
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Luisa Brandi | -- | 1968 | 2022-06-28 12:00:33 | | | |
| 2 | Lindsay Dong | -3 word(s) | 1965 | 2022-06-29 03:44:10 | | |
Video Upload Options
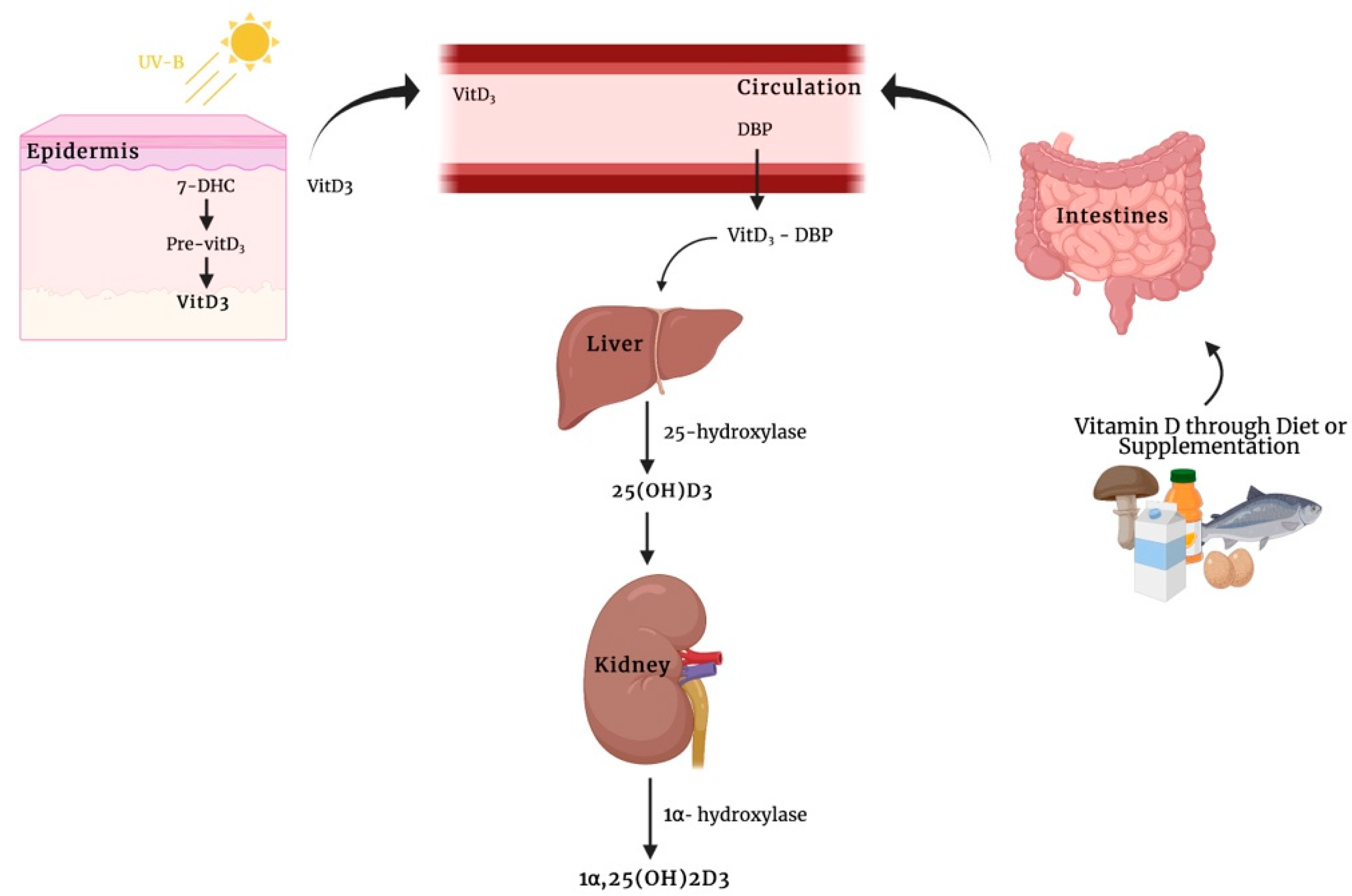
Classically, a secosteroid hormone, vitamin D, has been implicated in calcium and phosphate homeostasis and has been associated with the pathogenesis of rickets and osteomalacia in patients with severe nutritional vitamin D deficiency. The spectrum of known vitamin D-mediated effects has been expanded in recent years. However, the mechanisms of how exactly this hormone elicits its biological function are still not fully understood. The interaction of this metabolite with the vitamin D receptor (VDR) and, subsequently, with the vitamin D-responsive element in the region of specific target genes leading to the transcription of genes whose protein products are involved in the traditional function of calcitriol (known as genomic actions). Moreover, in addition to these transcription-dependent mechanisms, it has been recognized that the biologically active form of vitamin D3, as well as its immediate precursor metabolite, calcifediol, initiate rapid, non-genomic actions through the membrane receptors that are bound as described for other steroid hormones. So far, among the best candidates responsible for mediating rapid membrane response to vitamin D metabolites are membrane-associated VDR (VDRm) and protein disulfide isomerase family A member 3 (Pdia3).
1. Introduction
2. Rapid, Non-Genomic Steroid Actions
3. Mechanisms of Membrane-Associated Proteins for 1α,25(OH)2D3-Mediated Rapid, Non-Genomic Actions
4. 25(OH)D3-Mediated Rapid, Non-Genomic Actions
References
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95.
- Clinckspoor, I.; Verlinden, L.; Mathieu, C.; Bouillon, R.; Verstuyf, A.; Decallonne, B. Vitamin D in thyroid tumorigenesis and development. Prog. Histochem. Cytochem. 2013, 48, 65–98.
- Naveh-Many, T.; Marx, R.; Keshet, E.; Pike, J.W.; Silver, J. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin D3 in the parathyroid in vivo. J. Clin. Investig. 1990, 86, 1968–1975.
- Valero Zanuy, M.; Hawkins Carranza, F. Metabolismo, fuentes endógenas y exógenas de vitamina D. Rev. Esp. Enferm. Metab. Oseas 2007, 16, 63–70.
- DeLuca, H.F. The Metabolism and Functions of Vitamin D. In Steroid Hormone Resistance: Mechanisms and Clinical Aspects; Chrousos, G.P., Loriaux, D.L., Lipsett, M.B., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1986; pp. 361–375. ISBN 978-1-4684-5101-6.
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S.
- Feldman, D.; Malloy, P.J.; Gross, C. Chapter 9—Vitamin D: Biology, Action, and Clinical Implications. In Osteoporosis, 2nd ed.; Marcus, R., Feldman, D., Kelsey, J., Eds.; Academic Press: San Diego, LA, USA, 2001; pp. 257–303. ISBN 978-0-12-470862-4.
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776.
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66, S73–S87.
- Schmidt, B.M.; Gerdes, D.; Feuring, M.; Falkenstein, E.; Christ, M.; Wehling, M. Rapid, nongenomic steroid actions: A new age? Front. Neuroendocrinol. 2000, 21, 57–94.
- Selye, H. Correlations between the chemical structure and the pharmacological actions of the steroids. Endocrinology 1942, 30, 437–453.
- Spach, C.; Streeten, D.H.P. Retardation of Sodium Exchange in Dog Erythrocytes by Physiological Concentrations of Aldosterone, In Vitro. J. Clin. Investig. 1964, 43, 217–227.
- Donati, S.; Palmini, G.; Romagnoli, C.; Aurilia, C.; Miglietta, F.; Falsetti, I.; Marini, F.; Zonefrati, R.; Galli, G.; Marcucci, G.; et al. In Vitro Non-Genomic Effects of Calcifediol on Human Preosteoblastic Cells. Nutrients 2021, 13, 4227.
- Lou, Y.-R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170.
- Gerdes, D.; Christ, M.; K. Haseroth, K.; Notzon, A.; Falkenstein, E.; Wehling, M. Nongenomic Actions of Steroids-From the Laboratory to Clinical Implications. J. Pediat. Endocrinol. Metab. 2000, 13, 853–878.
- Nemere, I.; Yoshimoto, Y.; Norman, A.W. Calcium transport in perfused duodena from normal chicks: Enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology 1984, 115, 1476–1483.
- Fleet, J.C. Rapid, Membrane-Initiated Actions of 1,25 Dihydroxyvitamin D: What Are They and What Do They Mean? J. Nutr. 2004, 134, 3215–3218.
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: A review of the roles of phospholipase A2 activating protein and Ca (2+)/calmodulin-dependent protein kinase II. J. Steroid Biochem. Mol. Biol. 2015, 147, 81–84.
- Dwivedi, P.P.; Hii, C.S.T.; Ferrante, A.; Tan, J.; Der, C.J.; Omdahl, J.L.; Morris, H.A.; May, B.K. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J. Biol. Chem. 2002, 277, 29643–29653.
- Nutchey, B.K.; Kaplan, J.S.; Dwivedi, P.P.; Omdahl, J.L.; Ferrante, A.; May, B.K.; Hii, C.S.T. Molecular action of 1,25-dihydroxyvitamin D3 and phorbol ester on the activation of the rat cytochrome P450C24 (CYP24) promoter: Role of MAP kinase activities and identification of an important transcription factor binding site. Biochem. J. 2005, 389, 753–762.
- Dwivedi, P.; Gao, X.; Tan, J.; Evdokiou, A.; Ferrante, A.; Morris, H.; May, B.; Hii, C. A role for the phosphatidylinositol 3-kinase--protein kinase C zeta-Sp1 pathway in the 1,25-dihydroxyvitamin D3 induction of the 25-hydroxyvitamin D3 24-hydroxylase gene in human kidney cells. Cell. Signal. 2010, 22, 543–552.
- Norman, A.W. Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548.
- Dormanen, M.C.; Bishop, J.E.; Hammond, M.W.; Okamura, W.H.; Nemere, I.; Norman, A.W. Nonnuclear effects of the steroid hormone 1 alpha,25(OH)2-vitamin D3: Analogs are able to functionally differentiate between nuclear and membrane receptors. Biochem. Biophys. Res. Commun. 1994, 201, 394–401.
- Selles, J.; Boland, R. Evidence on the participation of the 3′,5′-cyclic AMP pathway in the non-genomic action of 1,25-dihydroxy-vitamin D3 in cardiac muscle. Mol. Cell. Endocrinol. 1991, 82, 229–235.
- Baran, D.T.; Ray, R.; Sorensen, A.M.; Honeyman, T.; Holick, M.F. Binding characteristics of a membrane receptor that recognizes 1 alpha,25-dihydroxyvitamin D3 and its epimer, 1 beta,25-dihydroxyvitamin D3. J. Cell. Biochem. 1994, 56, 510–517.
- Wu, S.; Ren, S.; Chen, H.; Chun, R.F.; Gacad, M.A.; Adams, J.S. Intracellular vitamin D binding proteins: Novel facilitators of vitamin D-directed transactivation. Mol. Endocrinol. 2000, 14, 1387–1397.
- Chapron, B.D.; Chapron, A.; Phillips, B.; Okoli, M.C.; Shen, D.D.; Kelly, E.J.; Himmelfarb, J.; Thummel, K.E. Reevaluating the role of megalin in renal vitamin D homeostasis using a human cell-derived microphysiological system. ALTEX 2018, 35, 504–515.
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D (3). Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900.
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884.
- Nemere, I.; Dormanen, M.C.; Hammond, M.W.; Okamura, W.H.; Norman, A.W. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J. Biol. Chem. 1994, 269, 23750–23756.
- Nemere, I.; Farach-Carson, M.C.; Rohe, B.; Sterling, T.M.; Norman, A.W.; Boyan, B.D.; Safford, S.E. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7392–7397.
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 281–285.
- Hettinghouse, A.; Liu, R.; Liu, C.-J. Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacol. Ther. 2018, 181, 34–48.
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217.
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55.
- Vazquez, G.; de Boland, A.R.; Boland, R. Stimulation of Ca2+ release-activated Ca2+ channels as a potential mechanism involved in non-genomic 1,25(OH)2-vitamin D3-induced Ca2+ entry in skeletal muscle cells. Biochem. Biophys. Res. Commun. 1997, 239, 562–565.




