Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pawan K Agrawal | -- | 2281 | 2022-07-06 15:40:39 | | | |
| 2 | Conner Chen | Meta information modification | 2281 | 2022-07-07 02:33:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Agrawal, P.K.; Agrawal, C.; Blunden, G. Artemisia Extracts and Artemisinin-Based Antimalarials for COVID-19 Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/24869 (accessed on 07 February 2026).
Agrawal PK, Agrawal C, Blunden G. Artemisia Extracts and Artemisinin-Based Antimalarials for COVID-19 Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/24869. Accessed February 07, 2026.
Agrawal, Pawan K., Chandan Agrawal, Gerald Blunden. "Artemisia Extracts and Artemisinin-Based Antimalarials for COVID-19 Management" Encyclopedia, https://encyclopedia.pub/entry/24869 (accessed February 07, 2026).
Agrawal, P.K., Agrawal, C., & Blunden, G. (2022, July 06). Artemisia Extracts and Artemisinin-Based Antimalarials for COVID-19 Management. In Encyclopedia. https://encyclopedia.pub/entry/24869
Agrawal, Pawan K., et al. "Artemisia Extracts and Artemisinin-Based Antimalarials for COVID-19 Management." Encyclopedia. Web. 06 July, 2022.
Copy Citation
Artemisia annua (“sweet wormwood”, “qinghao”), a member of the Asteraceae family, has been traditionally used safely over the centuries to treat a variety of fevers, and notably, ‘‘intermittent fevers” and chills-related conditions, including respiratory tract infections. It also exhibit positive effects against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and COVID-19 related symptoms. A. annua is a source of artemisinin, which is active against malaria, and also exhibits potential to fight against the SARS-CoV-2 infection by inhibiting its invasion, and replication, as well as reducing oxidative stress and inflammation, and mitigating lung damage.
Artemisia
artemisinins
antiviral
1. Introduction
One of the biggest breakthroughs in fighting the COVID-19 pandemic has been the development of vaccines that provide the best strategies to prevent infection against COVID-19. Several vaccines have been approved by the U.S. Food and Drug Administration (FDA). These have been shown to be highly effective and are available to the public for emergency use authorization (EUA) and for protection against COVID-19 [1]. These vaccines are safe and effective, since, in the rare instances of breakthrough infections (where a person has been vaccinated against COVID-19), patients are significantly less likely to become hospitalized. While vaccines prevent disease occurrence, infected individuals still need other treatment options.
Viral infection happens when a virus inserts its genetic code into a host cell, forcing it to replicate, produce more viral genomic material, and then leads to the death of the host cell. During a viral infection, this process can happen at enormous rates, which leads to viral fever affecting primarily the respiratory tract system, harmful inflammation, and excessive aberrant immunological responses as the body’s immune system tries to seek out and destroy viral material and, at a later stage, it can lead to potentially deadly complications [2][3][4]. By preventing virus entry and/or its replication or clearing of cells into which the virus has already entered, effective treatments with antivirals can help to slow the spread of a person’s infection, potentially reducing the length and severity of symptoms. Thus, safe and effective antivirals responsible for restricting viral entry and/or disruption of the replication process are crucial to the pandemic response [5][6][7][8][9][10][11][12][13].
Despite vaccine developments, COVID-19 treatment still remains largely supportive with an urgent need to identify effective anticoronavirals. An attractive approach is repurposing drugs already licensed for other diseases. In this respect, several studies have been undertaken to test whether antimalarial drugs could treat COVID-19. Teas of Artemisia annua L. plants have been employed to treat malaria [14][15][16] in many African countries. Scientists are currently testing A. annua’s potential against SARS-CoV-2, as it provides a basis for a large variety of derivatives used as antimalarial drugs, collectively called “artemisinins”.
2. Traditional Use and Bioactive Compounds of Artemisia
Artemisia annua (“sweet wormwood”, “qinghao”), a member of the Asteraceae family, has been traditionally used safely over the centuries to treat a variety of fevers, and notably, ‘‘intermittent fevers” and chills-related conditions, including respiratory tract infections [17][18][19][20]. One of the most bioactive compounds identified is a sesquiterpenoid lactone, artemisinin (1), which contains an unusual 1,2,4-trioxane moiety with an endoperoxide group. This compound has been identified as an active ingredient to treat malaria infections. This unusual endoperoxide bridge is the key active site for its drug mechanism of action and provides a structural chemical base for the synthesizing of a large variety of compounds, such as dihydroartemisinin (2), β-artemether (3), and artesunate (4) (Figure 1), exhibiting greater potency, improved water solubility, and improved pharmacological properties [21]. These artemisinin derivatives are the components of artemisinin-based combination therapies (ACTs), which have been approved as front-line drugs for treating Plasmodium falciparum malaria [22][23][24]. They also show additional pharmacological benefits such as anticancer, anti-inflammatory, and immunomodulatory properties [25][26][27][28][29][30][31][32][33]. In addition, A. annua has been extensively investigated and more than 600 chemical constituents have been identified [18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40].
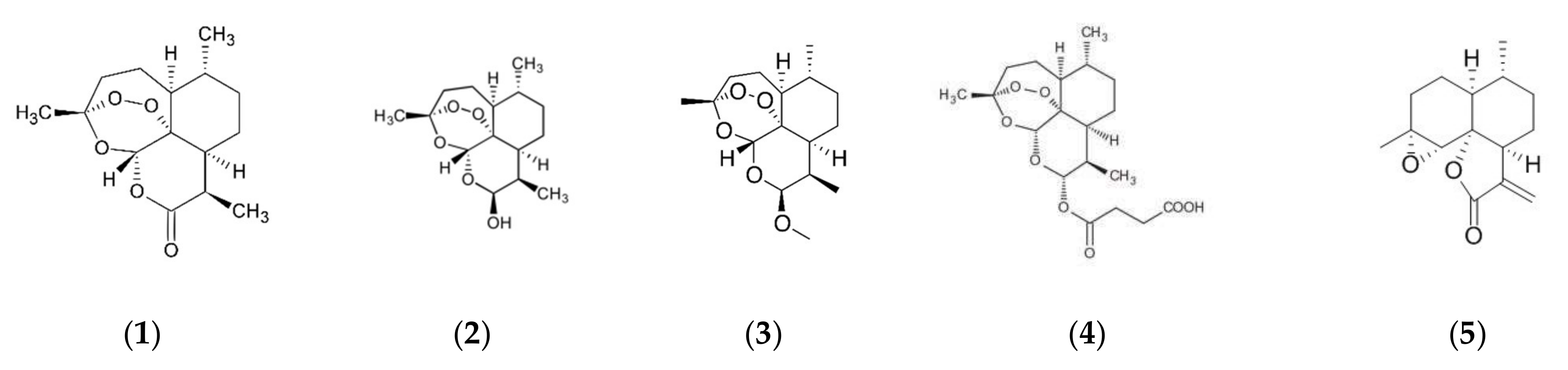
Figure 1. Chemical structure of artemisinin (1), dihydroartemisinin (2), artemether (3), artesunate (4), and arteannuanin B (5).
The artemisinin derivatives artesunate and artemether are the key ingredients of the WHO-recommended antimalaria combination therapies [41][42]. A. annua extracts and their constituents are active against several viruses, including SARS-CoV [43][44][45], suggesting the usefulness of artemisinin’s potential for drug repurposing.
3. Anti-Viral and Immune-Stimulatory Potential of Artemisia
In 2002, Lin et al., reported the use of A. annua against SARS coronavirus [46]. Interestingly, in a Vero cell-based, 3-(4,5-dimethylthiazol-2-yl-)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay for virus-induced cytopathic effect (CPE) screening analysis of medicinal plant extracts with antiviral potentials against SAR-CoV viral strain BJ001, A. annua, alongside three other plants, demonstrated a substantial inhibitory effect [46]. The ethanolic extract of whole plants of A. annua showed potent antiviral activities with 50% effective concentration (EC50) values of 34.5 (±2.6) and 39.2 (±4.1) μg/mL against the SARS-CoV-1 viral strains BJ-001 and BJ-006, respectively, with a CC50 value of 1053.0 ± 92.8 μg/mL in a cytotoxicity assay [47]. Ethnopharmacological studies of Artemisia and its constituents have also revolved around their retroviral properties [43][47][48][49][50], capacity to minimize the replication of herpes viruses [43][51][52][53][54], and activity against bovine viral diarrhoea, Epstein–Barr virus, hepatitis B virus, and hepatitis C virus [55][56][57][58][59][60][61][62]. Interestingly, derivatization enhanced the antiviral activity of artemisinin as its derivatives, i.e., artesunate, artemether, and arteether, including dimer and trimer molecules, exhibited potent antiviral activities [62]. For example, artesunate effectively inhibits human cytomegalovirus (HCMV), human herpes simplex virus (HSV), hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and polyomavirus BK [43][63][64]. Dihydroartemisinin has also shown inhibitory effects on HCMV and Zika virus [65][66], whereas artemisone, alone and in combination with other anti-HCMV agents, has been proven to be a potent HCMV inhibitor [67][68]. Artemisinin inhibited the replication of hepatitis C replicon, a single-stranded RNA virus, similar to SARS-CoV-2 [69]. A recent review by Efferth provided up-to-date information about the inhibition of viruses by artemisinin-type compounds [62].
The presence of flavonoids, such as quercetin and rutin, in Artemisia species can be associated with inhibition of activity of the main protease (Mpro), also known as chymotrypsin-like protease (CLpro), an enzyme intrinsic for replication of SARS-CoV-2 [70][71][72][73][74]. The presence of various bioactive components in A. annua seems to be responsible for its adoption as a therapeutic option against coronavirus infection. Artemisia also contains a high concentration of zinc, which has been reported to have an immunomodulation effect on the host response [75]. It should also be noted that the antioxidant ability of Artemisia has been shown to enhance immune defence [30][76]. The tea infusion of A. annua has shown potent anti-HIV activity, with a half maximal inhibitory concentration (IC50) of 2.0–14.8 µg/mL in vitro. The tea infusion was lacking in artemisinin, suggesting that the anti-HIV activity may be associated with other compounds [50].
Thus, the antiviral and antimalarial significance of A. annua and artemisinin derivatives have led to exploring their diverse pharmaceutical potentials [76][77]. Furthermore, earlier pharmacokinetic, pharmacodynamic, and cytotoxicity studies have identified additional factors that made them potential candidates for drug repurposing [78][79][80][81]. Thus, the COVID-19 pandemic outbreak has attracted attention on the efficacy and repurposing of the multifunctional properties of Artemisia and artemisinin-derived products as promising therapeutic drugs for the possible treatment of SARS-COV-2 [81][82].
Some antimalarial and/or antiviral agents, such as chloroquine (CQ), hydroxychloroquine (HCQ), and redmesivir, have been repurposed for their possible use against COVID-19 [83]. However, these may have caused cardiotoxicity concerns, as well as other after-administration side effects [84]. However, notably, artemisinin has been reported to possess a better and lower toxicity profile [85].
4. Artemisia Extracts and COVID-19
In response to the pandemic, in April 2020, a herbal tea or decoction based on Artemisia, developed by the Malagasy Institute for Applied Research (IMRA), and branded as “COVID-Organics”, was launched as a cure for COVID-19. It contains 62% Artemisia annua and a mixture, in confidential proportions, of Malagasy medicinal plants used in the composition of traditional remedies, such as antiseptics and bronchial fluidizers. President Rajoelina of Madagascar said that trials conducted on the COVID-Organics drink showed its effectiveness against the disease [86]. However, the use of a tonic containing unknown quantities of artemisinin and other constituents, over a large population, certainly raises fears of malarial parasites developing resistance. Moreover, its widespread unregulated usage as remedies for malaria, such as in tea, could result in reduced access to effective medicines and possible resistance of P. falciparum to artemisinin-based combination therapies (ACTs) [87][88][89]. Since May 2020, IMRA has been preparing an injectable form of Artemisia-derived products for patients in respiratory distress. In a recent study by Nie et al., it was shown that several Artemisia extracts, as well as Covid-Organics, at concentrations that did not affect cell viability, inhibited SARS-CoV-2 and feline coronavirus (FCoV) infection [90].
In a study related to the efficacy of A. annua extracts in high-throughput antiviral in vitro assays in VeroE6 cells, Gilmore et al., found that the leaves, after being extracted with either pure ethanol or distilled water, showed antiviral activity and the activity increased considerably when the ethanol extract was combined with coffee [91]. Extracts were added to VeroE6 cells either 1.5 h prior to infection (pretreatment (pt)] or 1 h post infection (treatment (t)), followed by a two-day incubation of the virus with extracts. The EC50 values were 173 µg/mL (pt) and 142 µg/mL (t) for the ethanolic extract; 390 µg/mL (pt) and 260 µg/mL (t) for the aqueous extract; and 176 µg/mL (pt) and 128 µg/mL (t) for the ethanolic extract and coffee, respectively [91]. With all extracts, almost complete virus inhibition was achieved at high concentrations: Cell viability assays revealed median cytotoxic concentrations (CC50) of 1044 µg/mL (A. annua ethanolic extract), 632 µg/mL (A. annua + coffee ethanolic extract), and 2721 µg/mL (A. annua aqueous extract). Selectivity indexes (SI), determined by dividing CC50 by EC50, revealed similar results. For the A. annua ethanolic extract the SIs were 6 (pt) and 7 (t), for the A. annua + coffee ethanolic extract 3 (pt) and 5 (t), and for the A. annua aqueous extract 7 (pt) and 10 (t), respectively [92]. The use of dried A. annua leaves has also been suggested as a potential therapeutic and inexpensive option for treating SARS-CoV-2 infection [92].
Recently, hot water extract obtained from dried leaves of A. annua, obtained from four different parts of the world, was tested against SARS-CoV-2, and two variants, B1.1.7 and B1.351, showed IC50 values corresponding to <12 μM artemisinin [93][94][95]. It was also noticed that the antiviral effect of the extracts decreased in inverse correlation with the artemisinin content. The failure of the IC50 to decrease as the concentration of artemisinin and/or flavonoids increased, indicated that these were not the only active factors, but may, in fact, be antagonists of the bioactive component. The plant possesses compounds that inhibit inflammation and the formation of scar-like tissues known as fibrosis, which also affect patients with COVID-19, but this warrants further investigation [93][94][95].
In South Africa, teas of Artemisia afra were used without in vitro or clinical data [96]. A. afra, in contrast to A. annua, does not contain artemisinin. Due to fears that artemisinin combination therapies against malaria may become ineffective if artemisinin-based treatments are used against COVID-19 [97], the WHO recently called for investigations into the efficacy of plant-based traditional medicines [98]. Human clinical trials will be required to answer the question whether the traditional medicines may indeed have an effect in either preventing or treating COVID-19 infections.
A study by Zhou et al. [99] related to the in vitro efficacy of A. annua ethanolic and aqueous extracts, artemisinin, artesunate, and artemether against SARS-CoV-2 spike glycoprotein revealed that treatment with extracts and compounds inhibited SARS-CoV-2 infection of VeroE6 cells, human hepatoma Huh7.5 cells, and human lung cancer A549-hACE2 cells. In treatment assays, the range of 50% effective concentrations (EC50) ranged between 83 and 260 µg/mL for A. annua extracts [99].
The aqueous fraction of A. annua, after the extraction of artemisinin, has been shown to regulate the expression of proinflammatory cytokines, matrix metalloproteinases, and NF-κB; to promote cell cycle arrest; to drive reactive oxygen species production; and to induce Bak or Bax-apoptosis [17]. It has also been reported that among the three different ethanol extracts (50%, 70%, and 95%), only the 70% and 95% extracts showed any positive antiviral activity, and the 70% extract was considered to be optimum for further investigation, as the 95% ethanol extract could be associated with cellular toxicity [100].
In a recent study, hot-water extracts of A. annua were found to be active against SARS-CoV-2 and its alpha, beta, gamma, delta, and kappa variants. The A. annua cultivar with the lowest artemisinin content had the lowest (most effective) IC50 against gamma, delta, and kappa variants, thus, demonstrating the potential of the extracts as treatments against this virus [101]. However, clinical studies are required to further evaluate the utility of these teas/drinks/extracts for COVID-19 prevention.
5. Artemisia Supplement and Formulation
The Max Planck Institute of Colloids and Interfaces (Germany) is collaborating with a company in the USA, ArtemiLife Inc., to explore the effect of A. annua plant extract and artemisinin derivatives against SARS-CoV-2 [102]. ArtemiLife is also marketing A. annua herbal tea and coffee directly to consumers, but is careful to note that its tea and coffee are “not intended to diagnose, treat, cure or prevent any disease” and cautions that common side effects may include hearing loss and liver problems. However, it also claims that drinking two servings per day will help consumers “maintain an active shield,” thus, “protecting well-being.” The firm’s coffee contains 0.4 g A. annua per serving, and its tea containes 0.23 g. The dried leaves of A. annua usually contain around 1% artemisinin, therefore, consuming the drinks would offer much lower doses than typical ACTs [103].
The product, ARTIVeda/PulmoHeal, delivered in a gelatin capsule, is an Ayurvedic drug against COVID-19. The drug is a formulated extract of Artemisia for oral delivery of artemisinin for growth factor-β (TGF-β) inhibition. It targets COVID-19 by suppressing both viral replication and clinical symptoms, i.e., both viral and immune driven pathologies (ARDS and cytokine storm) that arise from viral infection. With treatment, viral replication is suppressed, IFNβ is induced, and innate and adaptive immune responses are suppressed. The clinical studies on patients with mild and moderate COVID-19 have suggested that administration of artemisinin 500 mg capsules once daily for 5 days may lead to a faster recovery [103].
In a controlled Phase II trial, patients with COVID-19 received ArtemiC, a medical spray (containing artemisinin, curcumin, frankincense resin from the Boswellia sacra tree, and vitamin C, in a nanoparticular formulation for spray administration), in addition to standard care; improvement in the patients’ condition was recorded [104].
References
- Billingsley, A. FDA COVID-19 Vaccine Approval: Live Updates on Pfizer, Moderna, and J&J Vaccines. Available online: https://www.goodrx.com/conditions/covid-19/fda-covid-19-vaccine-approval-updates (accessed on 14 February 2022).
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the leading cause of death in the United States. JAMA 2021, 325, 123–124.
- Faust, J.S.; Krumholz, H.M.; Du, C.; Mayes, K.D.; Lin, Z.; Gilman, C.; Walensky, R.P. All-cause excess mortality and COVID-19-related mortality among US adults aged 25–44 years, March–July 2020. JAMA 2021, 325, 785–787.
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Feng, C.; Zhang, Z.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374.
- Muratov, E.N.; Amaro, R.; Andrade, C.H.; Brown, N.; Ekins, S.; Fourches, D.; Isayev, O.; Kozakov, D.; Medina-Franco, J.L.; Merz, K.M.; et al. A critical overview of computational approaches employed for COVID-19 drug discovery. Chem. Soc. Rev. 2021, 50, 9121–9151.
- Qazi, S.; Das, S.; Khuntia, B.K.; Sharma, V.; Sharma, S.; Sharma, G.; Raza, B.K. In silico molecular docking and molecular dynamic simulation analysis of phytochemicals from Indian foods as potential inhibitors of SARS-CoV-2 RdRp and 3CLpro. Nat. Prod. Commun. 2021, 16, 1–12.
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Pharmacological significance of hesperidin and hesperetin, two citrus flavonoids, as promising antiviral compounds for prophylaxis against and combating COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X211042540.
- Doharey, P.K.; Singh, V.; Rao Gedda, M.; Sahoo, A.K.; Varadwaj, P.K.; Sharma, B. In silico study indicates antimalarials as direct inhibitors of SARS-CoV-2-RNA dependent RNA polymerase. J. Biomol. Struct. Dyn. 2021, 1–18.
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444.
- Remali, J.; Aizat, W.M. A review on plant bioactive compounds and their modes of action against coronavirus infection. Front. Pharmacol. 2021, 11, 589044.
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Naringenin as a possible candidate against SARS-CoV-2 infection and in the pathogenesis of COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X211066723.
- Aherfi, S.; Pradines, B.; Devaux, C.; Honore, S.; Colson, P.; Scola, B.L.; Raoult, D. Drug repurposing against SARS-CoV-1, SARS-CoV-2 and MERS-CoV. Future Microbiol. 2021, 16, 1341–1370.
- Boukhatem, M.N.; Setzer, W.N. Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: Future perspectives. Plants 2020, 9, 800.
- Ogwang, P.E.; Ogwal, J.O.; Kasasa, S.; Olila, D.; Ejobi, F.; Kabasa, D.; Obua, C. Artemisia annua L. infusion consumed once a week reduces risk of multiple episodes of malaria: A randomized trial in a Ugandan community. Trop. J. Pharm. Res. 2012, 11, 445–453.
- Daddy, N.B.; Kalisya, L.M.; Bagire, P.G.; Watt, R.L.; Towler, M.J.; Weathers, P.J. Artemisia annua dried leaf tablets treated malaria resistant to ACT and i.v. artesunate: Case reports. Phytomedicine 2017, 32, 37–40.
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lu, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine 2019, 57, 49–56.
- Cheong, D.H.J.; Tan, D.W.S.; Wong, F.W.S.; Tran, T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020, 158, 104901.
- Sadiq, A.; Hayat, M.Q.; Ashraf, M. Ethnopharmacology of Artemisia annua L.: A review. In Artemisia annua—Pharmacology and Biotechnology; Aftab, T., Ferreira, J.F.S., Khan, M.M.A., Naeem, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–25.
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354.
- Kshirsagar, S.G.; Rao, R.V. Antiviral and immunomodulation effects of Artemisia. Medicina 2021, 57, 217.
- De Ridder, S.; van der Kooy, F.; Verpoorte, R. Artemisia annua as a self-reliant treatment for malaria in developing countries. J. Ethnopharmacol. 2008, 120, 302–314.
- Klayman, D.L. Artemisia annua: From weed to respectable antimalarial plant. In Human Medicinal Agents from Plants; Kinghorn, A.D., Balandrin, M.F., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 242–255.
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83.
- Pinheiro, L.C.S.; Feitosa, L.M.; Silveira, F.F.D.A.; Boechat, N. Current antimalarial therapies and advances in the development of semisynthetic artemisinin derivatives. An. Acad. Bras. Ciênc. 2018, 90, 1251–1271.
- An, J.; Minie, M.; Sasaki, T.; Woodward, J.J.; Elkon, K.B. Antimalarial drugs as immune modulators: New mechanisms for old drugs. Annu. Rev. Med. 2017, 68, 317–330.
- Shi, C.; Li, H.; Yang, Y.; Hou, L. Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediat. Inflamm. 2015, 2015, 435713.
- Alesaeidi, S.; Miraj, S. A systematic review of anti-malarial properties, immunosuppressive properties, anti-inflammatory properties, and anti-cancer properties of Artemisia annua. Electron. Physician 2016, 8, 3150–3155.
- Rao, R.V. Artemisia—Antiviral and Immunomodulation Effects. Available online: https://encyclopedia.pub/8738 (accessed on 16 February 2022).
- Khanal, P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Monatsh. Chem. 2021, 152, 387–400.
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170.
- Mesa, L.E.; Lutgen, P.; Velez, I.D.; Segura, A.M.; Sara, M.; Robledo, S.M. Artemisia annua L., potential source of molecules with pharmacological activity in human diseases. Am. J. Phytomed. Clin. Ther. 2015, 3, 436–445.
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650.
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med. 2021, 87, 584–599.
- Agrawal, P.K.; Vishwakarma, R.A.; Jain, D.C.; Roy, R. High field NMR spectroscopic studies of arteannuin B and a reappraisal of the structure of arteannuin C. Phytochemistry 1991, 30, 3469–3471.
- Agrawal, P.K.; Bishnoi, V. Sterol and taraxastane derivatives from Artemisia annua and a rational approach based upon C-13 NMR for the identification of skeletal type of amorphane sesquiterpenoids. Ind. J. Chem. 1996, 35B, 86–88.
- Singh, A.K.; Pathak, V.; Agrawal, P.K. Annphenone, a phenolic acetophenone from Artemisia Annu. Phytochemistry 1997, 44, 555–557.
- Brown, G.D. The biosynthesis of Artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698.
- Qin, D.P.; Li, H.B.; Pang, Q.Q.; Huang, Y.X.; Pan, D.B.; Su, Z.Z.; Yao, X.J.; Yao, X.S.; Xiao, W.; Yu, Y. Structurally diverse sesquiterpenoids from the aerial parts of Artemisia annua (Qinghao) and their striking systemically anti-inflammatory activities. Bioorg. Chem. 2020, 103, 104221.
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a traditional plant brought to light. Int. J. Mol. Sci. 2020, 21, 4986.
- Bisht, D.; Kumar, D.; Kumar, D.; Kamal Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474.
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; WHO: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf (accessed on 1 May 2022).
- Zeyuan, L. Artemisinin chemical research. In Artemisinin-Based and Other Antimalarials; Guoqiao, L., Ying, L., Zelin, L., Meiyi, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–175.
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811.
- Wang, X.; Zheng, B.; Ashraf, U.; Zhang, H.; Cao, C.; Li, Q.; Chen, Z.; Imran, M.; Chen, H.; Cao, S.; et al. Artemisinin inhibits the replication of flaviviruses by promoting the type I interferon production. Antivir. Res. 2020, 179, 104810.
- Desrosiers, M.R.; Mittelman, A.; Weathers, P.J. Dried leaf Artemisia annua improves bioavailability of artemisinin via cytochrome P450 inhibition and enhances artemisinin efficacy downstream. Biomolecules 2020, 10, 254.
- Lin, L.; Han, Y.; Yang, Z. Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western Medicine. Chin. J. Integr. Trad. Western Med. 2003, 23, 409–413.
- Li, S.Y.; Chen, C.; Zhang, H.Q.; Guo, H.Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.N.; Yu, J.; Xiao, P.G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23.
- Jana, S.; Iram, S.; Thomas, J.; Hayat, M.Q.; Pannecouque, C.; Dehaen, W. Application of the triazolization reaction to afford dihydroartemisinin derivatives with anti-HIV activity. Molecules 2017, 22, 303.
- Laila, U.; Akram, M.; Shariati, M.A.; Hashmi, A.M.; Akhtar, N.; Tahir, I.M.; Ghauri, A.O.; Munir, N.; Riaz, M.; Akhter, N.; et al. Role of medicinal plants in HIV/AIDS therapy. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1063–1073.
- Lubbe, A.; Seibert, I.; Klimkait, T.; Van der Kooy, F. Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua. J. Ethnopharmacol. 2012, 141, 854–859.
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J. Clin. Virol. 2009, 46, 24–28.
- Naesens, L.; Bonnafous, P.; Agut, H.; De Clercq, E. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 2006, 37, S69–S75.
- Nagamune, K.; Moreno, S.N.; Sibley, L.D. Artemisinin-resistant mutants of Toxoplasma gondii have altered calcium homeostasis. Antimicrob. Agents Chemother. 2007, 51, 3816–3823.
- Karamoddini, M.; Emami, S.; Ghannad, M.; Sani, E.; Sahebkar, A. Antiviral activities of aerial subsets of Artemisia species against Herpes Simplex virus type 1 (HSV1) in vitro. Asian Biomed. 2017, 5, 63–68.
- Dai, R.; Xiao, X.; Peng, F.; Li, M.; Gong, G. Artesunate, an antimalarial drug, has a potential to inhibit HCV replication. Virus Genes 2016, 52, 22–28.
- Paeshuyse, J.; Coelmont, L.; Vliegen, I.; Van hemel, J.; Vandenkerckhove, J.; Peys, E.; Sas, B.; De Clercq, E.; Neyts, J. Hemin potentiates the anti-hepatitis C virus activity of the antimalarial drug artemisinin. Biochem. Biophys. Res. Commun. 2006, 348, 139–144.
- Qian, R.S.; Li, Z.; Yu, J.; Ma, D.J. The immunologic and antiviral effect of qinghaosu. J. Trad. Chin. Med. 1982, 2, 271–276.
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castano, B.; Macias, R.I.R.; Briz, O.; Marin, J.J.G. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “In Vitro” replicative system. Antiviral. Res. 2005, 68, 75–83.
- Romero, M.R.; Serrano, M.A.; Vallejo, M.; Efferth, T.; Alvarez, M.; Marin, J.J. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 2006, 72, 1169–1174.
- Haq, F.U.; Roman, M.; Ahmad, K.; Rahman, S.U.; Shah, S.M.; Suleman, N.; Ahmad, I.; Ullah, W. Artemisia annua: Trials are needed for COVID-19. Phytother. Res. 2020, 34, 2423–2424.
- Canivet, C.; Menasria, R.; Rhéaume, C.; Piret, J.; Boivin, G. Valacyclovir combined with artesunate or rapamycin improves the outcome of herpes simplex virus encephalitis in mice compared to antiviral therapy alone. Antivir. Res. 2015, 123, 105–113.
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737.
- Raffetin, A.; Bruneel, F.; Roussel, C.; Thellier, M.; Buffet, P.; Caumes, E.; Jauréguiberry, S. Use of artesunate in non-malarial indications. Med. Mal. Infect. 2018, 48, 238–249.
- Shapira, M.Y.; Resnick, I.B.; Chou, S.; Neumann, A.U.; Lurain, N.S.; Stamminger, T.; Caplan, O.; Saleh, N.; Efferth, T.; Marschall, M.; et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2008, 46, 1455–1457.
- Flobinus, A.; Taudon, N.; Desbordes, M.; Labrosse, B.; Simon, F.; Mazeron, M.C.; Schnepf, N. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J. Antimicrob. Chemother. 2014, 69, 34–40.
- Han, Y.; Pham, H.T.; Xu, H.; Quan, Y.; Mesplede, T. Antimalarial drugs and their metabolites are potent Zika virus inhibitors. J. Med. Virol. 2019, 91, 1182–1190.
- Oiknine-Djian, E.; Bar-On, S.; Laskov, I.; Lantsberg, D.; Haynes, R.K.; Panet, A.; Wolf, D.G. Artemisone demonstrates synergistic antiviral activity in combination with approved and experimental drugs active against human cytomegalovirus. Antivir. Res. 2019, 172, 104639.
- Oiknine-Djian, E.; Weisblum, Y.; Panet, A.; Wong, H.N.; Haynes, R.K.; Wolf, D.G. The artemisinin derivative artemisone is a potent inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2018, 62, e00288-18.
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le, T.N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS ONE 2013, 8, e81783.
- Jo, S.; Kim, H.; Kim, S.; Shin, D.H.; Kim, M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Design 2019, 94, 2023–2030.
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151.
- Solnier, J.; Fladerer, J.P. Flavonoids: A complementary approach to conventional therapy of COVID-19? Phytochem. Rev. 2021, 20, 773–795.
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Commun. 2020, 15, 1934578X20976293.
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A potential antiviral for repurposing as a SARS-CoV-2 main protease (Mpro) inhibitor. Nat. Prod. Commun. 2021, 16, 1934578X21991723.
- Honscheid, A.; Rink, L.; Haase, H. T-lymphocytes: A target for stimulatory and inhibitory effects of zinc ions. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 132–144.
- Brisibe, E.A.; Umoren, U.E.; Brisbe, F.; Magalhaes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246.
- Liu, X.; Cao, J.; Huang, G.; Zhao, Q.; Shen, J. Biological activities of artemisinin derivatives beyond malaria. Curr. Top. Med. Chem. 2019, 19, 205–222.
- Li, J.; Zhang, C.; Gong, M.; Wang, M. Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria: Pharmacodynamic and pharmacokinetic studies. Phytother. Res. 2018, 32, 1415–1420.
- Rath, K.; Taxis, K.; Walz, G.; Gleiter, C.H.; Li, S.M.; Heide, L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L (annual wormwood). Am. Trop. Med. Hyg. 2004, 70, 128–132.
- Radulovic, N.S.; Randjelovic, P.J.; Stojanovic, N.M.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Ilic, I.R.; Djordjevic, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49.
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388.
- Zyad, A.; Tilaoui, M.; Jaafari, A.; Oukerrou, M.A.; Mouse, H.A. More insights into the pharmacological effects of artemisinin. Phytother. Res. 2018, 32, 216–229.
- Law, S.; Leung, A.W.; Xu, C. Is the traditional Chinese herb “Artemisia annua” possible to fight against COVID-19? Integr. Med. Res. 2020, 9, 100474.
- Bolarin, J.A.; Oluwatoyosi, M.A.; Orege, J.I.; Ayeni, E.A.; Ibrahim, Y.A.; Adeyemi, S.B.; Tiamiyu, B.B.; Gbadegesin, L.A.; Akinyemi, T.O.; Odoh, V.K.; et al. Therapeutic drugs for SARS-CoV-2 treatment: Current state and perspective. Int. Immunopharmacol. 2020, 90, 107228.
- Yang, B.; Zhou, S.; Li, C.; Wang, Y. Toxicity and side effects of artemisiae annuae CQ-189. J. Chin. Mater. Med. 2010, 35, 204–207.
- Nordling, L. Unproven herbal remedy against COVID-19 could fuel drug-resistant malaria, scientists warn. Science 2020.
- Kapepula, P.M.; Kabengele, J.K.; Kingombe, M.; Bambeke, F.V.; Tulkens, P.M.; Kishabongo, A.S.; Decloedt, E.; Zumla, A.; Tiberi, S.; Suloeman, F.; et al. Artemisia spp. derivatives for COVID-19 treatment: Anecdotal use, political hype, treatment potential, challenges, and road map to randomized clinical trials. Am. J. Trop. Med. Hyg. 2020, 103, 960–964.
- Gendrot, M.; Duflot, I.; Boxberger, M.; Delandre, O.; Jardot, P.; Bideau, M.L.; Andreani, J.; Fonta, I.; Mosnier, J.; Rolland, C.; et al. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: In vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int. J. Infect. Dis. 2020, 99, 437–440.
- Danis, M. Proposal for treatment of malaria with Artemisia leaves. Bull. Acad. Natl. Med. 2019, 203, 122–123.
- Nie, C.; Trimpert, J.; Moon, S.; Haag, R.; Gilmore, K.; Kaufer, B.B.; Seeberger, P.H. In vitro efficacy of Artemisia extracts against SARS-CoV-2. Virol. J. 2021, 18, 182.
- Gilmore, K.; Zhou, Y.; Ramirez, S.; Pham, L.V.; Fanhoe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; Bukh, J.; Osterrieder, K.; et al. In vitro efficacy of Artemisinin-based treatments against SARS-CoV-2. bioRxiv 2020.
- Artemisia Derivative Affects Replication of SARS-CoV-2. Available online: https://www.medindia.net/news/extract-of-artemisia-affects-replication-of-sars-cov-2-199351-1.html (accessed on 10 April 2022).
- Artemisinin Raises Hopes and Fears amid COVID-19. Available online: https://cen.acs.org/biological-chemistry/infectious-disease/Artemisinin-raises-hopes-fears-amid-COVID-19/98/i21 (accessed on 10 April 2022).
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts prevent in vitro replication of SARS-CoV-2. bioRxiv 2020.
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016.
- Dandara, C.; Dzobo, K.; Chirikure, S. COVID-19 Pandemic and Africa: From the situation in Zimbabwe to a case for precision herbal medicine. Omics 2020, 25, 209–212.
- Tih, F. WHO Holds Meeting with African Traditional Medicine Experts. 2020. Available online: https://www.aa.com.tr/en/africa/who-holds-meeting-with-african-traditional-medicine-experts/1838004 (accessed on 31 January 2021).
- World Health Organization. Regional Office for Africa. WHO Supports Scientifically-Proven Traditional Medicine. 2020. Available online: https://www.afro.who.int/news/who-supports-scientifically-proven-traditional-medicine (accessed on 31 January 2021).
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnoe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571.
- Runestad, T. Available online: https://www.naturalproductsinsider.com/herbs-botanicals/herb-discovered-have-activity-against-sars-cov-2-virus (accessed on 10 November 2021).
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Towler, M.J.; Weathers, P.J. Artemisia annua L. hot-water extracts show potent activity in vitro against Covid-19 variants including delta. J. Ethnopharmacol. 2022, 284, 114797.
- MPIKG. Max Planck Institute for Colloids and Interfaces Press Release. Available online: https://www.mpikg.mpg.de/6288044/news_publicatio n_14663263_transferred?c=132305 (accessed on 10 May 2020).
- Trieu, V.; Saund, S.; Rahate, P.V.; Barge, V.B.; Naik, S.; Windlass, H.; Uckun, F.M. Targeting TGF-β pathway with COVID-19 drug candidate ARTIVeda/PulmoHeal accelerates recovery from mild-moderate COVID-19. Clin. Investig. 2021, 11, 10–18.
- Health Care. MGC Pharmaceutical’s (ASX:MXC) ArtemiC Combats COVID-19. Available online: https://themarketherald.com.au/mgc-pharmaceuticals-asxmgc-artemic-combats-covid-19-2020-12-15/ (accessed on 15 December 2020).
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
07 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




