Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
In the spinal cord, there are three types of inhibitory neurons and terminals: GABAergic, GABA/glycine coreleasing, and glycinergic. These neurons and terminals are arranged in the spinal cord neural circuit and are involved in many vital roles, such as regulating somatic sense, locomotive movement, and respiratory rhythms.
- GABA transporter (GAT)
- glycine
- glycine receptor
1. GABAergic and Glycinergic Network in the Mature Spinal Cord
GABAergic and glycinergic synapses are schematically illustrated in Figure 1A,B, respectively. GABA and glycine are synthesized within the neurons and transported from the extracellular space, including the synaptic cleft. The neurotransmitters are loaded into synaptic vesicles and released from the axon terminals. After diffusion in the synaptic cleft, they bind to GABA or glycine receptors at the postsynaptic membrane. In the mature spinal cord, activation of GABA or glycine receptors induces hyperpolarization of the membrane potential and negatively regulates neuronal activity. The action of these neurotransmitters is terminated by their removal from the synaptic cleft into presynaptic terminals and astrocytic sheets that surround the synapses [3,11,12,13].
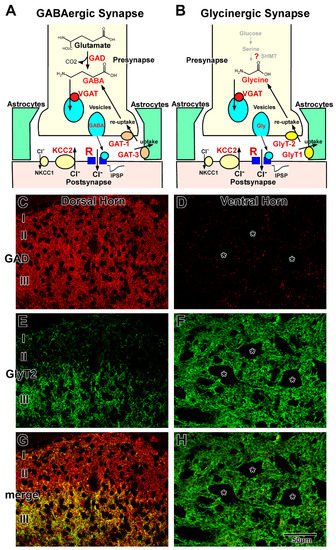
Figure 1. GABAergic and glycinergic transmission in the adult spinal cord. (A,B) are Schematic illustrations of GABAergic (A) and glycinergic (B) synapses. Various key molecules are involved in GABAergic and glycinergic transmission. C and D are Immunohistochemistry for GAD (C,D), GlyT2 (E,F), and both (G,H) in the dorsal (C,E,G) and ventral (D,F,H) horns. In lamina I and II, GABAergic terminals are dominant (C), whereas GAD and GlyT2 double-positive colocalizing terminals (yellow) are dominant in lamina III of the dorsal horn (G). In the ventral horn, glycinergic GlyT2-positive terminals (green) are dominant (F), but GABAergic GAD-positive terminals (red) are scarce (F,H).
2. GABAergic Transmission
GABAergic transmission at the synapse is illustrated in Figure 1A. GABA is synthesized from glutamate by two isoforms of glutamic acid decarboxylase (GAD65 and GAD67) [14]. The primary source of glutamate may be derived from glutamine, which is transported back from astrocytes by glutamine transporter [15]. GABA is loaded into vesicles by vesicular GABA transporter (VGAT), also known as vesicular inhibitory amino acid transporter (VIAAT) [16,17]. GABA is released via the fusion of vesicles with the presynaptic membrane at the nerve terminals and binds to GABA receptors on the postsynaptic membrane.
GABA receptors are classified into three groups based on their pharmacological and biochemical characteristics: GABAA, GABAB, and GABAC. Most of the fast synaptic transmission is mediated by GABAA receptors in the mammalian CNS [1,18]. The GABAA receptor is a member of the ligand-gated ion channel receptor family and is thought to be composed of five heteromeric subunits belonging to seven different subunit families: α1–6, β1–3, γ1–3, δ, ε, π, and θ [1,2,18,19]. Native GABAA receptors contain at least one α, one β, and one γ subunit. The subunit composition varies among the brain regions [20,21,22]. Different subunit compositions exhibit their own characteristic pharmacological and electrophysiological properties [1,2,19,23,24]. The GABAC receptor is also an ion-channel type receptor composed of only single or multiple ρ subunits: ρ1 and ρ2. The GABAC receptor is identified as a bicuculline and baclofen insensitive GABA receptor and is considered a pharmacological variant of GABAA receptors [18,25,26]. The binding of GABA to the GABAA and GABAC receptors induces the influx of chloride ions (Cl−) and mediate hyperpolarization of the postsynaptic membrane potential. The GABAB receptor, which consists of two subunits, GABAB1 and GABAB2, is a metabotropic receptor, activates G proteins, negatively regulates the second messenger system, and responds to slow-acting inhibition of channel and receptor functions [27,28,29,30,31,32]. GABAergic transmission is terminated by the reuptake of GABA into the nerve terminals or uptake into the surrounding astrocytes by plasma membrane GABA transporters (GATs) [33].
GATs are high-affinity Na+ and Cl−-dependent transporters that cotransport GABA with Na+ and Cl− [34,35]. In the CNS, there are three types of GATs: GAT-1, GAT-2, and GAT-3. GAT-2 is localized in leptomeningeal ependymal cells and the choroid plexus [36]. GAT-1 and GAT-3 function at the membranes of axon terminals containing GABAergic vesicles and astrocytic sheets surrounding GABAergic synapses, respectively [37,38,39,40]. In the astrocytes, GABA is degraded into succinate, followed by entering the Krebs cycle [15]. The 2-oxoglutarate in the Krebs cycle is converted to glutamate and glutamine. Astrocytic glutamine is transported back to neurons by a glutamine transporter. This GABA/glutamine cycle may play critical roles in GABA metabolism between neurons and astrocytes [41].
3. Glycinergic Transmission
Glycinergic transmission at the synapse is illustrated in Figure 1B. The metabolic pathway of glycine in neurons is still unclear. Although serine hydroxymethyltransferase (SHMT) has been shown to be able to synthesize in the neurons [42,43,44], little is known about the relationship between SHMT and glycine levels [45]. In general, high-affinity uptake systems, mediated by glycine transporter 2 (GlyT2), are considered the principal regulators of intracellular glycine concentrations. The GlyT2 knockout mice die from lack of glycine during the second postnatal week [46,47], which suggests that de novo synthesis by SHMT is not sufficient for glycinergic neurotransmission [4,12,48], and glycine in the neurons may be dominantly transported from extracellular space through blood–brain barrier. Thus, GlyT2 is a reliable marker for glycine-immunoreactive neurons [45]. After being loaded into vesicles via VGAT (VIAAT) [16,17], glycine is released by exocytosis from the nerve terminals and binds to glycine receptors on the postsynaptic membrane. The glycine receptor is a ligand-gated ion channel receptor that consists of five subunits belonging to two subunit families: α1–3 and β in the mammalian CNS [3,49,50]. The α subunit has a strychnine binding site, and the β subunit binds to the scaffolding protein gephyrin. The composition varies among the CNS regions. Different subunit compositions exhibit their own characteristic electrophysiological properties [50,51]. Glycine binding to the receptor induces an influx of Cl− (Figure 1A), as observed in GABA binding (Figure 1B). The glycinergic action is terminated by reuptake into the nerve terminals and uptake into the surrounding glia through plasma membrane glycine transporters (GlyTs) [52]. GlyTs are high-affinity Na+ and Cl−-dependent transporters that co-transport glycine with Na+ and Cl−. There are two types of GlyTs in the CNS: GlyT1 and GlyT2. In the spinal cord, as well as in other brain regions, GlyT1 is localized at the astrocytic sheets that surround glycinergic synapses, and GlyT2 is localized at the membranes of axon terminals that contain glycinergic vesicles [53,54]. In the astrocyte surrounding glycinergic synapses, glycine may be degraded by the glycine cleavage system [55,56].
4. GABAergic and Glycinergic Transmission in the Mature Spinal Cord
GABA or GAD immunohistochemistry [57,58,59] and GAD-green fluorescent protein (GFP) labeling [60] demonstrated that the density of GABAergic neurons is high in the dorsal horn and moderate in the middle part of the gray matter central part, whereas GABAergic neurons are scarce or in the ventral horn. The distribution of GABAergic terminals is almost the same as that of GABAergic neurons, with high density observed in the dorsal horn (Figure 1C) and low density observed in the ventral horn (Figure 1D). In contrast, glycine immunohistochemistry [61] and GlyT2 expression analysis [8,10] demonstrated that glycinergic neurons and terminals are homogeneously distributed in the gray matter, except for in the superficial layer of the dorsal horn (Figure 1E,F). In lamina I and II, the density of glycinergic neurons and terminals was lower than that observed in the other laminae (Figure 1E) [61,62]. Furthermore, double staining of GABA/GAD and glycine/GlyT2 demonstrated that colocalizing (functionally coreleasing) neurons and terminals are often detected in the spinal cord (Figure 1G,H) [5,6,7], and electrophysiological studies confirmed that the two neurotransmitters are loaded into the same synaptic vesicles and released simultaneously [63,64]. Coreleasing terminals have been abundantly detected in the deep part of the dorsal horn and middle part of the gray matter. In general, GABAergic neurons and their terminals are dominant in lamina I and II (Figure 1G). Coreleasing neurons and terminals are dominant in lamina III to VI (Figure 1G). Glycinergic neurons and their terminals are dominant in the lamina VII–IX (Figure 1H) [9,53,65,66,67]. Total inhibitory terminals detected as VGAT (VIAAT)-positive dots are homogeneously and ubiquitously distributed in the gray matter [57]. GABA and glycine play distinct roles in motor and sensory functions in the complex network in the dorsal [68,69] and ventral horns [70]. Fast GABAergic transmission is dominantly mediated by GABAA receptors consisting of α3β3γ2 subunits in the dorsal horn and α2(α5)β3γ2 subunits in the motor neurons [21,71]. Glycinergic transmission is mediated by glycine receptors consisting of α1β (two α1 and three β) subunits [51,72]. GABAA and glycine receptors colocalize at the postsynaptic membrane of the coreleasing terminals [73]. GABAB Receptors consisting of B1, 1a and 1b, and B2 mainly play roles in the dorsal horn and motor neuron pool [74,75]. [3H]-baclofen binding assay demonstrated that GABAB receptor activity may be higher in the dorsal horn than in other areas [74]. GABAc receptors, containing only ρ2 subunits, play roles in the dorsal horn, whereas motor neurons express those consisting of both ρ1 and ρ2 subunits [76]. Released GABA is removed into presynaptic terminals by GAT-1 and into astrocytic sheets by GAT-3. In contrast, uptake of released glycine into the astrocytic processes is mediated by GlyT1, and reuptake of glycine into the presynaptic terminals is mediated by GlyT2. Because GAT-1 distribution is identical to that of the GABAergic terminals, GAT-1 is abundantly localized in the dorsal horn and sparsely localized in the ventral horn [77]. In contrast, GlyT2 is homogeneously distributed throughout the gray matter [53,78]. Although the localizations of GABAergic and glycinergic terminals are different, both GAT-3 and GlyT1 are homogeneously distributed throughout the astrocytic sheets surrounding synapses, suggesting that astrocytic uptake may ubiquitously occur regardless of terminal distribution.
5. Regulation of GABAergic and Glycinergic Action by Chloride Transporters
In the CNS, the change in membrane potential exerted by GABA and glycine is determined by the intracellular chloride ion concentration ([Cl−]i), which is regulated by the balance of two different chloride cotransporters, Na+-K+-Cl− cotransporter 1 (NKCC1) and K+-Cl− cotransporter 2 (KCC2) (Figure 2A) [79,80,81]. NKCC1 increases the [Cl−]i, whereas KCC2 decreases the [Cl−]i. When the action of NKCC1 is relatively high or KCC2 is absent, [Cl−]i is high, and GABA and glycine induce depolarization of the membrane potential (Figure 2A, left). In contrast, when KCC2 expression is high compared with the expression of NKCC1, [Cl−]i is low, and GABA and glycine act in an inhibitory fashion (Figure 2A, right). In the mature CNS, which includes the spinal cord, KCC2 is highly expressed on the membranes of neuronal cell bodies and dendrites (Figure 2C) [57], and its expression level is very high compared with that of NKCC1 [79,80,81]. Thus, in the mature spinal cord, the [Cl−]i is low enough for GABA and glycine to act as inhibitory neurotransmitters [57,82].
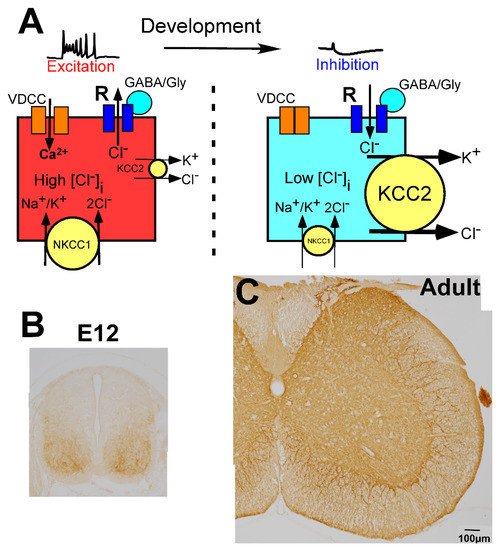
Figure 2. Molecular mechanisms underlying developmental changes in the action of GABA and glycine. (A) Schematic illustration of GABA action depending on intracellular Cl− concentration ([Cl−]i), which is regulated by K+, Cl− cotransporter 2 (KCC2), and Na+, K+, Cl− cotransporter 1 (NKCC1). In the immature stage, KCC2 expression is low and [Cl−]i is high, thus, GABA binding to GABA receptors (R) induces the efflux of chloride ions (Cl−) and mediates excitation (left). In contrast, after maturation, KCC2 expression is high and [Cl−]i is low, thus, GABA mediates inhibition. When GABA/glycine elevates the membrane potential in the immature spinal cord, Ca2+ enters through the activated voltage-dependent calcium channel (VDCC) (left). (B,C) Developmental localization of KCC2. KCC2 is weakly localized in the ventral horn at E12, but the dorsal part was negative (B). In the adult spinal cord, KCC2 is expressed throughout the gray matter (C).
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020834
This entry is offline, you can click here to edit this entry!
