Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chitoshi Takayama | -- | 1839 | 2022-06-28 12:00:14 | | | |
| 2 | Conner Chen | Meta information modification | 1839 | 2022-06-29 03:59:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shimizu-Okabe, C.; Kobayashi, S.; Kim, J.; Kosaka, Y.; Sunagawa, M.; Okabe, A.; Takayama, C. GABAergic and Glycinergic Network. Encyclopedia. Available online: https://encyclopedia.pub/entry/24569 (accessed on 07 February 2026).
Shimizu-Okabe C, Kobayashi S, Kim J, Kosaka Y, Sunagawa M, Okabe A, et al. GABAergic and Glycinergic Network. Encyclopedia. Available at: https://encyclopedia.pub/entry/24569. Accessed February 07, 2026.
Shimizu-Okabe, Chigusa, Shiori Kobayashi, Jeongtae Kim, Yoshinori Kosaka, Masanobu Sunagawa, Akihito Okabe, Chitoshi Takayama. "GABAergic and Glycinergic Network" Encyclopedia, https://encyclopedia.pub/entry/24569 (accessed February 07, 2026).
Shimizu-Okabe, C., Kobayashi, S., Kim, J., Kosaka, Y., Sunagawa, M., Okabe, A., & Takayama, C. (2022, June 28). GABAergic and Glycinergic Network. In Encyclopedia. https://encyclopedia.pub/entry/24569
Shimizu-Okabe, Chigusa, et al. "GABAergic and Glycinergic Network." Encyclopedia. Web. 28 June, 2022.
Copy Citation
In the spinal cord, there are three types of inhibitory neurons and terminals: GABAergic, GABA/glycine coreleasing, and glycinergic. These neurons and terminals are arranged in the spinal cord neural circuit and are involved in many vital roles, such as regulating somatic sense, locomotive movement, and respiratory rhythms.
GABA transporter (GAT)
glycine
glycine receptor
1. GABAergic and Glycinergic Network in the Mature Spinal Cord
GABAergic and glycinergic synapses are schematically illustrated in Figure 1A,B, respectively. GABA and glycine are synthesized within the neurons and transported from the extracellular space, including the synaptic cleft. The neurotransmitters are loaded into synaptic vesicles and released from the axon terminals. After diffusion in the synaptic cleft, they bind to GABA or glycine receptors at the postsynaptic membrane. In the mature spinal cord, activation of GABA or glycine receptors induces hyperpolarization of the membrane potential and negatively regulates neuronal activity. The action of these neurotransmitters is terminated by their removal from the synaptic cleft into presynaptic terminals and astrocytic sheets that surround the synapses [1][2][3][4].
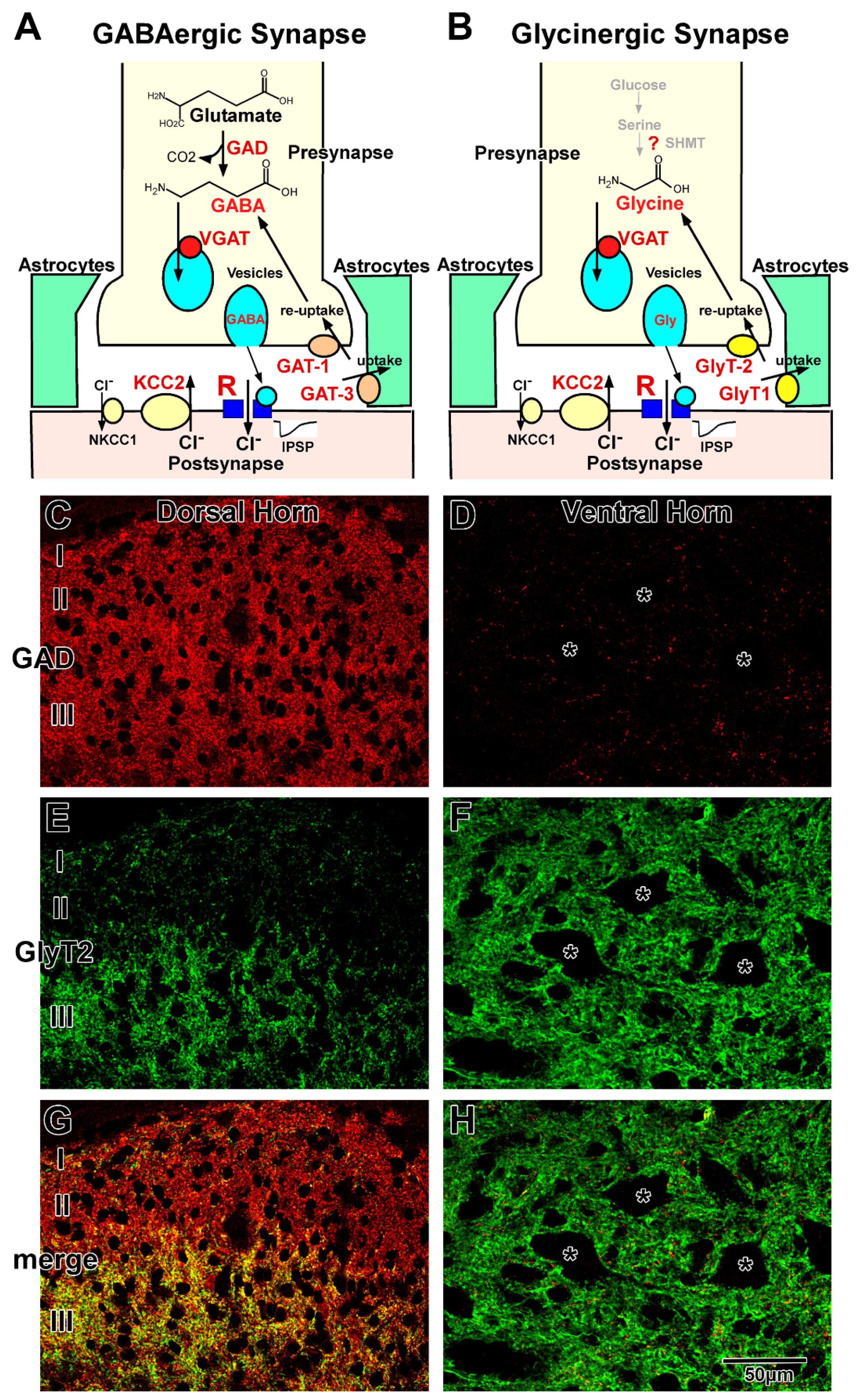
Figure 1. GABAergic and glycinergic transmission in the adult spinal cord. (A,B) are Schematic illustrations of GABAergic (A) and glycinergic (B) synapses. Various key molecules are involved in GABAergic and glycinergic transmission. C and D are Immunohistochemistry for GAD (C,D), GlyT2 (E,F), and both (G,H) in the dorsal (C,E,G) and ventral (D,F,H) horns. In lamina I and II, GABAergic terminals are dominant (C), whereas GAD and GlyT2 double-positive colocalizing terminals (yellow) are dominant in lamina III of the dorsal horn (G). In the ventral horn, glycinergic GlyT2-positive terminals (green) are dominant (F), but GABAergic GAD-positive terminals (red) are scarce (F,H).
2. GABAergic Transmission
GABAergic transmission at the synapse is illustrated in Figure 1A. GABA is synthesized from glutamate by two isoforms of glutamic acid decarboxylase (GAD65 and GAD67) [5]. The primary source of glutamate may be derived from glutamine, which is transported back from astrocytes by glutamine transporter [6]. GABA is loaded into vesicles by vesicular GABA transporter (VGAT), also known as vesicular inhibitory amino acid transporter (VIAAT) [7][8]. GABA is released via the fusion of vesicles with the presynaptic membrane at the nerve terminals and binds to GABA receptors on the postsynaptic membrane.
GABA receptors are classified into three groups based on their pharmacological and biochemical characteristics: GABAA, GABAB, and GABAC. Most of the fast synaptic transmission is mediated by GABAA receptors in the mammalian CNS [9][10]. The GABAA receptor is a member of the ligand-gated ion channel receptor family and is thought to be composed of five heteromeric subunits belonging to seven different subunit families: α1–6, β1–3, γ1–3, δ, ε, π, and θ [9][10][11][12]. Native GABAA receptors contain at least one α, one β, and one γ subunit. The subunit composition varies among the brain regions [13][14][15]. Different subunit compositions exhibit their own characteristic pharmacological and electrophysiological properties [9][11][12][16][17]. The GABAC receptor is also an ion-channel type receptor composed of only single or multiple ρ subunits: ρ1 and ρ2. The GABAC receptor is identified as a bicuculline and baclofen insensitive GABA receptor and is considered a pharmacological variant of GABAA receptors [10][18][19]. The binding of GABA to the GABAA and GABAC receptors induces the influx of chloride ions (Cl−) and mediate hyperpolarization of the postsynaptic membrane potential. The GABAB receptor, which consists of two subunits, GABAB1 and GABAB2, is a metabotropic receptor, activates G proteins, negatively regulates the second messenger system, and responds to slow-acting inhibition of channel and receptor functions [20][21][22][23][24][25]. GABAergic transmission is terminated by the reuptake of GABA into the nerve terminals or uptake into the surrounding astrocytes by plasma membrane GABA transporters (GATs) [26].
GATs are high-affinity Na+ and Cl−-dependent transporters that cotransport GABA with Na+ and Cl− [27][28]. In the CNS, there are three types of GATs: GAT-1, GAT-2, and GAT-3. GAT-2 is localized in leptomeningeal ependymal cells and the choroid plexus [29]. GAT-1 and GAT-3 function at the membranes of axon terminals containing GABAergic vesicles and astrocytic sheets surrounding GABAergic synapses, respectively [30][31][32][33]. In the astrocytes, GABA is degraded into succinate, followed by entering the Krebs cycle [6]. The 2-oxoglutarate in the Krebs cycle is converted to glutamate and glutamine. Astrocytic glutamine is transported back to neurons by a glutamine transporter. This GABA/glutamine cycle may play critical roles in GABA metabolism between neurons and astrocytes [34].
3. Glycinergic Transmission
Glycinergic transmission at the synapse is illustrated in Figure 1B. The metabolic pathway of glycine in neurons is still unclear. Although serine hydroxymethyltransferase (SHMT) has been shown to be able to synthesize in the neurons [35][36][37], little is known about the relationship between SHMT and glycine levels [38]. In general, high-affinity uptake systems, mediated by glycine transporter 2 (GlyT2), are considered the principal regulators of intracellular glycine concentrations. The GlyT2 knockout mice die from lack of glycine during the second postnatal week [39][40], which suggests that de novo synthesis by SHMT is not sufficient for glycinergic neurotransmission [3][41][42], and glycine in the neurons may be dominantly transported from extracellular space through blood–brain barrier. Thus, GlyT2 is a reliable marker for glycine-immunoreactive neurons [38]. After being loaded into vesicles via VGAT (VIAAT) [7][8], glycine is released by exocytosis from the nerve terminals and binds to glycine receptors on the postsynaptic membrane. The glycine receptor is a ligand-gated ion channel receptor that consists of five subunits belonging to two subunit families: α1–3 and β in the mammalian CNS [1][43][44]. The α subunit has a strychnine binding site, and the β subunit binds to the scaffolding protein gephyrin. The composition varies among the CNS regions. Different subunit compositions exhibit their own characteristic electrophysiological properties [44][45]. Glycine binding to the receptor induces an influx of Cl− (Figure 1A), as observed in GABA binding (Figure 1B). The glycinergic action is terminated by reuptake into the nerve terminals and uptake into the surrounding glia through plasma membrane glycine transporters (GlyTs) [46]. GlyTs are high-affinity Na+ and Cl−-dependent transporters that co-transport glycine with Na+ and Cl−. There are two types of GlyTs in the CNS: GlyT1 and GlyT2. In the spinal cord, as well as in other brain regions, GlyT1 is localized at the astrocytic sheets that surround glycinergic synapses, and GlyT2 is localized at the membranes of axon terminals that contain glycinergic vesicles [47][48]. In the astrocyte surrounding glycinergic synapses, glycine may be degraded by the glycine cleavage system [49][50].
4. GABAergic and Glycinergic Transmission in the Mature Spinal Cord
GABA or GAD immunohistochemistry [51][52][53] and GAD-green fluorescent protein (GFP) labeling [54] demonstrated that the density of GABAergic neurons is high in the dorsal horn and moderate in the middle part of the gray matter central part, whereas GABAergic neurons are scarce or in the ventral horn. The distribution of GABAergic terminals is almost the same as that of GABAergic neurons, with high density observed in the dorsal horn (Figure 1C) and low density observed in the ventral horn (Figure 1D). In contrast, glycine immunohistochemistry [55] and GlyT2 expression analysis [56][57] demonstrated that glycinergic neurons and terminals are homogeneously distributed in the gray matter, except for in the superficial layer of the dorsal horn (Figure 1E,F). In lamina I and II, the density of glycinergic neurons and terminals was lower than that observed in the other laminae (Figure 1E) [55][58]. Furthermore, double staining of GABA/GAD and glycine/GlyT2 demonstrated that colocalizing (functionally coreleasing) neurons and terminals are often detected in the spinal cord (Figure 1G,H) [59][60][61], and electrophysiological studies confirmed that the two neurotransmitters are loaded into the same synaptic vesicles and released simultaneously [62][63]. Coreleasing terminals have been abundantly detected in the deep part of the dorsal horn and middle part of the gray matter. In general, GABAergic neurons and their terminals are dominant in lamina I and II (Figure 1G). Coreleasing neurons and terminals are dominant in lamina III to VI (Figure 1G). Glycinergic neurons and their terminals are dominant in the lamina VII–IX (Figure 1H) [47][64][65][66][67]. Total inhibitory terminals detected as VGAT (VIAAT)-positive dots are homogeneously and ubiquitously distributed in the gray matter [51]. GABA and glycine play distinct roles in motor and sensory functions in the complex network in the dorsal [68][69] and ventral horns [70]. Fast GABAergic transmission is dominantly mediated by GABAA receptors consisting of α3β3γ2 subunits in the dorsal horn and α2(α5)β3γ2 subunits in the motor neurons [14][71]. Glycinergic transmission is mediated by glycine receptors consisting of α1β (two α1 and three β) subunits [45][72]. GABAA and glycine receptors colocalize at the postsynaptic membrane of the coreleasing terminals [73]. GABAB Receptors consisting of B1, 1a and 1b, and B2 mainly play roles in the dorsal horn and motor neuron pool [74][75]. [3H]-baclofen binding assay demonstrated that GABAB receptor activity may be higher in the dorsal horn than in other areas [74]. GABAc receptors, containing only ρ2 subunits, play roles in the dorsal horn, whereas motor neurons express those consisting of both ρ1 and ρ2 subunits [76]. Released GABA is removed into presynaptic terminals by GAT-1 and into astrocytic sheets by GAT-3. In contrast, uptake of released glycine into the astrocytic processes is mediated by GlyT1, and reuptake of glycine into the presynaptic terminals is mediated by GlyT2. Because GAT-1 distribution is identical to that of the GABAergic terminals, GAT-1 is abundantly localized in the dorsal horn and sparsely localized in the ventral horn [77]. In contrast, GlyT2 is homogeneously distributed throughout the gray matter [47][78]. Although the localizations of GABAergic and glycinergic terminals are different, both GAT-3 and GlyT1 are homogeneously distributed throughout the astrocytic sheets surrounding synapses, suggesting that astrocytic uptake may ubiquitously occur regardless of terminal distribution.
5. Regulation of GABAergic and Glycinergic Action by Chloride Transporters
In the CNS, the change in membrane potential exerted by GABA and glycine is determined by the intracellular chloride ion concentration ([Cl−]i), which is regulated by the balance of two different chloride cotransporters, Na+-K+-Cl− cotransporter 1 (NKCC1) and K+-Cl− cotransporter 2 (KCC2) (Figure 2A) [79][80][81]. NKCC1 increases the [Cl−]i, whereas KCC2 decreases the [Cl−]i. When the action of NKCC1 is relatively high or KCC2 is absent, [Cl−]i is high, and GABA and glycine induce depolarization of the membrane potential (Figure 2A, left). In contrast, when KCC2 expression is high compared with the expression of NKCC1, [Cl−]i is low, and GABA and glycine act in an inhibitory fashion (Figure 2A, right). In the mature CNS, which includes the spinal cord, KCC2 is highly expressed on the membranes of neuronal cell bodies and dendrites (Figure 2C) [51], and its expression level is very high compared with that of NKCC1 [79][80][81]. Thus, in the mature spinal cord, the [Cl−]i is low enough for GABA and glycine to act as inhibitory neurotransmitters [51][82].
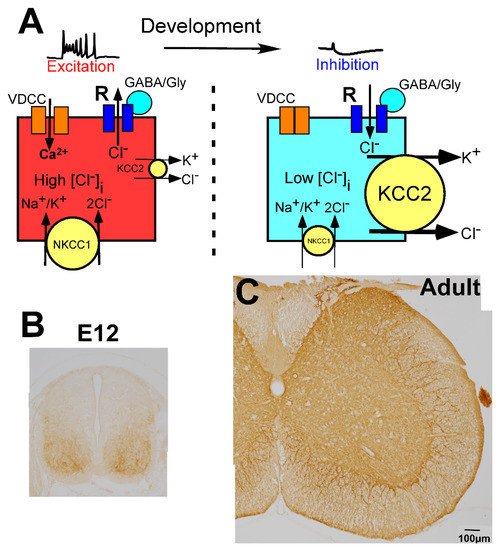
Figure 2. Molecular mechanisms underlying developmental changes in the action of GABA and glycine. (A) Schematic illustration of GABA action depending on intracellular Cl− concentration ([Cl−]i), which is regulated by K+, Cl− cotransporter 2 (KCC2), and Na+, K+, Cl− cotransporter 1 (NKCC1). In the immature stage, KCC2 expression is low and [Cl−]i is high, thus, GABA binding to GABA receptors (R) induces the efflux of chloride ions (Cl−) and mediates excitation (left). In contrast, after maturation, KCC2 expression is high and [Cl−]i is low, thus, GABA mediates inhibition. When GABA/glycine elevates the membrane potential in the immature spinal cord, Ca2+ enters through the activated voltage-dependent calcium channel (VDCC) (left). (B,C) Developmental localization of KCC2. KCC2 is weakly localized in the ventral horn at E12, but the dorsal part was negative (B). In the adult spinal cord, KCC2 is expressed throughout the gray matter (C).
References
- Kirsch, J. Glycinergic transmission. Cell Tissue Res. 2006, 326, 535–540.
- Barker, J.L.; Behar, T.; Li, Y.X.; Liu, Q.Y.; Ma, W.; Maric, D.; Maric, I.; Schaffner, A.E.; Serafini, R.; Smith, S.V.; et al. GABAergic cells and signals in CNS development. Perspect. Dev. Neurobiol. 1998, 5, 305–322.
- Zafra, F.; Aragon, C.; Gimenez, C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997, 14, 117–142.
- Martin, D.L.; Rimvall, K. Regulation of gamma-aminobutyric acid synthesis in the brain. J. Neurochem. 1993, 60, 395–407.
- Varju, P.; Katarova, Z.; Madarasz, E.; Szabo, G. GABA signalling during development: New data and old questions. Cell Tissue Res. 2001, 305, 239–246.
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653.
- Bedet, C.; Isambert, M.F.; Henry, J.P.; Gasnier, B. Constitutive phosphorylation of the vesicular inhibitory amino acid transporter in rat central nervous system. J. Neurochem. 2000, 75, 1654–1663.
- Sagne, C.; El Mestikawy, S.; Isambert, M.F.; Hamon, M.; Henry, J.P.; Giros, B.; Gasnier, B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997, 417, 177–183.
- Macdonald, R.L.; Olsen, R.W. GABAA receptor channels. Annu. Rev. Neurosci. 1994, 17, 569–602.
- Mehta, A.K.; Ticku, M.K. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999, 29, 196–217.
- Olsen, R.W.; Tobin, A.J. Molecular biology of GABAA receptors. FASEB J. 1990, 4, 1469–1480.
- Sieghart, W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995, 47, 181–234.
- Laurie, D.J.; Seeburg, P.H.; Wisden, W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J. Neurosci. 1992, 12, 1063–1076.
- Wisden, W.; Gundlach, A.L.; Barnard, E.A.; Seeburg, P.H.; Hunt, S.P. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res. Mol. Brain Res. 1991, 10, 179–183.
- Wisden, W.; Laurie, D.J.; Monyer, H.; Seeburg, P.H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992, 12, 1040–1062.
- Pritchett, D.B.; Sontheimer, H.; Shivers, B.D.; Ymer, S.; Kettenmann, H.; Schofield, P.R.; Seeburg, P.H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 1989, 338, 582–585.
- Vicini, S. New perspectives in the functional role of GABA(A) channel heterogeneity. Mol. Neurobiol. 1999, 19, 97–110.
- Bormann, J.; Feigenspan, A. GABAC receptors. Trends Neurosci. 1995, 18, 515–519.
- Bormann, J. The ‘ABC’ of GABA receptors. Trends Pharmacol. Sci. 2000, 21, 16–19.
- Bormann, J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988, 11, 112–116.
- Connors, B.W.; Malenka, R.C.; Silva, L.R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J. Physiol. 1988, 406, 443–468.
- LeVine, H., 3rd. Structural features of heterotrimeric G-protein-coupled receptors and their modulatory proteins. Mol. Neurobiol. 1999, 19, 111–149.
- Nicoll, R.A. The coupling of neurotransmitter receptors to ion channels in the brain. Science 1988, 241, 545–551.
- Jembrek, M.J.; Vlainic, J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959.
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABAB Receptor-Structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093.
- Cherubini, E.; Conti, F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001, 24, 155–162.
- Kanner, B.I. Sodium-coupled neurotransmitter transport: Structure, function and regulation. J. Exp. Biol. 1994, 196, 237–249.
- Gadea, A.; Lopez-Colome, A.M. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J. Neurosci. Res. 2001, 63, 461–468.
- Conti, F.; Zuccarello, L.V.; Barbaresi, P.; Minelli, A.; Brecha, N.C.; Melone, M. Neuronal, glial, and epithelial localization of gamma-aminobutyric acid transporter 2, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in the cerebral cortex and neighboring structures. J. Comp. Neurol. 1999, 409, 482–494.
- Minelli, A.; Brecha, N.C.; Karschin, C.; DeBiasi, S.; Conti, F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci. 1995, 15, 7734–7746.
- Minelli, A.; DeBiasi, S.; Brecha, N.C.; Zuccarello, L.V.; Conti, F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 1996, 16, 6255–6264.
- Itouji, A.; Sakai, N.; Tanaka, C.; Saito, N. Neuronal and glial localization of two GABA transporters (GAT1 and GAT3) in the rat cerebellum. Brain Res. Mol. Brain Res. 1996, 37, 309–316.
- Takayama, C.; Inoue, Y. Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Brain Res. Dev. Brain Res. 2005, 158, 41–49.
- Kolker, S. Metabolism of amino acid neurotransmitters: The synaptic disorder underlying inherited metabolic diseases. J. Inherit. Metab. Dis. 2018, 41, 1055–1063.
- Verleysdonk, S.; Martin, H.; Willker, W.; Leibfritz, D.; Hamprecht, B. Rapid uptake and degradation of glycine by astroglial cells in culture: Synthesis and release of serine and lactate. Glia 1999, 27, 239–248.
- Beyoglu, D.; Idle, J.R. The glycine deportation system and its pharmacological consequences. Pharmacol. Ther. 2012, 135, 151–167.
- Zeilhofer, H.U.; Wildner, H.; Yevenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235.
- Xu, T.L.; Gong, N. Glycine and glycine receptor signaling in hippocampal neurons: Diversity, function and regulation. Prog. Neurobiol. 2010, 91, 349–361.
- Gomeza, J.; Ohno, K.; Hulsmann, S.; Armsen, W.; Eulenburg, V.; Richter, D.W.; Laube, B.; Betz, H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 2003, 40, 797–806.
- Latal, A.T.; Kremer, T.; Gomeza, J.; Eulenburg, V.; Hulsmann, S. Development of synaptic inhibition in glycine transporter 2 deficient mice. Mol. Cell. Neurosci. 2010, 44, 342–352.
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793.
- Chalphin, A.V.; Saha, M.S. The specification of glycinergic neurons and the role of glycinergic transmission in development. Front. Mol. Neurosci. 2010, 3, 11.
- Laube, B.; Maksay, G.; Schemm, R.; Betz, H. Modulation of glycine receptor function: A novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol. Sci. 2002, 23, 519–527.
- Lynch, J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004, 84, 1051–1095.
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309.
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333.
- Sunagawa, M.; Shimizu-Okabe, C.; Kim, J.; Kobayashi, S.; Kosaka, Y.; Yanagawa, Y.; Matsushita, M.; Okabe, A.; Takayama, C. Distinct development of the glycinergic terminals in the ventral and dorsal horns of the mouse cervical spinal cord. Neuroscience 2017, 343, 459–471.
- Zafra, F.; Aragon, C.; Olivares, L.; Danbolt, N.C.; Gimenez, C.; Storm-Mathisen, J. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 1995, 15, 3952–3969.
- Sato, K.; Yoshida, S.; Fujiwara, K.; Tada, K.; Tohyama, M. Glycine cleavage system in astrocytes. Brain Res. 1991, 567, 64–70.
- Kikuchi, G.; Motokawa, Y.; Yoshida, T.; Hiraga, K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 246–263.
- Kosaka, Y.; Kin, H.; Tatetsu, M.; Uema, I.; Takayama, C. Distinct development of GABA system in the ventral and dorsal horns in the embryonic mouse spinal cord. Brain Res. 2012, 1486, 39–52.
- Todd, A.J.; Maxwell, D.J. GABA in the mammalian spinal cord. In GABA in the Nervous System; Martin, D.L., Olsen, R.W., Eds.; Lippincotto Willams & Wilkins: Philadelphia, PA, USA, 2000; pp. 439–457.
- Ottersen, O.P.; Storm-Mathisen, J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J. Comp. Neurol. 1984, 229, 374–392.
- Tamamaki, N.; Yanagawa, Y.; Tomioka, R.; Miyazaki, J.; Obata, K.; Kaneko, T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003, 467, 60–79.
- Campistron, G.; Buijs, R.M.; Geffard, M. Glycine neurons in the brain and spinal cord. Antibody production and immunocytochemical localization. Brain Res. 1986, 376, 400–405.
- Hossaini, M.; French, P.J.; Holstege, J.C. Distribution of glycinergic neuronal somata in the rat spinal cord. Brain Res. 2007, 1142, 61–69.
- Zeilhofer, H.U.; Studler, B.; Arabadzisz, D.; Schweizer, C.; Ahmadi, S.; Layh, B.; Bosl, M.R.; Fritschy, J.M. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 2005, 482, 123–141.
- Allain, A.E.; Bairi, A.; Meyrand, P.; Branchereau, P. Expression of the glycinergic system during the course of embryonic development in the mouse spinal cord and its co-localization with GABA immunoreactivity. J. Comp. Neurol. 2006, 496, 832–846.
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145.
- Vaaga, C.E.; Borisovska, M.; Westbrook, G.L. Dual-transmitter neurons: Functional implications of co-release and co-transmission. Curr. Opin. Neurobiol. 2014, 29, 25–32.
- Jonas, P.; Bischofberger, J.; Sandkuhler, J. Corelease of two fast neurotransmitters at a central synapse. Science 1998, 281, 419–424.
- Wojcik, S.M.; Katsurabayashi, S.; Guillemin, I.; Friauf, E.; Rosenmund, C.; Brose, N.; Rhee, J.S. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 2006, 50, 575–587.
- Ishibashi, H.; Yamaguchi, J.; Nakahata, Y.; Nabekura, J. Dynamic regulation of glycine-GABA co-transmission at spinal inhibitory synapses by neuronal glutamate transporter. J. Physiol. 2013, 591, 3821–3832.
- Todd, A.J.; Sullivan, A.C. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J. Comp. Neurol. 1990, 296, 496–505.
- Ornung, G.; Shupliakov, O.; Linda, H.; Ottersen, O.P.; Storm-Mathisen, J.; Ulfhake, B.; Cullheim, S. Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: A postembedding electron microscopic study. J. Comp. Neurol. 1996, 365, 413–426.
- Dougherty, K.J.; Sawchuk, M.A.; Hochman, S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience 2009, 163, 909–919.
- Bardoni, R.; Takazawa, T.; Tong, C.K.; Choudhury, P.; Scherrer, G.; Macdermott, A.B. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci. 2013, 1279, 90–96.
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284.
- Cioffi, C.L. Modulation of Glycine-Mediated Spinal Neurotransmission for the Treatment of Chronic Pain. J. Med. Chem. 2018, 61, 2652–2679.
- Goulding, M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat. Rev. Neurosci. 2009, 10, 507–518.
- Paul, J.; Zeilhofer, H.U.; Fritschy, J.M. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol. 2012, 520, 3895–3911.
- Malosio, M.L.; Marqueze-Pouey, B.; Kuhse, J.; Betz, H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991, 10, 2401–2409.
- Todd, A.J.; Watt, C.; Spike, R.C.; Sieghart, W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996, 16, 974–982.
- Dorfman, V.B.; Vega, M.C.; Coirini, H. Age-related changes of the GABA-B receptor in the lumbar spinal cord of male rats and penile erection. Life Sci. 2006, 78, 1529–1534.
- Sands, S.A.; Purisai, M.G.; Chronwall, B.M.; Enna, S.J. Ontogeny of GABA(B) receptor subunit expression and function in the rat spinal cord. Brain Res. 2003, 972, 197–206.
- Rozzo, A.; Armellin, M.; Franzot, J.; Chiaruttini, C.; Nistri, A.; Tongiorgi, E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur. J. Neurosci. 2002, 15, 1747–1758.
- Kim, J.; Kosaka, Y.; Shimizu-Okabe, C.; Niizaki, A.; Takayama, C. Characteristic development of the GABA-removal system in the mouse spinal cord. Neuroscience 2014, 262, 129–142.
- Jursky, F.; Nelson, N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J. Neurochem. 1995, 64, 1026–1033.
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727.
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739.
- Payne, J.A.; Rivera, C.; Voipio, J.; Kaila, K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003, 26, 199–206.
- Baccei, M.L.; Fitzgerald, M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J. Neurosci. 2004, 24, 4749–4757.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
702
Revisions:
2 times
(View History)
Update Date:
29 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




