Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianlin Han | -- | 4022 | 2022-06-29 09:59:18 | | | |
| 2 | Conner Chen | Meta information modification | 4022 | 2022-06-30 07:54:51 | | | | |
| 3 | Conner Chen | + 3 word(s) | 4025 | 2022-07-01 08:08:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Han, J.; Escorihuela, J.; Fustero, S.; Landa, A.; Soloshonok, V.A.; Sorochinsky, A. Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes. Encyclopedia. Available online: https://encyclopedia.pub/entry/24616 (accessed on 07 February 2026).
Han J, Escorihuela J, Fustero S, Landa A, Soloshonok VA, Sorochinsky A. Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes. Encyclopedia. Available at: https://encyclopedia.pub/entry/24616. Accessed February 07, 2026.
Han, Jianlin, Jorge Escorihuela, Santos Fustero, Aitor Landa, Vadim A. Soloshonok, Alexander Sorochinsky. "Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes" Encyclopedia, https://encyclopedia.pub/entry/24616 (accessed February 07, 2026).
Han, J., Escorihuela, J., Fustero, S., Landa, A., Soloshonok, V.A., & Sorochinsky, A. (2022, June 29). Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes. In Encyclopedia. https://encyclopedia.pub/entry/24616
Han, Jianlin, et al. "Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes." Encyclopedia. Web. 29 June, 2022.
Copy Citation
The proline-catalyzed asymmetric Michael addition reaction of acetaldehyde with α,β-unsaturated nitroalkenes as synthetically useful routes to β-substituted derivatives of γ-Aminobutyric acid (GABA).
pharmaceuticals
neurological drugs
γ-aminobutyric-acid derivatives
asymmetric Michael addition
1. Introduction
Tailor-made amino acids [1] play an indispensable role in the development of modern pharmaceuticals and drug formulations [2][3][4][5][6]. Thus, over 20% of newly approved small-molecule drugs contain structural fragments of AAs [7][8][9]. In particular, γ-aminobutyric-acid (GABA) derivatives bearing β-alkyl or β-aryl substituents, which include baclofen [10], phenibut [11][12][13], tolibut [14], and pregabalin [15][16][17], are recognized as an essential class of marketed pharmaceuticals for the treatment of neurological diseases (Figure 1). Similarly, piracetam-based GABA derivatives such as phenylpiracetam [18], brivaracetam [19][20][21], and rolipram [22] are also developed as pharmaceuticals. Introduction of β-alkyl or β-aryl substituent in the GABA backbone allows for the improvement of the lipophilic character of these compounds.
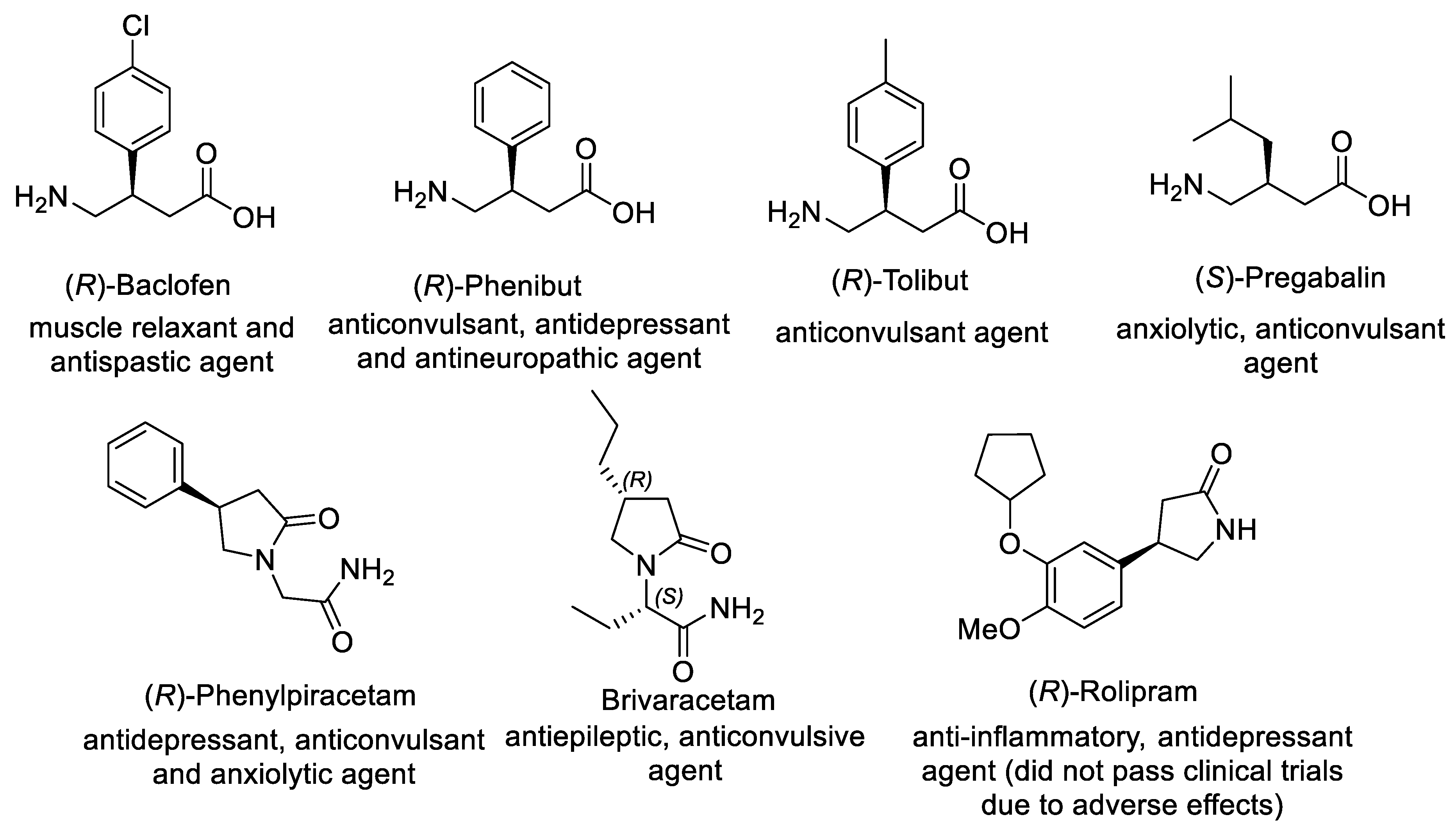
Figure 1. Representative β-substituted GABA derivatives with clinical applications.
Significantly, biological activity of β-substituted GABA derivatives depends on their absolute configuration. For example, (R)-enantiomers of Baclofen (antispastic agent and muscle relaxant) and Phenibut (tranquilizer and anticonvulsant) are considerably more active than the corresponding (S)-enantiomers, while anticonvulsant activity of Pregabalin (anti-epilepsy drug) is primarily related to (S)-enantiomer [23]. Consequently, considerable efforts were devoted to developing asymmetric synthesis of β-substituted GABA derivatives, including chemical and biocatalytic resolution, asymmetric reduction, desymmetrization, aldol addition, and nucleophilic substitution [24][25][26]. For the past decade, the asymmetric Michael addition of carbon nucleophile to α,β-unsaturated compound-bearing nitro or carbonyl groups gained impressive progress, providing straightforward access to γ-nitrocarbonyl compounds as key chiral intermediates that can be converted into β-substituted GABA derivatives via subsequent transformation of functional groups.
2. Michael Addition of Carbonyl Compounds to α,β-Unsaturated Nitroalkenes
The proline-catalyzed asymmetric Michael addition reaction [27] of acetaldehyde with α,β-unsaturated nitroalkenes attracted considerable attention as synthetically useful routes to β-substituted derivatives of GABA. Thus, the addition of acetaldehyde to the nitroolefins (E)-1 and (E)-2 (Scheme 1) was carried out in the presence of enantiomerically pure (S)-diphenylprolinol silyl ether 3 as the catalyst [28][29]. After optimization of reaction conditions, the asymmetric Michael addition proceeded efficiently with 10–20 mol% of organocatalyst (S)-3 in such solvents as MeCN, DMF/i-PrOH, and 1,4-dioxane to afford γ-nitro aldehydes (S)-4 and (R)-5 in reasonable yield and excellent enantiomeric excess. Oxidation of γ-nitro aldehydes (S)-4 and (R)-5 was successfully performed in aqueous t-BuOH using NaClO2 and NaH2PO4 with 2-methyl-2-butene as a chlorine scavenger to afford carboxylic acids (S)-6 and (R)-7 in good to excellent yields [30]. The reduction of the nitro acid (S)-6 with Raney Ni in MeOH gave, after treatment with aqueous HCl, (S)-Baclofen 8 as hydrochloride salt in 91% yield. (R)-Pregabalin 9 was also synthesized by the reduction of the nitro group in (R)-7 under Pd/C in 93% yield. The mechanism of the asymmetric Michael addition reaction of acetaldehyde with nitroalkenes promoted by diphenylprolinol silyl ether involves the formation of the enamine as a nucleophile. Thus, the organocatalyst would react with the acetaldehyde forming anti-enamine with the double bond oriented away from the diphenylsiloxymethyl group. In this case, the (diphenylmethyl)trimethylsiloxy group provides the formation of anti-enamine and shielding one face of the enamine double bond. The anti-enamine would add stereoselectively to the nitroolefin via the acyclic synclinal transition state proposed by Seebach [31] as shown in Scheme 1.
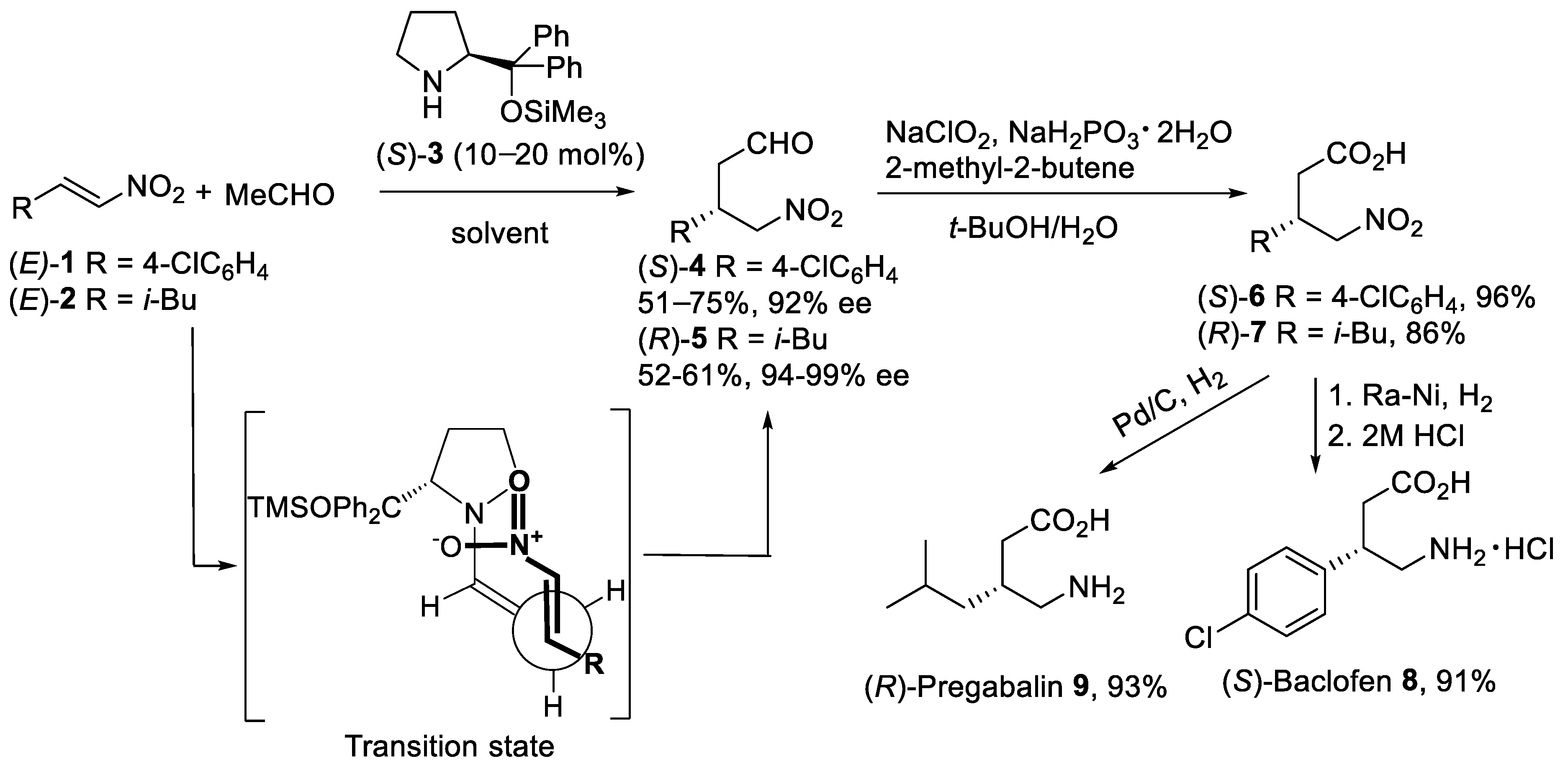
Scheme 1. Organocatalytic asymmetric Michael reaction of acetaldehyde with nitroalkenes (E)-1 and (E)-2.
In a similar way, the nitro olefin (E)-10 (Scheme 2), easily available from isovanillin via O-alkylation and Henry condensation, reacted with acetaldehyde in the presence of a catalytic amount of diphenylprolinol silyl ether (R)-3 (10 mol%) affording corresponding nitro aldehyde adduct which upon oxidation with oxone and esterification successfully transformed into ester derivative (R)-11 in 85% yield [32]. The nitroester (R)-11 underwent intramolecular reductive lactamization under H2 in presence of catalytic amount of Pd/C to furnish (R)-Rolipram 12 in 93% yield and >99% ee. The present method was also utilized to prepare enantiomerically pure (S)-Rolipram using (S)-diphenylprolinol silyl ether-mediated asymmetric Michael addition reaction as the key step. Recently, water-soluble diarylprolinol silyl ether containing the dimethylamine functionality was found to be very effective for the Michael additions of the acetaldehyde with nitroolefins. These reactions took place in brine with good yields and high enantioselectivities for a broad range of nitroolefins [33][34][35].
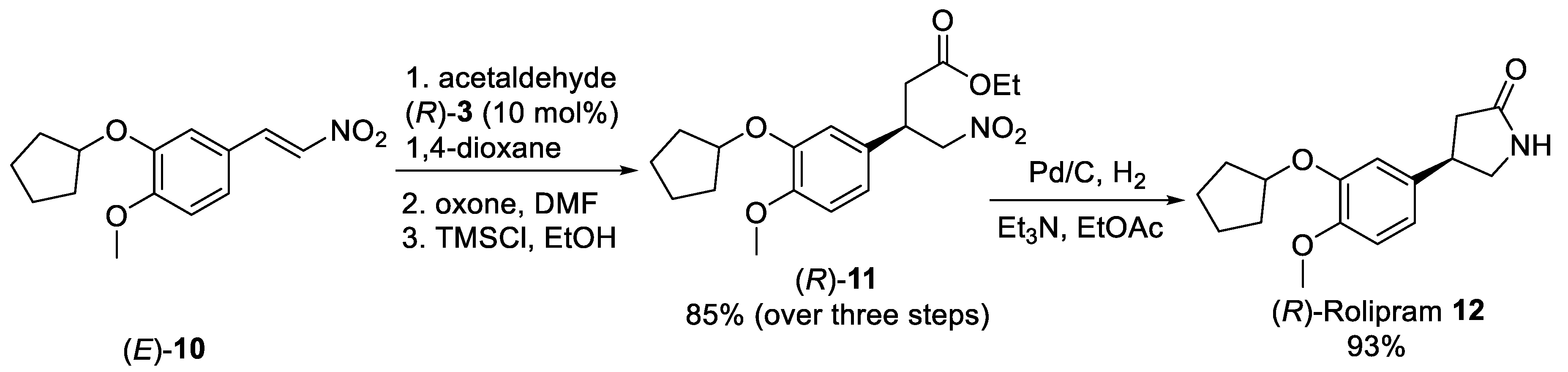
Scheme 2. Synthetic approach of (R)-Rolipram 12 employing the organocatalyzed asymmetric Michael addition acetaldehyde to nitro olefin (E)-10.
Enantioselective conjugate addition of malonates and their equivalents to nitroolefins promoted by bifunctional organocatalysts bearing a hydrogen-bonding donor group and Lewis base (tertiary amine) is considered as one of the most simple and efficient routes for constructing chiral β-substituted derivatives of GABA and their lactam analogs, especially from the perspective of green chemistry. The success of bifunctional organocatalysts was based on their ability to increase the reactivity of both nitroolefins and nucleophiles as well as control the approach of nucleophiles to nitroolefins in the transition state [36][37]. For example, the Michael reaction of nitroalkene (E)-1 (Scheme 3) with diethyl malonate was performed in the presence of Takemoto thiourea catalyst (R,R)-13 bearing 3,5-bis(trifluoromethyl)benzene and tertiary amino group [38]. The use of 2 equiv of diethyl malonate in toluene and 10 mol% catalyst loading was required to provide the Michael adduct (R)-14 in 80% yield with 94% ee. A single recrystallization made it possible to increase the enantiomeric purity of the product (R)-14 to 99% ee. Then, reductive cyclization of (R)-14 with NaBH4/NiCl2 in methanol gave γ-lactam (3S,4R)-15 as thermodynamically more stable anti-diastereomer [39][40], which after hydrolysis and decarboxylation was converted into lactam (R)-16 in 84%. Finally, acidic hydrolysis of (R)-16 gave (R)-Baclofen 8 as hydrochloride salt in 94% yield. A high level of enantioselectivity in organocatalytic Michael addition reaction was achieved as a result of dual activation through deprotonation of the acidic proton of diethylmalonate by tertiary amino group of the organocatalyst and the hydrogen-bond formation between the nitro group and the thiourea moiety [41].
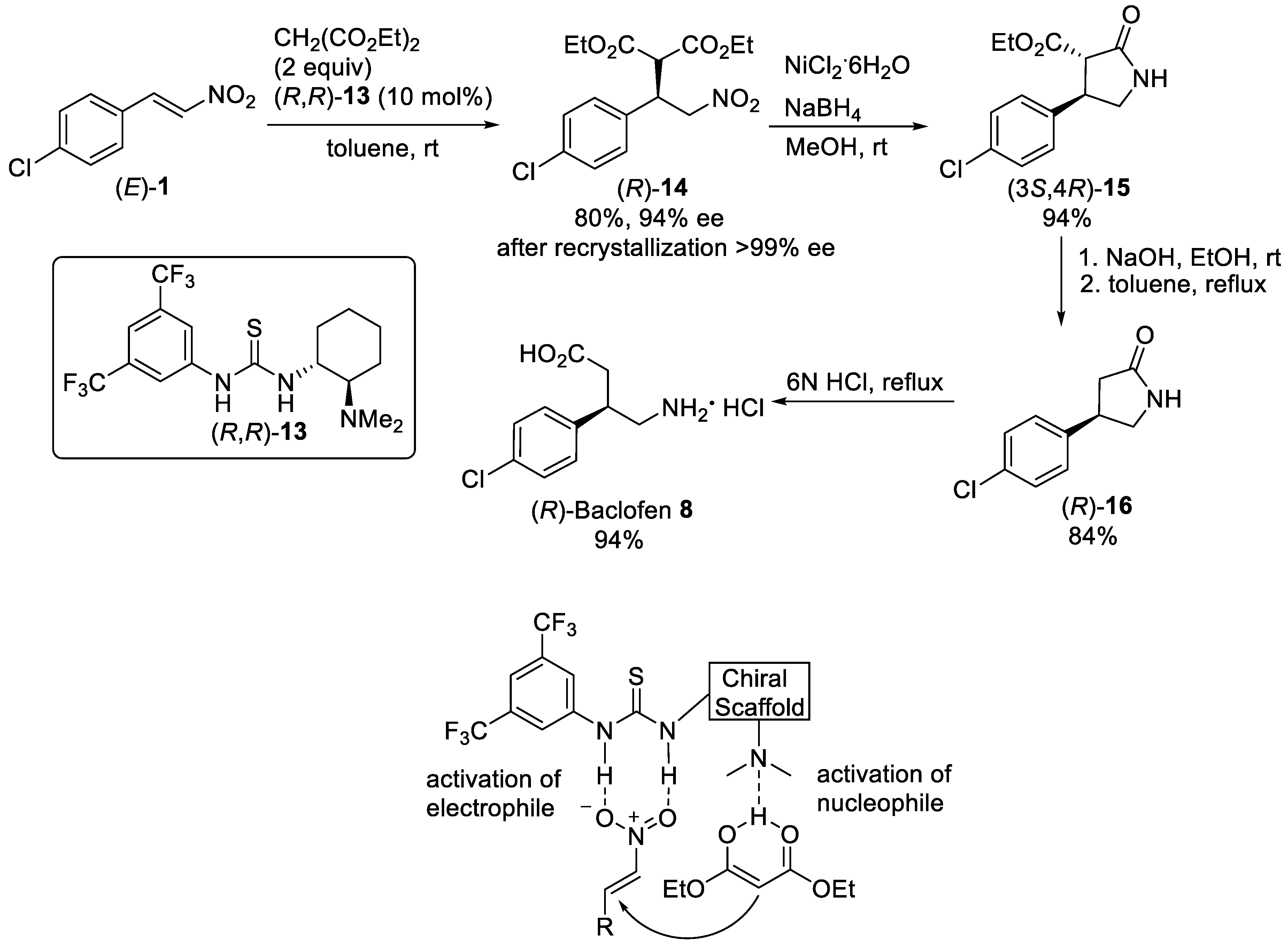
Scheme 3. Enantioselective Michael reaction of (E)-1 with diethyl malonate in the presence of thiourea (R,R)-13.
Under solvent-free conditions reducing the amount of diethyl malonate from 2 to 1 equiv did not significantly influence both the yield and enantioselectivity of Michael addition to nitroalkenes promoted by thiourea (R,R)-13 catalysts. Thus, Michael addition of diethyl malonate to alkyl-substituted nitroalkene (E)-2 (Scheme 4) in the presence of 10 mol% of (R,R)-13 under solvent-free conditions produced the nitro ester (S)-17 in 73% yield and 88% enantiomeric excess [42]. Hydrogenation of (S)-17 over Raney Ni provided the pyrrolidin-2-one (3S,4S)-18 [43] in 72% after crystallization, which was subjected to ester hydrolysis followed by decarboxylation to give γ-lactam (S)-19 in 90% yield and 98% enantiomeric excess. Hydrolysis of γ-lactam (S)-19 with 6N HCl gave the enantiomerically pure (S)-Pregabalin 9 hydrochloride in 95% yield. Additionally, treatment of pyrrolidin-2-one derivative (3S,4S)-18 with 6N HCl at reflux also directly produced the (S)-Pregabalin 9 hydrochloride in 92% yield.
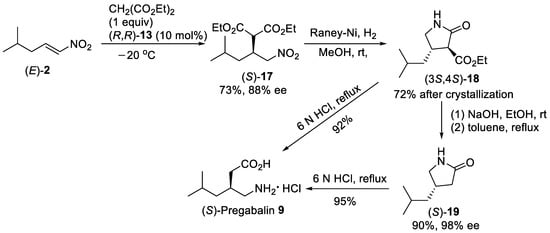
Scheme 4. Enantioselective Michael reaction of (E)-2 with diethyl malonate in the presence of thiourea (R,R)-13 under solvent-free conditions.
A series of Takamoto-type bifunctional organocatalysts were examined for the asymmetric Michael addition of malonate derivatives to nitroalkenes and showed very effective catalytic activity. For example, L-proline-derived bifunctional urea-pyrrolidine organocatalyst (S,R)-21 (Scheme 5) was demonstrated to catalyze the enantioselective Michael addition of diphenyl dithiomalonates to nitrostyrene (E)-20 in toluene as the best solvent at 25 °C [44]. The reaction performing with 5 mol% of (S,R)-21 was complete in 1.5 h, affording Michael adduct (R)-22 in 90% enantiomeric excess, which was improved to 98% after recrystallization from ethanol. Reduction of the nitro group using zinc in acetic acid and substoichiometric amounts of TiCl3 followed by intermolecular cyclization enabled the formation of the lactam (R)-23 in excellent yields of 90%. Hydrolysis of the lactam (R)-23 was finally achieved with 6N HCl and the resulting (R)-Phenibut 24 was isolated as hydrochloride in 85% yield. Additionally, mild basic hydrolysis-decarboxylation of Michael adduct (R)-22 provided γ-nitrothioester (R)-25 in 94% yield, which reduced and cyclized under the above described conditions (Zn/AcOH/TiCl3) to lactam (R)-26 that was isolated in 82% yield.
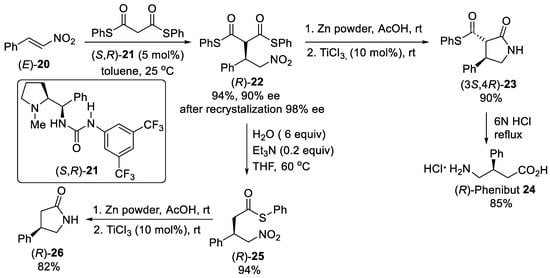
Scheme 5. Enantioselective Michael addition of diphenyl dithiomalonates to β-nitrostyrene (E)-20 catalyzed by L-proline-derived urea (S,R)-21.
When the addition of cyclohexyl Meldrum’s acid to aliphatic nitroalkene (E)-2 (Scheme 6) was carried out with only 0.2 mol% of N-sulfinyl urea catalysis (Ss,R,R)-27 in cyclopentyl methyl ether (CPME), complete conversion of starting nitroalkene (E)-2 proceeded at 35 °C for 48 h providing the Michael adduct (S)-28 in 92% ee [45]. Using n-sulfinyl urea catalysis (Ss,R,R)-27 bearing cyclic tertiary amine was found to be essential for achieving high conversion and enantioselectivity. Direct hydrolysis/decarboxylation of addition product (S)-28 without purification led to one mole scale synthesis of γ-nitroacid (S)-29 in 90% overall yield from nitroalkene (E)-2. (S)-Pregabalin 9 could be provided by heterogeneous catalytic hydrogenation of (S)-29.
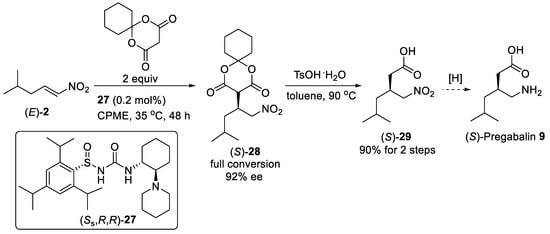
Scheme 6. Catalytic enantioselective addition of cyclohexyl Meldrum’s acid to nitroalkene (E)-2 using N-sulfinyl urea catalysis (Ss,R,R)-27.
Another urea catalyst (S,S)-31 (Scheme 7) containing the pyrrolidine moiety was designed to catalyze in brine media [46] the enantioselective addition of dithiomalonate to otherwise unreactive β-CF3-β,β-disubstituted nitroalkenes 30 providing corresponding Michael adducts 32 with a β-trifluoromethylated quaternary stereocenter [47]. In brine media the use of 15 mol% of urea catalyst (S,S)-31 as well as the addition of o-xylene as additive was required for obtaining high yield and enantioselectivity of adducts 32 at 0 °C. In the absence of cosolvent, the achieved enantioselectivity of Michael adducts 32 was lower. These results could be explained by enforced hydrophobic interactions between catalysts and substrates when the reaction was carried out in brine media. Under optimal conditions, trifluoromethylated nitroalkenes 30 having substituted phenyl and iso-butyl groups were converted into the corresponding products 32 in 55–97% yields with 67–96% ee. So-obtained Michael products 32 were further subjected to reductive cyclization to furnish γ-lactam thioesters 33. Hydrolysis of γ-lactam thioesters 33 followed by a decarboxylation provided β-trifluoromethylated analogues of rolipram 34c, phenibut 35a, baclofen 35b, and pregabalin 35d. The enantiopurity of γ-lactam thioester 33d and γ-lactams 34a,b could be improved by recrystallization.
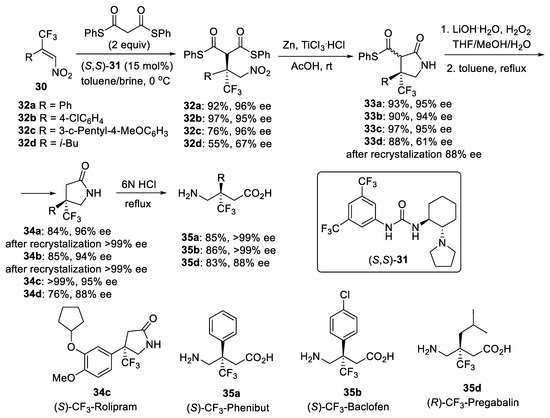
Scheme 7. Synthesis of GABA derivatives with a trifluoromethylated quaternary stereogenic center.
A three-component reaction of aromatic or aliphatic aldehydes, nitromethane and dimethyl malonate was found to catalyze by mesoporous siliceous material 36 (Scheme 8) incorporating urea-modified quinidine and propylamine groups in o-xylene at 70 °C leading to corresponding Michael adducts in reasonable yield [48][49]. The organic–inorganic hybrid catalyst 36 enabled access to Michael adducts with modest-to-good enantiomeric excess (50–70%). After removing the excess nitromethane by distillation, the o-xylene solution of Michael adducts was subjected to heterogeneous catalytic hydrogenation of the nitro group followed by cyclization and thermal decarboxylation to give γ-lactams with retention of enantiopurity. Thus, after column chromatography and recrystallization, (R)-Rolipram 12 and precursors of Phenibut (R)-26, Baclofen (R)-16 and Pregabalin (S)-19 were obtained in two-pot multicomponent operations with enantiopurity ranging from 81% ee to 97% ee. The solid hybrid catalyst could be reused three times without loss of activity.
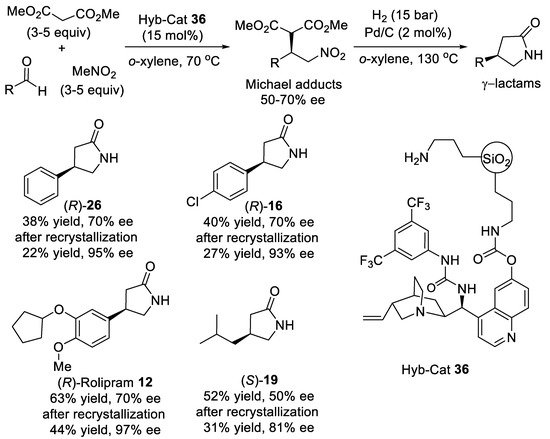
Scheme 8. Asymmetric three-component reaction of aldehydes, nitromethane, and malonate.
Conformationally rigid cyclobutene-ring-derivative squaramide (R,S)-37 (Scheme 9) was shown to function in a complementary manner to thioureas as an effective hydrogen-bonding bifunctional organocatalyst for the Michael addition of dimethyl malonate to aromatic nitroalkene (E)-1 [50][51]. It was supposed that two H-bonds formed between the squaramide (R,S)-37 organocatalyst, incorporating tertiary nitrogen as Lewis base and chiral scaffold, and the nitroalkene substrate. Performing the reaction in dichloromethane as solvent with 5 mol% catalyst loading afforded the Michael product (R)-38 in 84% yield and 86% ee, albeit in a long reaction time. Polymer-immobilized squaramide was also effective for the Michael addition of dimethyl malonate to nitroalkene (E)-1, but significant loss of activity was observed in the second and third cycles. The adduct (R)-38 was further transformed to (R)-baclofen 8 hydrochloride in 56% overall yield through the procedure exemplified in Scheme 1.
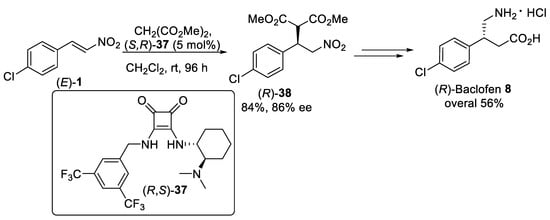
Scheme 9. Michael addition of dimethyl malonate to β-nitrostyrene (E)-1 using squaramide organocatalysis (R,S)-37.
Squaramide (R,R)-39 (Scheme 10), which possess an piperidine unit, was useful for the activation of Meldrum’s acid in enantioselective Michael addition to aliphatic nitroalkenes. The reaction of Meldrum’s acid and nitroalkene (E)-2 was carried out with catalyst loading of 5 mol% in dichloromethane as the best solvent to obtain the nitro derivative (S)-40 in 83% yield and 94% enantiomeric excess [52]. Squaramide (R,R)-39 showed higher catalytic activity and enantioselectivity compared to quinidine-based thioureas organocatalysts [53]. Single-step catalytic hydrogenation and acid-catalyzed deprotection of (S)-40 over Raney Ni in acetic acid than decarboxylation of the resulting malonic-acid derivative (S)-41 with 6N HCl afforded (S)-Pregabalin 9 hydrochloride in 52% yield for three steps.
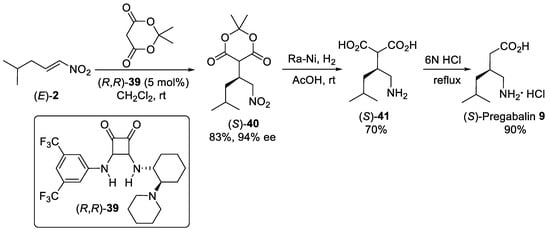
Scheme 10. The Michael addition of Meldrum’s acid to nitroalkene (E)-2 catalyzed by squaramide (R,R)-39 catalyst.
The use of catalyst 42 (Scheme 11A) incorporating squaramide and hydroquinidine units allowed one to perform an enantioselective Michael addition of malonate to nitroolefin (E)-2 in brine due to the “hydrophobic hydration effect” [54][55]. Under these conditions, hydrophobic hydroquinidine-squaramide catalyst 42 directed the reaction towards the corresponding Michael adduct (S)-43 with high yield and enantioselectivity [43]. After the extraction of the Michael adduct (S)-43 with methylcyclohexane, catalyst 42 was recoveried in quantitative yield (>99%) by simple filtration permitted the catalyst recycling. Hydrogenation of the crude Michael adduct (S)-43 using Raney Ni followed by hydrolysis of resulting γ-lactam (3S,4R)-18 with 6N HCl afforded (S)-pregabalin 9 in enantiomerically pure form after simple recrystallization from 2-propanol/water. The developed procedure in brine media was also applied for the scalable synthesis of enantiomerically pure (S)-Rolipram 12 (Scheme 11B) from aryl nitroolefin (E)-10 using 10 mol% of hydroquinine-based squaramide catalyst 44.
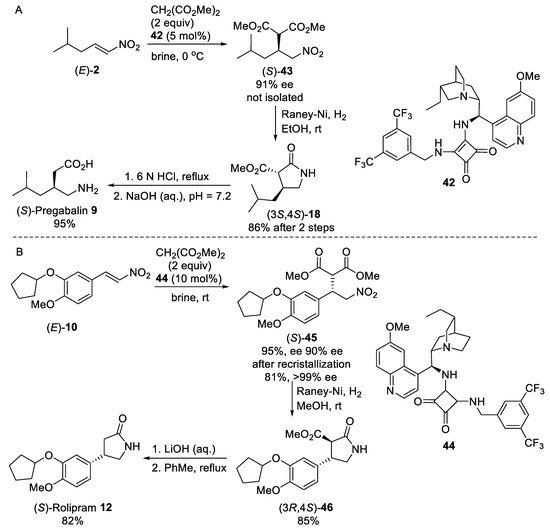
Scheme 11. Enantioselective syntheses of (S)-Pregabalin 9 (A) and (S)-Rolipram 12 (B) under “on water” conditions.
The hydrophobic dihydroquinine-squaramide derivative 44 (Scheme 11B) also demonstrated its efficiency in brine media for enantioselective Michael addition of the diethyl dithiomalonates to (E)-α-methyl-β-nitrostyrenes 47 (Scheme 12) [56]. In these cases, a high loading of 44 (15 mol%) and lowering the reaction temperature to 0 °C were required for the reaction to proceed with high enantioselectivities. At the same time, no reaction was observed when catalyst 44 was used in organic solvents. The resulting adducts (S)-48 could further be converted into GABA analogs bearing an all-carbon quaternary stereocenter at the β-position. After the separation of the catalyst, the crude products (S)-48 were subjected to reduction affording γ-lactam thioesters 49, which were hydrolyzed with 6N HCl into β-methylated analogs of Phenibut (S)-50a and Baclofen (S)-50b as hydrochloride salts. At the same time, the β-methylated analog of Rolipram (S)-50c was prepared by basic hydrolysis and decarboxylation of corresponding γ-lactam thioester 49.
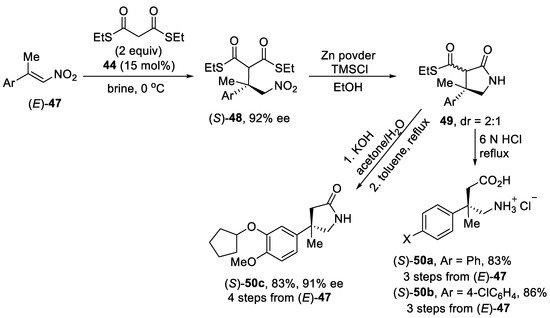
Scheme 12. Enantioselective Michael addition reaction of β,β-disubstituted nitroalkenes (E)-47 with dithiomalonate using dihydroquinine-squaramide catalyst.
An enantioselective decarboxylative Michael addition between malonic-acid half-thioester [57] and β-nitroolefins providing access to γ-nitro thioester was demonstrated to catalyze with cinchona-based squaramide [58] and cinchona-based urea [59] organocatalysts under mild reaction conditions. Performing the reaction of malonic-acid half-thioester 51 (Scheme 13) and β-nitrostyrene (E)-1 with loading of 5 mol% of bifunctional quinine-based squaramide 52 as the most active and selective organocatalyst in methyl tert-butyl ether (MTBE) at 45 °C resulted in addition product (S)-53 in high yield and excellent enantioselecitivity within 22 h [58]. It is noteworthy that using E or Z isomers of the starting β-nitrostyrene 1 afforded the (S)-enantiomer of the Michael adduct 53 with the same ee value. The nitro thioester (S)-53 was converted with Raney Ni and H3PO4 in THF by intramolecular cyclization and recrystallization into enantiomerically pure γ-butyrolactam (S)-16. Finally, hydrolysis of resulting γ-butyrolactam (S)-16 could be performed with 6N HCl under reflux to deliver the (S)-Baclofen 8 as its hydrochloride salt in 78% yield.
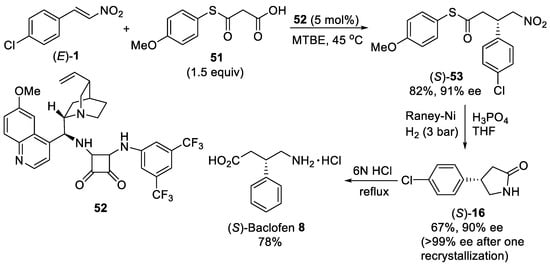
Scheme 13. Enantioselective Michael addition of malonic-acid half-thioester 51 to β-nitrostyrene (E)-1. Synthesis of (S)-baclofen 8.
The bifunctional bisalkaloid organocatalyst 54 (Scheme 14) was developed and successfully tested for the enantioselective conjugate addition of malonates to nitroalkenes [60]. The best result in terms of reactivity and selectivity was achieved using dimethyl malonate, 1 mol% of organocatalyst 54 in THF at room temperature. Under optimal conditions, the Michael reaction of dimethyl malonate with β-nitrostyrene (E)-1 proceeded well to give the corresponding adduct (R)-38 with excellent yield and enantioselectivity (after recrystallization). The obtained adduct (R)-38 was converted into (R)-Baclofen 8 hydrochloride according to the synthetic sequence demonstrated in Scheme 3.
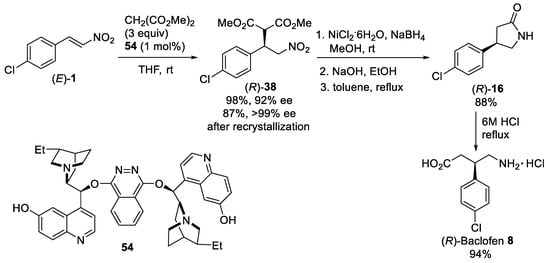
Scheme 14. Asymmetric conjugate addition of dimethyl malonate to β-nitrostyrene (E)-1. Synthesis of (R)-Baclofen 8 hydrochloride.
The highly stereoselective (>99% ee) conjugate addition of acetophenone to β-nitrostyrenes (E)-1 and (E)-20 (Scheme 15) was completed using primary amine-thiourea organocatalyst [61] based on (S)-di-tert-butyl aspartate. With the optimal organocatalyst 55, derived from (1R,2R)-diphenylethylenediamine the Michael addition provided adducts (S)-56 and (S)-57 at low catalyst loading (5 mol%) in excellent yield [62]. After Bayer–Villiger oxidation of (S)-56 and (S)-57, corresponding γ-nitro esters (S)-58 and (S)-59 could be transformed into the (S)-Baclofen 8 and (S)-Phenibut 24 according to described above procedures. The enantiomer of 55 was also utilized as organocatalyst for the efficient synthesis of (R)-Baclofen 8.
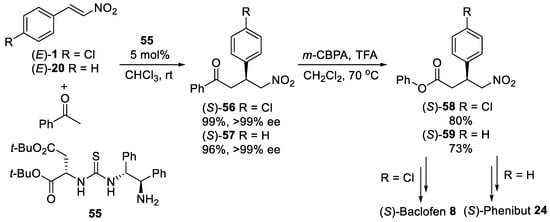
Scheme 15. Michael reaction between acetophenone and β-nitrostyrenes using primary amine-thiourea organocatalyst 55.
Catalytic asymmetric version of the Michael addition reaction between malonates and nitroalkenes was also achieved by using chiral metal−ligand complexes [63][64]. For example, the highly enantioselective Michael addition of tert-butyl phenyl malonate to the β-nitrostyrene (E)-20 (Scheme 16) was developed in toluene at room temperature using the easily accessible chiral bis-(cyclohexyldiamine)-based Ni(II) complex 60 (2 mol%) [65]. This process offered a route for the synthesis of β-nitro derivative (S)-61 in 92% yield and 93% ee (1.8:1 diastereoisomeric ratio). Reduction and cyclization of (S)-61 with the more reactive ester group afforded the γ-lactam (3R,4S)-62 as one diastereomer. Final hydrolysis and decarboxylation of the γ-lactam (3R,4S)-62 with 6N HCl under reflux produced the (S)-Phenibut 24 as the hydrochloride salt in quantitative yield. Later, several heterogeneous catalysts incorporating chiral bis(cyclohexyldiamine)-based Ni(II) complexes were developed for the asymmetric Michael addition of malonates to both aromatic [66][67] and aliphatic [68][69][70] nitroalkenes. These catalysts demonstrated good activities, enantioselectivities, reusability, and applicability for the multistep sequential flow synthesis of the (S)-Pregabalin [69].
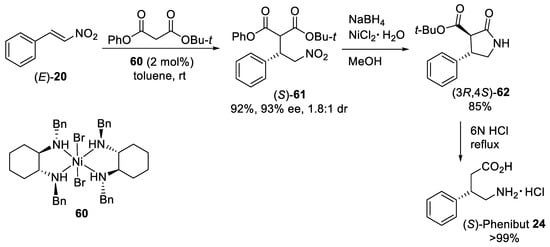
Scheme 16. Enantioselective Michael additions of tert-butyl phenyl malonate to β-nitrostyrene (E)-20 catalyzed by diamine−Ni(II) complex 60.
On the basis of mechanistic studies of conjugate addition reactions catalyzed by chiral nickel(II)-diamine complexes is proposed that the malonate displaces one diamine ligand of the catalyst generating the chiral enolate I (Scheme 17) [65]. Coordination of the nitroalkene to the nickel center of I leads to the intermediate II, and subsequent addition of enolate to the bound nitroalkene affords the 1,4-addition intermediate III. Then intermolecular proton transfer and displacement of the Michael product with another molecule of the malonate regenerates chiral enolate I.
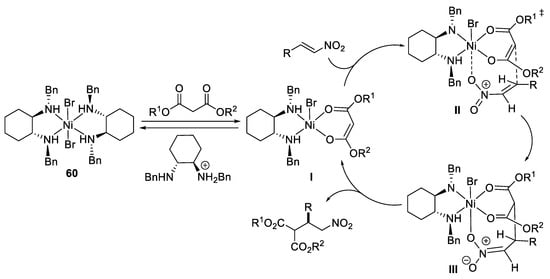
Scheme 17. Proposed mechanism reaction.
The application of the chiral nickel(II)-diamine catalysts was extended to 1,4-selective asymmetric addition of malonates to nitroenynes [71]. The 1,4-addition of di-tert-butyl malonate to nitroenyne 63 (Scheme 18) in the presence of 2 mol% of Ni(II) complex 64 as the catalyst proceeded regioselectively under mild conditions, affording β-alkynyl nitro acid (R)-65 in good yield and high enantioselectivity (91% ee). Enantiomerical purity of product (R)-65 could be improved to 99% ee by single recrystallization. This protocol allowed to obtain β-alkynyl acid (R)-66 after decarboxylation of (R)-65 in the presence of TsOH under reflux. Reaction of β-alkynyl acid (R)-66 with oxalyl chloride and MeOH gave β-alkynyl ester (R)-67 in 64% yield, which by reduction of the nitro group followed by the treatment with di-tert-butyl dicarbonate (Boc)2O afforded the N-Boc β-alkynyl-γ-amino ester (R)-68. The ester (R)-68, as the common intermediate, was converted to the β-alkynyl-γ-amino acid (R)-69 and β-alkyny-γ-lactam (R)-70 using standard procedures without loss of enantiomeric purity.
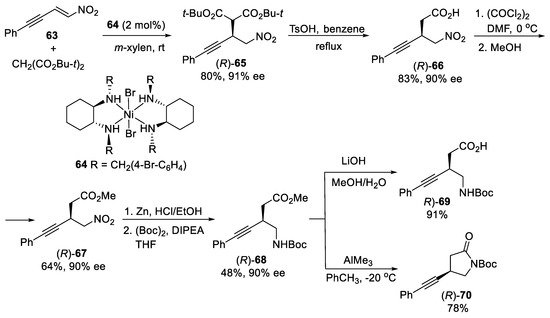
Scheme 18. Asymmetric synthesis of β-alkynyl-γ-amino acid (R)-69 and β-alkyny-γ-lactam (R)-70.
The catalytic asymmetric decarboxylative 1,4-addition reaction of malonic-acid half-thioester to nitroalkene (E)-10 (Scheme 19) was realized by employing heterobimetallic system [72] with transition metal, rare-earth metal, and dinucleating amino-phenol ligand (S,S)-71. Screening of the transition metal and rare-earth metal combination revealed that Ni/La/ligand (S,S)-71 catalyst in the presence of phosphine oxide 72 as an achiral additive gave the best catalytic activity and selectivity delivering the addition product (S)-73 in 80% yield and 93% ee [73]. The synthetic utility of the asymmetric decarboxylative 1,4-addition reaction was demonstrated by the reduction and cyclization of (S)-73 with Zn and (CH3)3SiCl to (S)-Rolipram 12 in 83% yield.
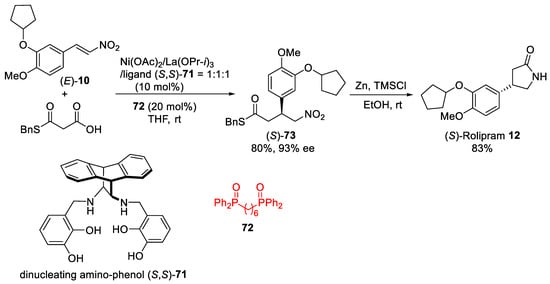
Scheme 19. Ni–La system for decarboxylative 1,4-addition of malonic-acid half-thioester to nitroalkene (E)-10.
Additionally, asymmetric 1,4-addition of dimethyl malonate to β-nitrostyrene (E)-1 catalyzed by polymer-supported CaCl2-pyridinebisoxazoline (Pybox) complex [74] was developed and implemented in continuous-flow synthesis of (S)-Baclofen precursor (3R,4S)-74 using a series of different heterogeneous catalysts (Figure 2) [75]. In the first step, amine-modified silica gel/molecular sieves 4Å column reactor was used for condensation of p-chlorobenzaldehyde and nitromethane into β-nitrostyrene (E)-1 in toluene. Then sequential-flow enantioselective asymmetric 1,4-addition of dimethyl malonate to β-nitrostyrene (E)-1 proceeded into the second column reactor with polymer-supported CaCl2-Pybox chiral catalyst to give adduct (S)-38 with high enantioselectivity (92% ee). Further, the third column reactor containing dimethylpolysilane (DMPS)-modified Pt catalyst supported on activated carbon (AC) and calcium phosphate (CP) was successfully employed for flow chemoselective hydrogenation and intramolecular cyclization of adduct (S)-38 to give 5 g of lactam (3R,4S)-73 in 93–96% overall yield based on nitromethane with 92% ee during the 69-h process. Finally, (S)-Baclofen 8 was obtained according to the standard procedure. Slightly modifying the continuous flow system using polymer-supported CaCl2-Pybox chiral catalyst was also employed in the synthesis of (R)-Phenibut [76] and (R)-Rolipram [77]. However, the polymer-supported CaCl2-Pybox chiral catalyst demonstrated poor enantioselectivities in reactions of nitroolefins bearing primary aliphatic substituents.
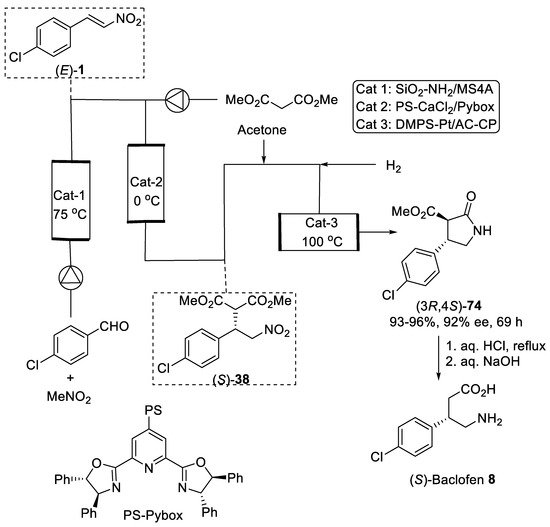
Figure 2. Sequential flow synthesis of Baclofen precursor (3R,4S)-74.
3. Michael Additions of Cyanide or Nitroalkanes to α,β-Unsaturated Carbonyl Compounds
Asymmetric conjugate addition of cyanide and nitroalkanes to α,β-unsaturated carbonyl substrates was another practical route for the synthesis of β-substituted GABA derivatives. For example, the Michael addition of diethylaluminum cyanide to substrate (R)-75 (Scheme 20) bearing oxazolidinone chiral auxiliary [78] was conducted as the key step in the synthesis of (S)-Pregabalin 9 from commercially available starting materials [79]. Conjugate addition was performed in toluene at 0 °C to produce addition adduct (4R,3′S)-76 in satisfactory yield and moderate dr (87:13). The diastereomerically pure (4R,3′S)-76 was provided after purification with column chromatography on silica gel in a 57% yield. The observed stereoselectivity was attributed to the approach of the diethylaluminum cyanide mainly from the less hindered Si face opposite to phenyl group in the oxazolidinone auxiliary. The removal of oxazolidinone chiral auxiliary by treating with LiOH and H2O2 in aqueous THF and reduction of the cyano group by hydrogenolysis under Raney Ni afforded (S)-pregabalin 9 in 95% yield for two steps. Moreover, acetone cyanohydrin was also effectively used as cyanide source for diastereoselective conjugate addition to α,β-unsaturated oxazolidinone (S)-77 (Scheme 20) [80]. The hydrocyanation of (S)-77 cleanly proceeded with two equivalents of acetone cyanohydrin and 10 mol% of Sm(III) isopropoxide as catalyst in toluene to give the addition adduct (4S,3′R)-78 in 75% yield and 88:12 dr. The diastereomerically pure product (4S,3′R)-78 was chromatographically isolated in 66% yield. The catalytic hydrogenation of the cyano group with simultaneous cleavage of oxazolidinone chiral auxiliary over platinum oxide afforded the lactam (R)-79 in 75% yield and 96% ee, which acidic hydrolysis led to the (R)-Pregabalin 9 as hydrochloride in 95% yield with retention of the enantiomeric purity. A similar route was employed for the synthesis of (S)-Baclofen 8 using aryl-substituted substrate (S)-80, acetone cyanohydrin and Sm(Oi-Pr)3 as catalyst (Scheme 20) [80]. Under standard conditions, the diastereomerically pure nitrile adduct (4S,3′S)-81 was obtained in 62% yield. Selective reduction of the cyano group with such reagents as NaBH4 and NiCl2 and hydrolysis of resulting lactam (S)-82 provided (S)-Baclofen 8 in excellent yield and enantiomeric purity.
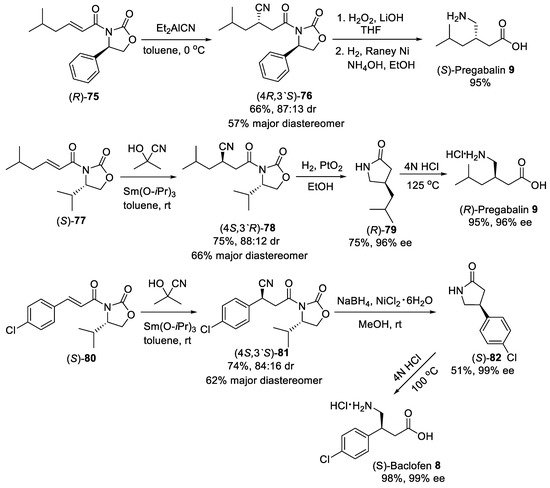
Scheme 20. Diastereoselective Michael addition of cyanide to α,β-unsaturated oxazolidinones.
Similarly, oxazolidinone as a chiral auxiliary was used to generate a new stereogenic center in the Michael addition between nitromethane and α,β-unsaturated oxazolidinone ®-75 with Cs2CO3 as a base providing a diastereomerically pure addition adduct (4R,3′S)-83 in 34% yield after two recrystallizations from isopropanol (Scheme 21) [81]. The oxazolidinone chiral auxiliary was removed by treating (4R,3′S)-83 with alkaline hydrogen peroxide to give the γ-nitroacid (S)-84. (S)-Pregabalin 9 was obtained after hydrogenation of γ-nitroacid (S)-84 using Pd/C in 26% overall yield for three steps from ®-75 with >99% ee.
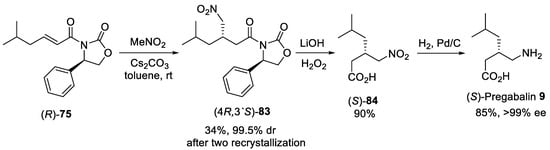
Scheme 21. Preparation of (S)-Pregabalin 9.
References
- Soloshonok, V.A.; Cai, C.; Hruby, V.J.; Van Meervelt, L. Asymmetric synthesis of novel highly sterically constrained (2S, 3S)-3-methyl-3-trifluoromethyl-and (2S, 3S, 4R)-3-trifluoromethyl-4-methylpyroglutamic acids. Tetrahedron 1999, 55, 12045–12058.
- Han, J.; Lyutenko, N.V.; Sorochinsky, A.E.; Okawara, A.; Konno, H.; White, S.; Soloshonok, V.A. Tailor-Made Amino Acids in Pharmaceutical Industry: Synthetic Approaches to Aza-Tryptophan Derivatives. Chem. Eur. J. 2021, 27, 17510–17528.
- Han, J.; Konno, H.; Sato, T.; Soloshonok, V.A.; Izawa, K. Tailor-made amino acids in the design of small-molecule blockbuster drugs. Eur. J. Med. Chem. 2021, 220, 113448.
- Han, J.; Konno, H.; Sato, T.; Izawa, K.; Soloshonok, V.A. Peptidomimetics and Peptide-Based Blockbuster Drugs. Curr. Org. Chem. 2021, 25, 1627–1658.
- Liu, J.; Han, J.; Izawa, K.; Sato, T.; White, S.; Meanwell, N.A.; Soloshonok, V.A. Cyclic tailor-made amino acids in the design of modern pharmaceuticals. Eur. J. Med. Chem. 2020, 208, 112736.
- Mei, H.; Han, J.; White, S.; Graham, D.J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N.A.; Soloshonok, V.A. Tailor-made amino acids and fluorinated motifs as prominent traits in modern pharmaceuticals. Chem. Eur. J. 2020, 26, 11349–11390.
- Liu, A.; Han, J.; Nakano, A.; Konno, H.; Moriwaki, H.; Abe, H.; Izawa, K.; Soloshonok, V.A. New pharmaceuticals approved by FDA in 2020: Small-molecule drugs derived from amino acids and related compounds. Chirality 2022, 34, 86–103.
- Yin, Z.; Hu, W.; Zhang, W.; Konno, H.; Moriwaki, H.; Izawa, K.; Han, J.; Soloshonok, V.A. Tailor-made amino acid-derived pharmaceuticals approved by the FDA in 2019. Amino Acids 2020, 52, 1227–1261.
- Soloshonok, V.A.; Izawa, K. (Eds.) Asymmetric Synthesis and Application of α-Amino Acids; ACS Symposium Series #1009; Oxford University Press: Washington, DC, USA, 2009.
- Kent, C.N.; Park, C.; Lindsley, C.W. Classics in chemical neuroscience: Baclofen. ACS Chem. Neurosci. 2020, 11, 1740–1755.
- Lapin, I. Phenibut (β-phenyl-GABA): A tranquilizer and nootropic drug. CNS Drug Rev. 2001, 7, 471–481.
- Zvejniece, L.; Vavers, E.; Svalbe, B.; Veinberg, G.; Rizhanova, K.; Liepins, V.; Kalvinsh, I.; Dambrova, M. R-phenibut binds to the α2–δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects. Pharmacol. Biochem. Behav. 2015, 137, 23–29.
- Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Comparative pharmacological activity of optical isomers of phenibut. Eur. J. Pharmacol. 2008, 583, 128–134.
- Tyurenkov, I.N.; Borodkina, L.E.; Bagmetova, V.V.; Berestovitskaya, V.M.; Vasil’eva, O.S. Comparison of nootropic and neuroprotective features of aryl-substituted analogs of gamma-aminobutyric acid. Bull. Exp. Biol. Med. 2016, 160, 465–469.
- Silverman, R.B. From basic science to blockbuster drug: The discovery of Lyrica. Angew. Chem. Int. Ed. 2008, 47, 3500–3504.
- Reinares, M.; Rosa, A.R.; Franco, C.; Goikolea, J.M.; Fountoulakis, K.; Siamouli, M.; Gonda, X.; Frangou, S.; Vieta, E. A systematic review on the role of anticonvulsants in the treatment of acute bipolar depression. Int. J. Neuropsychop. 2013, 16, 485.
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277.
- Zvejniece, L.; Zvejniece, B.; Videja, M.; Stelfa, G.; Vavers, E.; Grinberga, S.; Svalbe, B.; Dambrova, M. Neuroprotective and anti-inflammatory activity of DAT inhibitor R-phenylpiracetam in experimental models of inflammation in male mice. Infammopharmacology 2020, 28, 1283–1292.
- Malykh, A.G.; Sadaie, M.R. Piracetam and piracetam-like drugs. Drugs 2010, 70, 287–312.
- Kenda, B.M.; Matagne, A.C.; Talaga, P.E.; Pasau, P.M.; Differding, E.; Lallemand, B.I.; Frycia, A.M.; Moureau, F.G.; Klitgaard, H.V.; Gillard, M.R.; et al. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J. Med. Chem. 2004, 47, 530–549.
- von Rosenstiel, P. Brivaracetam (ucb 34714). Neurotherapeutics 2007, 4, 84–87.
- Zhu, J.; Mix, E.; Winblad, B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001, 7, 387–398.
- Gajcy, K.; Lochyński, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347.
- Ordóñez, M.; Cativiela, C.; Romero-Estudillo, I. An update on the stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 2016, 27, 999–1055.
- Ramesh, P.; Suman, D.; Reddy, K.S.N. Asymmetric Synthetic Strategies of (R)-(–)-Baclofen: An Antispastic Drug. Synthesis 2018, 50, 211–226.
- Martínez, C.A.; Hu, S.; Dumond, Y.; Tao, J.; Kelleher, P.; Tully, L. Development of a chemoenzymatic manufacturing process for pregabalin. Org. Process Res. Dev. 2008, 12, 392–398.
- Shagufta, K.; Ameer, F.Z.; Sajjad, A.; Rabia, A.; Iqra, K.; Wajiha, Q.; Attia, M. Recent trends in the development of novel catalysts for asymmetric Michael reaction. Curr. Org. Chem. 2020, 24, 1397–1458.
- García-García, P.; Ladépêche, A.; Halder, R.; List, B. Catalytic asymmetric Michael reactions of acetaldehyde. Angew. Chem. Int. Ed. 2008, 47, 4719–4721.
- Hayashi, Y.; Itoh, T.; Ohkubo, M.; Ishikawa, H. Asymmetric Michael reaction of acetaldehyde catalyzed by diphenylprolinol silyl ether. Angew. Chem. Int. Ed. 2008, 47, 4722–4724.
- Gotoh, H.; Ishikawa, H.; Hayashi, Y. Diphenylprolinol silyl ether as catalyst of an asymmetric, catalytic, and direct Michael reaction of nitroalkanes with α, β-unsaturated aldehydes. Org. Lett. 2007, 9, 5307–5309.
- Seebach, D.; Golinski, J. Synthesis of Open-Chain 2,3-Disubstituted 4-nitroketones by Diastereoselective Michael-addition of (E)-Enamines to (E)-Nitroolefins. A topological rule for C, C-bond forming processes between prochiral centres. Preliminary communication. Helv. Chim. Acta 1981, 64, 1413–1423.
- Kaur, R.; Pandey, S.K. Efficient synthesis of (−)-(R)-and (+)-(S)-rolipram. Tetrahedron Lett. 2017, 58, 4333–4335.
- Qiao, Y.; He, J.; Ni, B.; Headley, A.D. Asymmetric Michael reaction of acetaldehyde with nitroolefins catalyzed by highly water-compatible organocatalysts in aqueous media. Adv. Synth. Catal. 2012, 354, 2849–2853.
- Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. DoE-Driven Development of an Organocatalytic Enantioselective Addition of Acetaldehyde to Nitrostyrenes in Water. Chem. Eur. J. 2022, 28, e202104524.
- Palomo, C.; Landa, A.; Mielgo, A.; Oiarbide, M.; Puente, A.; Vera, S. Water-compatible iminium activation: Organocatalytic Michael reactions of carbon-centered nucleophiles with enals. Angew. Chem. Int. Ed. 2007, 46, 8431–8435.
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G.; Pastor, I.M.; Ramón, D.R. Recent advances in asymmetric organocatalyzed conjugate additions to nitroalkenes. Molecules 2017, 22, 895.
- Odagi, M.; Nagasawa, K. Recent Advances in Natural Products Synthesis Using Bifunctional Organocatalysts bearing a Hydrogen-Bonding Donor Moiety. Asian J. Org. Chem. 2019, 8, 1766–1774.
- Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. Enantio-and diastereoselective Michael reaction of 1, 3-dicarbonyl compounds to nitroolefins catalyzed by a bifunctional thiourea. J. Am. Chem. Soc. 2005, 127, 119–125.
- Perlikowska, R.; Piekielna, J.; Mazur, M.; Koralewski, R.; Olczak, J.; do Rego, J.C.; Fichna, J.; Modranka, J.; Janecki, T.; Janecka, A. Antinociceptive and antidepressant-like action of endomorphin-2 analogs with proline surrogates in position 2. Bioorg. Med. Chem. 2014, 22, 4803–4809.
- Sukhanova, A.A.; Nelyubina, Y.V.; Zlotin, S.G. Asymmetric synthesis of 3-prenyl-substituted pyrrolidin-2-ones. Mendeleev Commun. 2016, 26, 471–473.
- Steppeler, F.; Dominika Iwan, D.; Elżbieta Wojaczyńska, E.; Wojaczyński, J. Chiral thioureas—Preparation and significance in asymmetric synthesis and medicinal chemistry. Molecules 2020, 25, 401.
- Liu, J.; Wang, X.; Ge, Z.; Sun, Q.; Cheng, T.; Li, R. Solvent-free organocatalytic Michael addition of diethyl malonate to nitroalkenes: The practical synthesis of Pregabalin and γ-nitrobutyric acid derivatives. Tetrahedron 2011, 67, 636–640.
- Bae, H.Y.; Song, C.E. Unprecedented Hydrophobic Amplification in Noncovalent Organocatalysis “on Water”: Hydrophobic Chiral Squaramide Catalyzed Michael Addition of Malonates to Nitroalkenes. ACS Catal. 2015, 5, 3613–3619.
- Jin, H.; Kim, S.T.; Hwang, G.S.; Ryu, D.H. l-Proline Derived Bifunctional Organocatalysts: Enantioselective Michael Addition of Dithiomalonates to trans-β-Nitroolefins. J. Org. Chem. 2016, 81, 3263–3274.
- Kimmel, K.L.; Weaver, J.D.; Ellman, J.A. Enantio-and diastereoselective addition of cyclohexyl Meldrum’s acid to β-and α, β-disubstituted nitroalkenes via N-sulfinyl urea catalysis. Chem. Sci. 2012, 3, 121–125.
- Mondal, A.; Bhowmick, S.; Ghosh, A.; Chanda, T.; Bhowmick, K.C. Advances on asymmetric organocatalytic 1,4-conjugate addition reactions in aqueous and semi-aqueous media. Tetrahedron Asymmetry 2017, 28, 849–875.
- Sim, J.H.; Park, J.H.; Maity, P.; Song, C.E. Access to Chiral GABA Analogues Bearing a Trifluoromethylated All-Carbon Quaternary Stereogenic Center through Water-Promoted Organocatalytic Michael Reactions. Org. Lett. 2019, 21, 6715–6719.
- Leyva-Pérez, A.; García-García, P.; Corma, A. Multisite organic–inorganic hybrid catalysts for the direct sustainable synthesis of GABAergic drugs. Angew. Chem. Int. Ed. 2014, 53, 8687–8690.
- García-García, P.; Zagdoun, A.; Copèret, C.; Lesage, A.; Díaz, U.; Corma, A. In situ preparation of a multifunctional chiral hybrid organic–inorganic catalyst for asymmetric multicomponent reactions. Chem. Sci. 2013, 4, 2006–2012.
- Veverková, E.; Bilka, S.; Baran, R.; Šebesta, R. Squaramide-Catalyzed Michael Addition as a Key Step for the Direct Synthesis of GABAergic Drugs. Synthesis 2016, 48, 1474–1482.
- Marchetti, L.A.; Kumawat, L.K.; Mao, N.; Stephens, J.C.; Elmes, R.B.P. The versatility of squaramides: From supramolecular chemistry to chemical biology. Chem 2019, 5, 1398–1485.
- Baran, R.; Veverková, E.; Škvorcová, A.; Šebesta, R. Enantioselective Michael addition of 1, 3-dicarbonyl compounds to a nitroalkene catalyzed by chiral squaramides—A key step in the synthesis of pregabalin. Org. Biomol. Chem. 2013, 11, 7705–7711.
- Bassas, O.; Huuskonen, J.; Rissanen, K.; Koskinen, A.M.P. A simple organocatalytic enantioselective synthesis of pregabalin. Eur. J. Org. Chem. 2009, 2009, 1340–1351.
- Kitanosono, T.; Kobayashi, S. Synthetic Organic “Aquachemistry” that Relies on Neither Cosolvents nor Surfactants. ACS Cent. Sci. 2021, 7, 739–747.
- Bae, H.Y.; Some, S.; Oh, J.S.; Lee, Y.S.; Song, C.E. Hydrogen bonding mediated enantioselective organocatalysis in brine: Significant rate acceleration and enhanced stereoselectivity in enantioselective Michael addition reactions of 1,3-dicarbonyls to β-nitroolefins. Chem. Commun. 2011, 47, 9621–9623.
- Sim, J.H.; Song, C.E. Water-Enabled Catalytic Asymmetric Michael Reactions of Unreactive Nitroalkenes: One-Pot Synthesis of Chiral GABA-Analogs with All-Carbon Quaternary Stereogenic Centers. Angew. Chem. Int. Ed. 2017, 56, 1835–1839.
- Wang, Z.L. Recent advances in catalytic asymmetric decarboxylative addition reactions. Adv. Synth. Catal. 2013, 355, 2745–2755.
- Bae, H.Y.; Some, S.; Lee, J.H.; Kim, J.Y.; Song, M.J.; Lee, S.; Zhang, Y.J.; Song, C.E. Organocatalytic Enantioselective Michael-Addition of Malonic Acid Half-Thioesters to β-Nitroolefins: From Mimicry of Polyketide Synthases to Scalable Synthesis of γ-Amino Acids. Adv. Synth. Catal. 2011, 353, 3196–3202.
- Lubkoll, J.; Wennemers, H. Mimicry of Polyketide Synthases—Enantioselective 1, 4-Addition Reactions of Malonic Acid Half-Thioesters to Nitroolefins. Angew. Chem. Int. Ed. 2007, 46, 6841–6844.
- Li, F.; Li, Y.Z.; Jia, Z.S.; Xu, M.H.; Tian, P.; Lin, G.Q. Biscinchona alkaloids as highly efficient bifunctional organocatalysts for the asymmetric conjugate addition of malonates to nitroalkenes at ambient temperature. Tetrahedron 2011, 67, 10186–10194.
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071.
- Tsakos, M.; Kokotos, C.G.; Kokotos, G. Primary Amine-Thioureas with Improved Catalytic Properties for “Difficult” Michael Reactions: Efficient Organocatalytic Syntheses of (S)-Baclofen, (R)-Baclofen and (S)-Phenibut. Adv. Synth. Catal. 2012, 354, 740–746.
- Zheng, K.; Liu, X.; Feng, X. Recent advances in metal-catalyzed asymmetric 1,4-conjugate addition (ACA) of nonorganometallic nucleophiles. Chem. Rev. 2018, 118, 7586–7656.
- Hui, C.; Pu, F.; Xu, J. Metal-Catalyzed Asymmetric Michael Addition in Natural Product Synthesis. Chem. Eur. J. 2017, 23, 4023–4036.
- Evans, D.A.; Mito, S.; Seidel, D. Scope and mechanism of enantioselective Michael additions of 1,3-dicarbonyl compounds to nitroalkenes catalyzed by nickel (II)–diamine complexes. J. Am. Chem. Soc. 2007, 129, 11583–11592.
- Liu, K.; Jin, R.; Ceng, T.; Xu, X.; Gao, F.; Liu, G.; Li, H. Functionalized Periodic Mesoporous Organosilica: A Highly Enantioselective Catalyst for the Michael Addition of 1,3-Dicarbonyl Compounds to Nitroalkenes. Chem. Eur. J. 2012, 18, 15546–15553.
- Bissessar, D.; Achard, T.; Bellemin-Laponnaz, S. Robust and Recyclable Self-Supported Chiral Nickel Catalyst for the Enantioselective Michael Addition. Adv. Synth. Catal. 2016, 358, 1982–1988.
- Poe, S.L.; Kobašlija, M.; McQuade, D.T. Mechanism and application of a microcapsule enabled multicatalyst reaction. J. Am. Chem. Soc. 2007, 129, 9216–9221.
- Ishitani, H.; Kanai, K.; Yoo, W.J.; Yoshida, T.; Kobayashi, S. A Nickel-Diamine/Mesoporous Silica Composite as a Heterogeneous Chiral Catalyst for Asymmetric 1, 4-Addition Reactions. Angew. Chem. Int. Ed. 2019, 58, 13313–13317.
- Buendia, M.B.; Kegnæs, S.; Kramera, S. A Nickel-Bisdiamine Porous Organic Polymer as Heterogeneous Chiral Catalyst for Asymmetric Michael Addition to Aliphatic Nitroalkenes. Adv. Synth. Catal. 2020, 362, 5506–5512.
- Li, X.; Li, X.; Peng, F.; Shao, Z. Mutually Complementary Metal- and Organocatalysis with Collective Synthesis: Asymmetric Conjugate Addition of 1,3-Carbonyl Compounds to Nitroenynes and Further Reactions of the Products. Adv. Synth. Catal. 2012, 354, 2873–2885.
- Matsunaga, S.; Shibasaki, M. Recent advances in cooperative bimetallic asymmetric catalysis: Dinuclear Schiff base complexes. Chem. Commun. 2014, 50, 1044–1057.
- Furutachi, M.; Mouri, S.; Matsunaga, S.; Shibasaki, M. A Heterobimetallic Ni/La-salan Complex for Catalytic Asymmetric Decarboxylative 1,4-Addition of Malonic Acid Half-Thioester. Chem. Asian J. 2010, 5, 2351–2354.
- Tsubogo, T.; Yamashita, Y.; Kobayashi, S. Toward efficient asymmetric carbon–carbon bond formation: Continuous flow with chiral heterogeneous catalysts. Chem. Eur. J. 2012, 18, 13624–13628.
- Ishitani, H.; Furiya, Y.; Kobayashi, S. Enantioselective Sequential-Flow Synthesis of Baclofen Precursor via Asymmetric 1,4-Addition and Chemoselective Hydrogenation on Platinum/Carbon/Calcium Phosphate Composites. Chem. Asian. J. 2020, 15, 1688–1691.
- Ishitani, H.; Saito, Y.; Tsubogo, T.; Kobayashi, S. Synthesis of Nitro-Containing Compounds through Multistep Continuous Flow with Heterogeneous Catalysts. Org. Lett. 2016, 18, 1346–1349.
- Tsubogo, T.; Oyamada, H.; Kobayashi, S. Multistep continuous-flow synthesis of (R)-and (S)-rolipram using heterogeneous catalysts. Nature 2015, 520, 329–332.
- Zadsirjan, V.; Heravi, M.M. Oxazolidinones as Chiral Auxiliaries in the Asymmetric 1,4-Conjugate Addition Reaction Applied to the Total Synthesis of Natural Products: A Supplemental Mini-Review. Curr. Org. Synth. 2018, 15, 3–20.
- Tovar-Gudiño, E.; Morales-Nava, R.; Fernández-Zertuche, M. Diasteroselective conjugate addition of diethylaluminum cyanide to a conjugated N-enoyl system: An alternative synthesis of (S)-pregabalin. Can. J. Chem. 2014, 92, 45–48.
- Armstrong, A.; Convine, N.J.; Popkin, M.E. Diastereoselective conjugate addition of cyanide to α, β-unsaturated oxazolidinones: Enantioselective synthesis of ent-pregabalin and baclofen. Synlett 2006, 2006, 1589–1591.
- He, C.; Zhai, Z.; Zhou, Y.; Li, J.; Wang, G. A new synthetic route for the preparation of pregabalin. Synth. Commun. 2021, 51, 2034–2040.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.9K
Revisions:
3 times
(View History)
Update Date:
01 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




