Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jenni Ho | -- | 1493 | 2022-06-30 16:19:14 | | | |
| 2 | Peter Tang | Meta information modification | 1493 | 2022-07-01 03:01:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ho, J.; Chaiswing, L.; Clair, D.K.S. Extracellular Vesicles and Cancer Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/24696 (accessed on 07 February 2026).
Ho J, Chaiswing L, Clair DKS. Extracellular Vesicles and Cancer Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/24696. Accessed February 07, 2026.
Ho, Jenni, Luksana Chaiswing, Daret K. St. Clair. "Extracellular Vesicles and Cancer Therapy" Encyclopedia, https://encyclopedia.pub/entry/24696 (accessed February 07, 2026).
Ho, J., Chaiswing, L., & Clair, D.K.S. (2022, June 30). Extracellular Vesicles and Cancer Therapy. In Encyclopedia. https://encyclopedia.pub/entry/24696
Ho, Jenni, et al. "Extracellular Vesicles and Cancer Therapy." Encyclopedia. Web. 30 June, 2022.
Copy Citation
Oxidative stress plays a significant role in cancer development and cancer therapy, and is a major contributor to normal tissue injury. The unique characteristics of extracellular vesicles (EVs) have made them potentially useful as a diagnostic tool in that their molecular content indicates their cell of origin and their lipid membrane protects the content from enzymatic degradation. In addition to their possible use as a diagnostic tool, their role in how normal and diseased cells communicate is of high research interest. The most exciting area is the association of EVs, oxidative stress, and pathogenesis of numerous diseases.
extracellular vesicles
oxidative stress
4-hydroxy-2-nonenal
cancer
cancer therapy
1. Introduction
Extracellular vesicles (EVs) are membrane-enclosed particles that contain molecular content that is excreted from most cells and they are able to modulate downstream targets following uptake [1][2]. Isolated molecular content within EVs may provide insight into the interior state of a cell [1]. The lipid membrane of EVs protects the molecular content from enzymatic degradation, making these organelles promising potential diagnostic and drug delivery tools [3][4]. The role of EVs in normal and pathophysiological cell interactions is being extensively researched [5][6][7][8][9]. For example, while EVs have been identified as playing a role in normal cell-to-cell communication, when homeostasis is altered in a system (such as an increase in oxidative stress), EV content may change and alter downstream targets [2][8][10]. The role of EVs in normal and pathophysiological processes warrants continued research to determine the potential utilization of EVs in translational research, since EVs can be isolated from body fluids, providing biochemical insights into patients. However, the role of EVs and the molecular content of EVs following cancer therapy have not been fully elucidated, in particular, those EVs with increased levels of oxidative damage following cancer therapy.
Advancements in diagnostic methods, screening technology, and cancer treatments have led to a steady decline in the cancer death rate since the 1990s [11]. Specifically, a 2018 review of pediatric oncology patients showed that the death rates for both children (ages 0 to 14) and adolescents (15 to 19) had declined by more than half since 1975 (from 4.9 to 2.0 in children and from 5.9 to 2.9 in adolescents) [11]. Ironically, as cancer therapy becomes more effective, more patients are surviving cancer and living longer, but they are often living with one or more of the unintended consequences caused by therapy. A prominent consequence observed in pediatric cancer survivors is cancer therapy-induced cognitive impairment [12]. While the underlying pathogenesis of cognitive impairment is complex, a growing body of evidence implicates oxidative damage, which results from an imbalance in the reduction-oxidation (redox) regulatory system where the amount of oxidants exceeds the capacity of the antioxidant system to remove the excessive amount of reactive oxygen species (ROS), an important mechanism contributing to damage in the brain microenvironment [13]. In particular, the role of 4-hydroxy-2-nonenal (HNE, a highly reactive end-product of lipid peroxidation) in neurodegenerative disorders is well-established and HNE has been shown to be elevated in a number of different diseases [14][15][16][17][18].
The consequences of cancer therapy on pediatric and adolescent patients, who are still developing neurocognitive capabilities and who have the potential to live long and productive lives, is critically important to address [19][20]. It has been demonstrated that oxidative stress may be a contributing factor to the off-target tissue effects in patients receiving cancer therapy, since at least 50% of current chemotherapies are associated with increased ROS production, and radiation therapy utilizes free radicals to exert its therapeutic effects [21][22]. In addition to the direct impact of oxidative stress in contributing to off-target tissue side effects (particularly, to the brain [23][24]), pro-inflammatory cytokines such as TNF-α have also been demonstrated to contribute to the negative consequences of oxidative stress by increasing ROS production and mitochondrial dysfunction [25][26][27].
2. Extracellular Vesicles: Biogenesis and Characterization
EVs are membrane-enclosed particles that contain molecular components specific to their cell of origin and circulate freely throughout the body [28]. Originally believed to be a mechanism for the cell to dispose of unwanted molecular products that could not be degraded by other methods, EVs are now known to be conduits of cell-to-cell communication. Nucleic acids (e.g., mRNA and miRNA) are often among their contents, and they have the ability to alter the phenotype of their target cell following internalization [29]. The classification of EVs encompasses a highly heterogeneous group of extracellular particles. The two major groups of EVs are based primarily on their size but also on their biogenesis, contents, and the physiological roles that they play. Even though it remains a challenge to separate the two distinct groups during application [30], it is important to highlight the two main groups that form the generic term “extracellular vesicles”.
Exosomes constitute the first category of EVs. They typically range from 30 to 150 nm in diameter and are derived from the endosomal membrane budding inward, forming intraluminal vesicles (ILVs) during multivesicular endosome (MVE) maturation [1]. These liposomal particles are then secreted from the cell upon the fusion of MVE with the cell surface lipid bilayer. Due to their MVE origin (also known as multivesicular bodies, MVBs), they contain markers that are associated with the endosomal pathway [2]. Exosomes are secreted by various cell types in the body and may play a role in cell-to-cell communication and elimination of undesirable products within the cell, and are part of the normal and pathophysiological processes in the body [2].
Microvesicles are the second category of EVs. These molecules are larger in diameter (100 to 1000 nm) than exosomes and have a different route of biogenesis. Microvesicles are formed by budding of the plasma membrane and may possess markers such as integrins and P-selectin [1][2]. The outward budding of the plasma membrane due to apoptosis that creates an apoptotic body has long been known; however, the study of microvesicles budding from healthy cells is a more recent area of interest. The current understanding of microvesicle biogenesis is that flippases, floppases, scramblases, and calpain rearrange the composition of the phospholipid bilayer, allowing for physical bending of the membrane, and also allowing for microvesicle formation to occur more efficiently [1]. Figure 1 provides a schematic of the differences in biogenesis between the two different types of EVs.
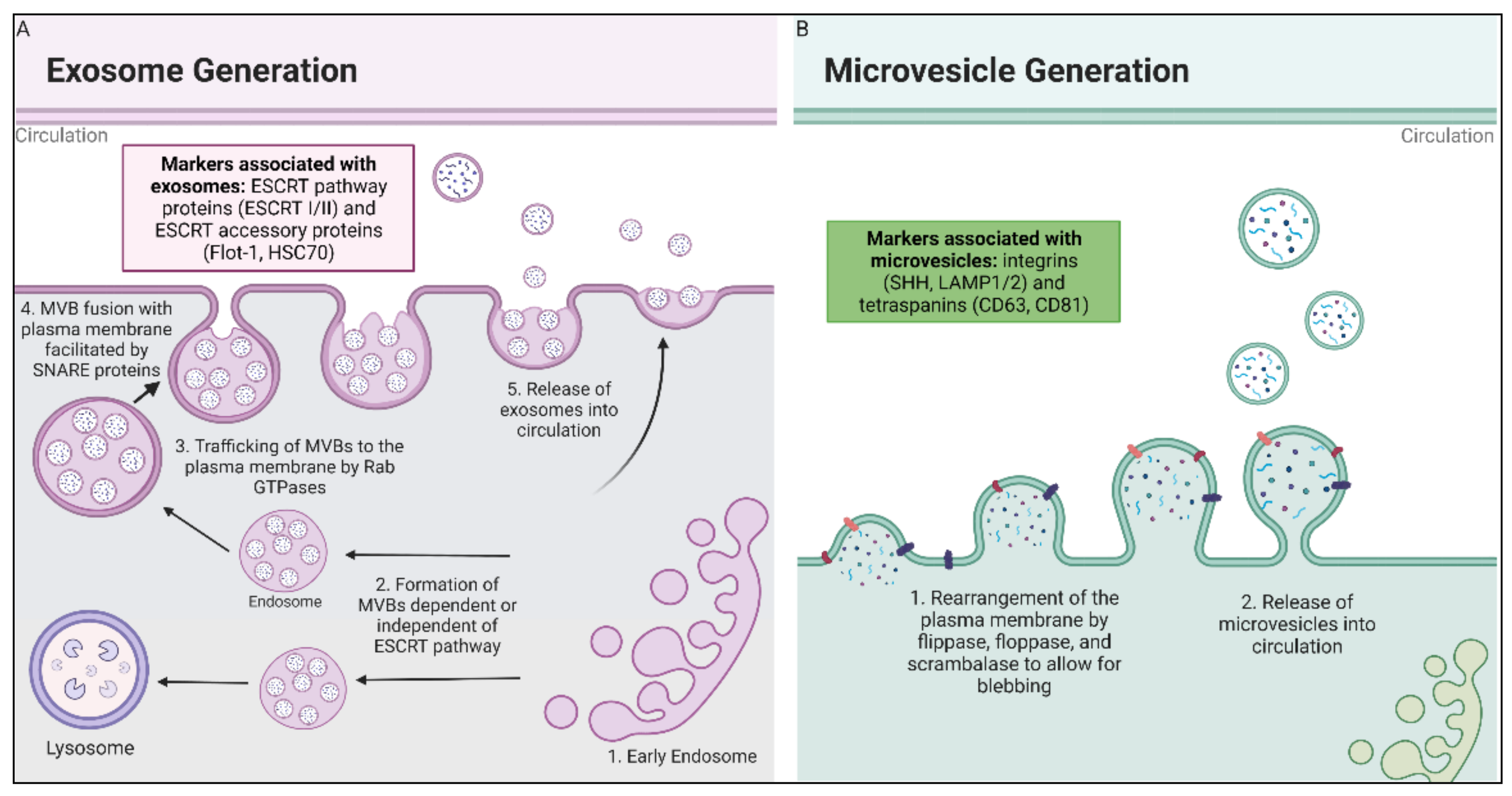
Figure 1. Biogenesis of exosomes and microvesicles. (A) Exosomes are derived from fusion of multivesicular endosomal bodies with the plasma membrane, which releases exosomes into extracellular space. Early endosomes are formed by the inward budding of either the plasma membrane or the Golgi Apparatus, where they then mature to late endosomes and become MVBs. The MVBs are then trafficked to the cell membrane, where they are fused with the plasma membrane by SNARE proteins [28][31][32]. (B) Microvesicles are formed by the rearrangement of the plasma membrane facilitated by flippase, floppase, scrambalase, and calpain, leading to the budding of the microvesicles from the cell membrane [33].
3. Oxidative Stress: Pro-Oxidants and Antioxidants
Oxidative stress results from increased ROS or reactive nitrogen species (RNS), where the ROS or RNS pro-oxidant conditions cannot be mitigated due to inefficient antioxidant systems or dysregulation of the redox pathways that control the balance between pro-oxidants and antioxidants. The resulting oxidative stress can cause oxidative damage, which has been demonstrated to increase with age and has been linked with numerous neurodegenerative diseases [16][17]. Oxidative stress has also been linked with increased risk of developing cancer, which is due, in part, to genetic mutations that favor the development of cancer and which contribute to a pro-tumorigenic microenvironment [34][35][36]. Therefore, the careful regulation of redox balance is critical for normal physiological function.
4. Role and Function of EVs: Current Understanding and Future Directions
The fundamental role of EVs in both normal and pathophysiological conditions has been demonstrated, and more research is underway to explore the potential use of EVs in the clinical setting. EVs play a variety of roles in maintaining normal physiological functions within the body. Their content allows for the delivery of effectors (e.g., transcription factors, nucleic acids, oncogenes) to recipient cells or for the proteins or lipids they contain to activate surface receptors [37]. Many of the earlier studies elucidating the role of EVs in both normal and pathological physiologies emphasized the use of EVs to transport nucleic acids to target cells, which results in alterations in gene expression. Valadi et al. demonstrated the various kinds of nucleic acids (i.e., mRNA, miRNA) found in exosomes released from both human and murine mast cells, and elucidated how delivery of these nucleic acids altered the protein expression in target cells after they were harvested [29]. The role of EVs in the immune system has also piqued interest in recent years; their ability to present antigens and trigger different immune responses is of interest to immunologists, who are investigating their potential use in the clinic [9]. Additionally, miRNAs within exosomes have been demonstrated to modulate inflammation responses, revealing their contribution to compensatory mechanisms that maintain homeostasis [38]. EVs have also been shown to be released from a variety of stem cell populations, where they help maintain plasticity and are able to promote proliferation and vascularization in damaged tissues [39]. Moreover, EVs have also been shown to take part in the coagulation cascade, neuronal communication in the brain, and cell phenotype modulation [8][37].
References
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 2180–2181.
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156.
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476.
- Peinado, H.; Alec kovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.M.; et al. Corrigendum: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2016, 22, 1502.
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624.
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011, 46, 409–418.
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593.
- Harmati, M.; Gyukity-Sebestyen, E.; Dobra, G.; Janovak, L.; Dekany, I.; Saydam, O.; Hunyadi-Gulyas, E.; Nagy, I.; Farkas, A.; Pankotai, T.; et al. Small extracellular vesicles convey the stress-induced adaptive responses of melanoma cells. Sci. Rep. 2019, 9, 15329.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Vitali, M.; Ripamonti, C.I.; Roila, F.; Proto, C.; Signorelli, D.; Imbimbo, M.; Corrao, G.; Brissa, A.; Rosaria, G.; de Braud, F.; et al. Cognitive impairment and chemotherapy: A brief overview. Crit. Rev. Oncol. Hematol. 2017, 118, 7–14.
- Halliwell, B.G.J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015.
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630.
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504.
- Sultana, R.; Butterfield, D.A. Role of oxidative stress in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 341–353.
- Di Domenico, F.; Tramutola, A.; Butterfield, D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017, 111, 253–261.
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012, 17, 1590–1609.
- Green, J.L.; Knight, S.J.; McCarthy, M.; De Luca, C.R. Motor functioning during and following treatment with chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 1261–1266.
- Anderson, F.S.; Kunin-Batson, A.S. Neurocognitive late effects of chemotherapy in children: The past 10 years of research on brain structure and function. Pediatr. Blood Cancer 2009, 52, 159–164.
- Chen, Y.; Jungsuwadee, P.; Vore, M.; Butterfield, D.A.; St Clair, D.K. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol. Interv. 2007, 7, 147–156.
- Hall, E.J. Radiobiology for the Radiologist; Medical Dept., Harper & Row: Hagerstown, MD, USA, 1973.
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25.
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205.
- Tangpong, J.; Cole, M.P.; Sultana, R.; Joshi, G.; Estus, S.; Vore, M.; St Clair, W.; Ratanachaiyavong, S.; St Clair, D.K.; Butterfield, D.A. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol. Dis. 2006, 23, 127–139.
- Ren, X.; Keeney, J.T.R.; Miriyala, S.; Noel, T.; Powell, D.K.; Chaiswing, L.; Bondada, S.; St Clair, D.K.; Butterfield, D.A. The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-alpha. Free Radic. Biol. Med. 2019, 134, 1–8.
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423.
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. (Weinh) 2020, 8, 2003505.
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Jahn, R.; Scheller, R.H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643.
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166.
- Pap, E.; Pallinger, E.; Pasztoi, M.; Falus, A. Highlights of a new type of intercellular communication: Microvesicle-based information transfer. Inflamm. Res. 2009, 58, 1–8.
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616.
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830.
- Kang, S.W.; Lee, S.; Lee, E.K. ROS and energy metabolism in cancer cells: Alliance for fast growth. Arch. Pharm. Res. 2015, 38, 338–345.
- El Andaloussi, S.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357.
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015, 6, 7321.
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
771
Revisions:
2 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




