuddanda, P.R.; Singh, S.; Velaga, S. Boswellic acid—Medicinal use of an ancient herbal remedy. J. Herb. Med. 2016, 6, 8.
- Chiavari, G.; Galletti, G.C.; Piccaglia, R.; Mohamud, M.A. Differentiation between Resins Boswellia carterii and Boswellia frereana (Frankincense) of Somali Origin. J. Essent. Oil Res. 1990, 3, 2.
- Wahab, S.M.; Aboutabl, E.A.; El-Zalabani, S.M.; Fouad, H.A.; De Pooter, H.L.; El-Fallaha, B. The essential oil of olibanum. Planta Med. 1987, 53, 382–384.
- Wang, W.; Zhu, Y.; Liu, L.; Ling, D.; Qin, X.; Tian, J. Analysis of the Chemical Constituents of Essential Oil of Boswellia carterii Birdwood from Somali. Chin. J. Pharm. Anal. 1993, 13, 3.
- Mohamed, A.A.; Ali, S.I.; Kabiel, H.F.; Hegazy, A.K.; Kord, M.A.; EL-Baz, F.K. Assessment of Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Boswellia carterii Resin. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 8.
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146.
- Mikhaeil, B.R.; Maatooq, G.T.; Badria, F.A.; Amer, M.M. Chemistry and immunomodulatory activity of frankincense oil. Z. Nat. C 2003, 58, 230–238.
- DeCarlo, A.; Johnson, S.; Okeke-Agulu, K.I.; Dosoky, N.S.; Wax, S.J.; Owolabi, M.S.; Setzer, W.N. Compositional analysis of the essential oil of Boswellia dalzielii frankincense from West Africa reveals two major chemotypes. Phytochemistry 2019, 164, 24–32.
- Dimas Kubmarawa, I.A.O.; Okorie, D.A.; Olawore, N.O.; Kasali, A.A. Constituents of the Essential Oils of Boswellia dalzielii Hutch. from Nigeria. J. Essent. Oil Res. 2011, 18, 2.
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.A.; Cazaux, S.; Bouajila, J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017, 55, 33–42.
- Gupta, I.; Parihar, A.; Malhotra, P.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001, 67, 391–395.
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101.
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523.
- Padhi, M.; Mahapatra, S. Boswellia serrata: A review of its traditional uses, phytochemistry and pharmacology. Int. Rev. Biophys. Chem. (IREBIC) 2013, 4, 74–83.
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375.
- Borrelli, F.; Capasso, F.; Capasso, R.; Ascione, V.; Aviello, G.; Longo, R.; Izzo, A.A. Effect of Boswellia serrata on intestinal motility in rodents: Inhibition of diarrhoea without constipation. Br. J. Pharmacol. 2006, 148, 553–560.
- Ammon, H.P. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006, 72, 1100–1116.
- Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 1998, 3, 511–514.
- Shah, B.A.; Qazi, G.N.; Taneja, S.C. Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 2009, 26, 72–89.
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 1997, 2, 37–43.
- Banno, N.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Tabata, K.; Nakamura, Y.; Nishimura, R.; Kimura, Y.; Suzuki, T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J. Ethnopharmacol. 2006, 107, 249–253.
- Langmead, L.; Rampton, D.S. Review article: Complementary and alternative therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 23, 341–349.
- Holtmeier, W.; Zeuzem, S.; Preiss, J.; Kruis, W.; Bohm, S.; Maaser, C.; Raedler, A.; Schmidt, C.; Schnitker, J.; Schwarz, J.; et al. Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn’s disease: Good safety profile but lack of efficacy. Inflamm. Bowel Dis. 2011, 17, 573–582.
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369.
- Krieglstein, C.F.; Anthoni, C.; Rijcken, E.J.; Laukotter, M.; Spiegel, H.U.; Boden, S.E.; Schweizer, S.; Safayhi, H.; Senninger, N.; Schurmann, G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal. Dis. 2001, 16, 88–95.
- Moussaieff, A.; Mechoulam, R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2010, 61, 1281–1293.
- Yu, G.; Xiang, W.; Zhang, T.; Zeng, L.; Yang, K.; Li, J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 225.
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae). Ann. Chim. 2007, 97, 837–844.
- Shao, Y.; Ho, C.T.; Chin, C.K.; Badmaev, V.; Ma, W.; Huang, M.T. Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med. 1998, 64, 328–331.
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267.
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S. Acetyl-11-keto-beta-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Res. Notes 2011, 4, 406.
- Khosravi Samani, M.; Mahmoodian, H.; Moghadamnia, A.; Poorsattar Bejeh Mir, A.; Chitsazan, M. The effect of Frankincense in the treatment of moderate plaque-induced gingivitis: A double blinded randomized clinical trial. Daru 2011, 19, 288–294.
- Winking, M.; Sarikaya, S.; Rahmanian, A.; Jodicke, A.; Boker, D.K. Boswellic acids inhibit glioma growth: A new treatment option? J. Neurooncol. 2000, 46, 97–103.
- Ammon, H.P. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867.
- Knaus, U.; Wagner, H. Effects of boswellic acid of Boswellia serrata and other triterpenic acids on the complement system. Phytomedicine 1996, 3, 77–80.
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2011, 162, 147–162.





