Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jinjing Jia | -- | 1755 | 2022-06-28 08:41:17 | | | |
| 2 | Camila Xu | Meta information modification | 1755 | 2022-06-28 08:57:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jia, J.; Wang, Y.; Mo, X.; Chen, D. Yes-Associated Protein in Psoriasis and Skin Tumor Pathogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/24551 (accessed on 08 February 2026).
Jia J, Wang Y, Mo X, Chen D. Yes-Associated Protein in Psoriasis and Skin Tumor Pathogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/24551. Accessed February 08, 2026.
Jia, Jinjing, Yuqian Wang, Xiumei Mo, Dacan Chen. "Yes-Associated Protein in Psoriasis and Skin Tumor Pathogenesis" Encyclopedia, https://encyclopedia.pub/entry/24551 (accessed February 08, 2026).
Jia, J., Wang, Y., Mo, X., & Chen, D. (2022, June 28). Yes-Associated Protein in Psoriasis and Skin Tumor Pathogenesis. In Encyclopedia. https://encyclopedia.pub/entry/24551
Jia, Jinjing, et al. "Yes-Associated Protein in Psoriasis and Skin Tumor Pathogenesis." Encyclopedia. Web. 28 June, 2022.
Copy Citation
Psoriasis and skin tumors (such as basal cell carcinoma, squamous cell carcinoma, and melanoma) are chronic diseases that endanger physical and mental health, and yet the causes are largely unknown and treatment options limited. Yes-associated protein (YAP) is a key member of the Hippo signaling pathway. It was originally identified in Drosophila, with a molecular weight of 65 kDa, and is also known as YAP65.
Yes-associated protein
Hippo signaling pathway
psoriasis
skin tumors
1. Introduction
Psoriasis is a chronic inflammatory disease of the skin, with an incidence rate of approximately 0.1–4% and affecting all ages. Given its high incidence, recurrence is common. Lesions are often located on exposed parts of the body, which results in significant psychological and social pressure on patients and seriously affects their physical and mental health and quality of life [1][2][3]. Patients with psoriasis are also at increased risk of metabolic syndrome [4][5][6]. However, the etiology and pathogenesis of psoriasis are not completely known. There is a general consensus that adverse external stimuli, such as infection, mental stress, trauma, and medications, result in abnormalities in metabolism and regulation of the immune system. Inflammatory cell chemotaxis, infiltration, and the release of inflammatory mediators under the joint action of internal factors, external factors (acting on certain genotypes), and other risk factors lead to pathological changes, such as excessive proliferation and abnormal differentiation of epidermal keratinocytes [7]. Although biological agents have achieved good results in treating psoriasis in recent years, significant limitations remain, such as high cost, inducibility of infectious diseases and tumors, and recurrence after medication withdrawal. Indeed, psoriasis often requires long-term or even lifelong maintenance treatment [8].
Skin tumors, such as melanoma and non-melanoma skin cancers (NMSCs), are cell proliferative diseases of the intradermal or subcutaneous tissues [9]. The most common types of NMSCs are basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), which often occur at exposed sites. Approximately 5.4 million cases of BCC and cSCC are diagnosed annually [10]. Because the malignancy of these cancers is low and most of them can be surgically removed, the prognosis is relatively good. However, because these tumors mainly occur in the head and face, they can damage the local skin and surrounding tissues, resulting in disfigurement; at later stages, they can have serious consequences due to the invasion of bones and nerves, consequently affecting the daily life of patients and causing serious harm to the physiological and mental health of patients [11].
2. Composition of the YAP and Hippo Signaling Pathways
Yes-associated protein (YAP) is a key member of the Hippo signaling pathway. It was originally identified in Drosophila, with a molecular weight of 65 kDa, and is also known as YAP65 [12]. YAP was confirmed to be encoded by the yap gene on human chromosome 11q22. It is a proline-rich protein that plays a role in controlling the cell number and organ size in Drosophila development by coordinating cell proliferation, the cell cycle, and apoptosis [13][14]. The Hippo signaling pathway includes three main components: upstream kinase cascade initiation, kinase cascade core, and transcription-related activity. The core components of the mammalian kinase cascade include Mst1/2, Sav1, LATS1/2, Mob1, YAP, and TAZ (transcriptional coactivator with the PDZ-binding motif). Dong et al. [15] first determined the signal transmission sequence of the Hippo–YAP pathway in mammals; when an external stimulation signal activates the Hippo pathway, Mst1/2 and Sav form a complex, which binds to LATS1/2 through the adaptor protein WW45, resulting in activation of LATS1/2, which subsequently phosphorylates YAP at the ser127 site. YAP transcriptional activity depends on its subcellular localization: YAP is active in its dephosphorylated state and can thus enter the nucleus and activate gene transcription by interacting with transcription factors TEAD (TEA domain family members) 1–4, Smads, p63/p73, Runx, and ErbB4. When phosphorylated through the upstream kinase cascade, YAP binds to the cytoplasmic 14-3-3 protein, is inactivated, and remains in the cytoplasm, where it cannot act as a transcription factor (Figure 1).
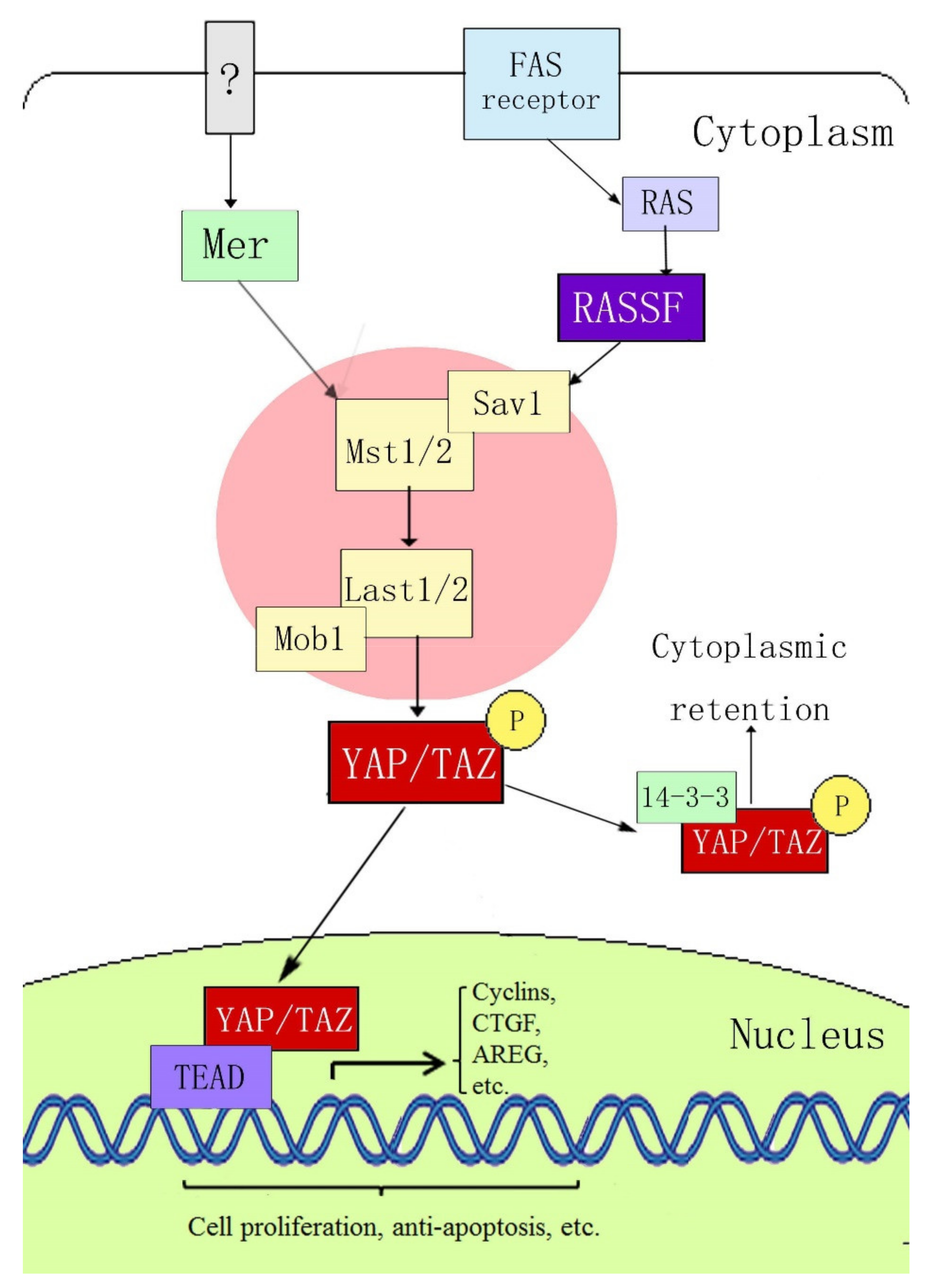 Figure 1. Hippo–YAP signaling pathway.
Figure 1. Hippo–YAP signaling pathway.3. Possible Mechanisms of YAP in Psoriasis and Skin Tumor Pathogenesis
YAP and its upstream pathway (the Hippo signaling pathway) play important roles in regulating cell proliferation and apoptosis to regulate the growth, development, and size of tissues and organs; epithelial mesenchymal transformation (EMT); intercellular contact inhibition; and stem cell self-renewal. Tumor research accounts for the vast majority of studies on YAP [16][17]. YAP is highly expressed in lung cancer [18], breast cancer [19], ovarian cancer [20][21], colon cancer [22], liver cancer [23], and other cancers. In some tumors, YAP expression is positively correlated with the survival rate and degree of tumor malignancy. Psoriasis and tumors are characterized by excessive cell proliferation, abnormal differentiation, vasodilation, and hyperplasia. Therefore, anything that can affect these pathophysiological mechanisms may be a key link in their pathogenesis.
3.1. YAP Regulates Apoptosis
The importance of the Hippo–YAP signaling pathway in regulating apoptosis has been confirmed by a number of studies. In Drosophila, the Yorkie (Yki) protein, a homologue of YAP, can inhibit the activity of the Drosophila apoptosis-related gene reaper (RPR) by promoting the binding of p53 to ASPP1 (the apoptosis-stimulating protein of p53). Yki can also inhibit the activation of defective head involvement (HID), both of which inhibit Drosophila apoptosis [24]. The Hippo–YAP signaling pathway can also increase the expression of Drosophila inhibitor of apoptosis protein 1 (DIP1) [25]. YAP also inhibits apoptosis in human periodontal ligament stem cells [26], human liver cancer cells [27], endometrial stromal cells [28], and meningioma cells [29].
YAP can also promote apoptosis in some cases. During DNA damage, the promyelocytic leukemia gene (PML) recruits YAP and p73 into the nucleus. YAP acts as a transcriptional activator to enhance binding with p73 and activates the transcription of apoptosis-related genes [30]. YAP accelerates amyloid-β-peptide (Aβ)-induced apoptosis through nuclear translocation, induces p73-mediated Bax expression and activation, and promotes the activation of the apoptosis-related protein caspase-3 [31]. In addition, YAP mediates c-Jun-dependent apoptosis [32].
3.2. YAP Regulates Cell Proliferation and Differentiation and Maintains the Three-Dimensional Structure of Skin
In mice and Drosophila, YAP overexpression induces the proliferation of undifferentiated intestinal progenitor cells. Reduced YAP expression induces the differentiation of intestinal progenitor cells. YAP activation or inhibition of upstream negative regulatory proteins can induce the activation or enhancement of the Notch and Wnt signaling pathways, which are related to the inhibition of stem cell differentiation and promotion of stem cell proliferation [33][34]. Schlegelmilch et al. [35] confirmed that YAP regulates epidermal stem cell proliferation and maintains the three-dimensional structure of the skin by interacting with the transcription factor TEAD; the epidermis of YAP-knockout mice became thinner, the stratum corneum decreased, and the arrangement of the epidermal structure became disordered. Zhang et al. [36] also reported high expression of YAP in monolayer basal epidermal progenitor cells, and that the expression of nuclear YAP decreased gradually with age, which was related to the proliferation potential of epidermal progenitor cells. YAP promotes the proliferation of basal epidermal progenitor cells and inhibits terminal differentiation. In vitro studies have shown that, when YAP is activated, the proliferation rate of primary mouse keratinocytes (MKSs) increases, whereas the rates of differentiation and apoptosis decrease, and these characteristics are reversed after inhibiting YAP expression. YAP acts as a molecular switch for epidermal stem/progenitor cell activation. The C-terminus of YAP was found to regulate the balance between stem/progenitor cell proliferation and differentiation in the postnatal interfollicular epidermis [37]. Overexpression of YAP can promote immortalized proliferation of human primary keratinocytes, hinder their normal differentiation process, increase the expression of the epithelial proliferation markers p63 and PCNA, and decrease the expression of the epidermal differentiation markers 14-3-3σ and LEKTI [38]. YAP knockout resulted in a reduced level of expression of transforming growth factor (TGF)-β, a decrease in the proliferation rate of epidermal basal cells, and hindrance of skin wound healing. Interestingly, skin wound healing is dependent on YAP expression [39]. YAP is mainly localized in the cytoplasm of differentiated cells [40]; however, when epithelial tumor cells lose polarity and exhibit enhanced invasiveness, YAP is mainly located in the nucleus. Therefore, the regulation of YAP subcellular localization is crucial for the conversion between proliferation and terminal differentiation [35].
3.3. YAP Regulates Cell Density and Intercellular Contact Inhibition
Contact inhibition refers to the biological characteristic in which cells stop growing because of mutual contact. It is an important regulatory mechanism that maintains the normal morphology of body tissues and prevents disordered cell proliferation in vivo. Contact inhibition allows the cells to stop proliferating when they accumulate in large numbers. Two notable characteristics of many tumor cell lines cultured in vitro are their lack of contact inhibition and the ability to grow without support [41]. When the intercellular density increases, YAP localizes to the cytoplasm, and the activities of cell proliferation-related genes and apoptosis-inhibiting genes are inhibited. In contrast, at a low cell density, YAP localizes to the nucleus and its proliferation-inducing activity is increased; that is, contact inhibition between cells occurs and is mediated by YAP [42]. The integrated membrane protein angiomotin (AMOT) and related proteins can inhibit YAP activity by chelating and binding with YAP in the cytoplasm, thereby inhibiting cell proliferation and intercellular contact and maintaining normal cell density [43]. Zhang et al. [44] found that at high cell densities, overexpression of 14-3-3ζ (an apoptosis inhibitor protein) in human umbilical cord mesenchymal stem cell-exosomes (hucMSC-EX) promoted the binding of LATS to YAP, enhanced the phosphorylation of YAP at ser127, and inhibited YAP activity. After transfecting YAP into the non-neoplastic breast epithelial cell line MCF10A by retrovirus, Overholtzer et al. [45] found that overexpression of YAP could abolish the cell contact inhibition effect, promote growth-factor-independent proliferation, inhibit apoptosis, promote epithelial mesenchymal transition (EMT), and anchor independent growth in soft agar. Taken together, these changes are indicative of malignant transformation of cells.
3.4. YAP can Regulate Angiogenesis
The main pathological feature of psoriasis is the painful expansion of superficial dermal vessels with a clinical manifestation, such as the Auspitz sign [46]. Tumor tissues require a nutrient supply, which is often accompanied by extensive angiogenesis [47]. Choi et al. [48] demonstrated that YAP is an important regulator of angiogenesis in mice; after phosphorylation, YAP is inactivated and then redistributed in a cell-contact-dependent manner through E-cadherin. In mice, YAP knockout is associated with a significant reduction in the number of endothelial buds in the common duct network and aortic ring. During angiogenesis, the vascular endothelial growth factor (VEGF)–VEGF receptor 2 (VEGFR2) signaling axis depends on YAP/TAZ activation [49]. VEGFR regulates YAP/TAZ through the Rho GTPase, mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K) pathways to regulate angiogenesis [50][51][52][53]. During postnatal development of human umbilical vein endothelial cells (HUVECs) and mouse retina, Ang2 is a key YAP target gene in endothelial cells that mediates the regulation of angiogenesis and vascular remodeling by YAP [54].
References
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212.
- Hwang, S.T.; Nijsten, T.; Elder, J.T. Recent Highlights in Psoriasis Research. J. Investig. Dermatol. 2017, 137, 550–556.
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994.
- Liu, L.; Cai, X.C.; Sun, X.Y.; Zhou, Y.Q.; Jin, M.Z.; Wang, J.; Ma, T.; Li, B.; Li, X. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: Current evidence. J. Eur. Acad. Dermatol. Venereol. 2022; Epub ahead of print.
- Kim, D.H.; Lee, J.Y.; Cho, S.I.; Jo, S.J. Risks of Comorbidities in Patients with Palmoplantar Pustulosis vs Patients with Psoriasis Vulgaris or Pompholyx in Korea. JAMA Dermatol. 2022, 27, e221081.
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163.
- Boehncke, W.H. Etiology and Pathogenesis of Psoriasis. Rheum. Dis. Clin. N. Am. 2015, 41, 665–675.
- Marson, J.W.; Snyder, M.L.; Lebwohl, M.G. Newer Therapies in Psoriasis. Med. Clin. N. Am. 2021, 105, 627–641.
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin cancer: Findings and role of high-resolution ultrasound. J. Ultrasound 2019, 22, 423–431.
- Souto, E.B.; da Ana, R.; Vieira, V.; Fangueiro, J.F.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Silva, A.M.; Staszewski, R.; Karczewski, J. Non-melanoma skin cancers: Physio-pathology and role of lipid delivery systems in new chemotherapeutic treatments. Neoplasia 2022, 30, 100810.
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci 2020, 21, 5394.
- Sudol, M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 1994, 9, 2145–2152.
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.G.; Hynes, R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA 2012, 109, E2441–E2450.
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79.
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133.
- Hooglugt, A.; van der Stoel, M.M.; Boon, R.A.; Huveneers, S. Endothelial YAP/TAZ Signaling in Angiogenesis and Tumor Vasculature. Front. Oncol. 2021, 10, 612802.
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589.
- Hsu, P.C.; Jablons, D.M.; Yang, C.T.; You, L. Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int. J. Mol. Sci. 2019, 20, 3821.
- Zhao, W.; Wang, M.; Cai, M.; Zhang, C.; Qiu, Y.; Wang, X.; Zhang, T.; Zhou, H.; Wang, J.; Zhao, W.; et al. Transcriptional co-activators YAP/TAZ: Potential therapeutic targets for metastatic breast cancer. Biomed. Pharmacother. 2021, 133, 110956.
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525.
- Xia, Y.; Chang, T.; Wang, Y.; Liu, Y.; Li, W.; Li, M.; Fan, H.Y. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS ONE 2014, 9, e91770.
- Wang, L.; Shi, S.; Guo, Z.; Zhang, X.; Han, S.; Yang, A.; Wen, W.; Zhu, Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013, 8, e65539.
- Wang, H.; Song, X.; Liao, H.; Wang, P.; Zhang, Y.; Che, L.; Zhang, J.; Zhou, Y.; Cigliano, A.; Ament, C.; et al. Overexpression of Mothers Against Decapentaplegic Homolog 7 Activates the Yes-Associated Protein/NOTCH Cascade and Promotes Liver Carcinogenesis in Mice and Humans. Hepatology 2021, 74, 248–263.
- Zhang, W.; Cohen, S.M. The Hippo pathway acts via p53 and microRNAs to control proliferation and proapoptotic gene expression during tissue growth. Biol. Open 2013, 2, 822–828.
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst Ortholog, hippo, Restricts Growth and Cell Proliferation and Promotes Apoptosis. Cell 2003, 114, 457–467.
- Jia, L.; Gu, W.; Zhang, Y.; Jiang, B.; Qiao, X.; Wen, Y. Activated Yes-Associated Protein Accelerates Cell Cycle, Inhibits Apoptosis, and Delays Senescence in Human Periodontal Ligament Stem Cells. Int. J. Med. Sci. 2018, 15, 1241–1250.
- Bai, N.; Zhang, C.; Liang, N.; Zhang, Z.; Chang, A.; Yin, J.; Li, Z.; Luo, N.; Tan, X.; Luo, N.; et al. Yes-associated protein (YAP) increases chemosensitivity of hepatocellular carcinoma cells by modulation of p53. Cancer Biol. Ther. 2013, 14, 511–520.
- Song, Y.; Fu, J.; Zhou, M.; Xiao, L.; Feng, X.; Chen, H.; Huang, W. Activated Hippo/Yes-Associated Protein Pathway Promotes Cell Proliferation and Anti-apoptosis in Endometrial Stromal Cells of Endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 1552–1561.
- Baia, G.S.; Caballero, O.L.; Orr, B.A.; Lal, A.; Ho, J.S.; Cowdrey, C.; Tihan, T.; Mawrin, C.; Riggins, G.J. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol. Cancer Res. 2012, 10, 904–913.
- Strano, S.; Monti, O.; Pediconi, N.; Baccarini, A.; Fontemaggi, G.; Lapi, E.; Mantovani, F.; Damalas, A.; Citro, G.; Sacchi, A.; et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol. Cell 2005, 18, 447–459.
- Zhang, H.; Wu, S.; Xing, D. YAP accelerates Aβ(25-35)-induced apoptosis through upregulation of Bax expression by interaction with p73. Apoptosis 2011, 16, 808–821.
- Danovi, S.A.; Rossi, M.; Gudmundsdottir, K.; Yuan, M.; Melino, G.; Basu, S. Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008, 15, 217–219.
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17, 2054–2060.
- Shaw, R.L.; Kohlmaier, A.; Polesello, C.; Veelken, C.; Edgar, B.A.; Tapon, N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 2010, 137, 4147–4158.
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795.
- Zhang, H.; Pasolli, H.A.; Fuchs, E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 2011, 108, 2270–2275.
- Beverdam, A.; Claxton, C.; Zhang, X.; James, G.; Harvey, K.F.; Key, B. Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J. Investig. Dermatol. 2013, 133, 1497–1505.
- D’Addario, I.; Abbruzzese, C.; lo Iacono, M.; Teson, M.; Golisano, O.; Barone, V. Overexpression of YAP1 induces immortalization of normal human keratinocytes by blocking clonal evolution. Histochem. Cell Biol. 2010, 134, 265–276.
- Lee, M.J.; Byun, M.R.; Furutani-Seiki, M.; Hong, J.H.; Jung, H.S. YAP and TAZ regulate skin wound healing. J. Investig. Dermatol. 2014, 134, 518–525.
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687.
- Mendonsa, A.M.; Na, T.Y.; Gumbiner, B.M. E-cadherin in contact inhibition and cancer. Oncogene 2018, 37, 4769–4780.
- Zeng, Q.; Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 2008, 13, 188–192.
- Mana-Capelli, S.; Paramasivam, M.; Dutta, S.; McCollum, D. Angiomotins link F-actin architecture to Hippo pathway signaling. Mol. Biol. Cell 2014, 25, 1676–1685.
- Zhang, B.; Shi, Y.; Gong, A.; Pan, Z.; Shi, H.; Yang, H.; Fu, H.; Yan, Y.; Zhang, X.; Wang, M.; et al. HucMSC Exosome-Delivered 14-3-3ζ Orchestrates Self-Control of the Wnt Response via Modulation of YAP During Cutaneous Regeneration. Stem Cells 2016, 34, 2485–2500.
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 2006, 103, 12405–12410.
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035.
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426.
- Choi, H.J.; Zhang, H.; Park, H.; Choi, K.S.; Lee, H.W.; Agrawal, V.; Kim, Y.M.; Kwon, Y.G. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 2015, 6, 6943.
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017, 127, 3441–3461.
- Fan, R.; Kim, N.G.; Gumbiner, B.M. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. USA 2013, 110, 2569–2574.
- Janse van Rensburg, H.J.; Lai, D.; Azad, T.; Hao, Y.; Yang, X. TAZ enhances mammary cell proliferation in 3D culture through transcriptional regulation of IRS1. Cell Signal. 2018, 52, 12–22.
- Zhao, Y.; Montminy, T.; Azad, T.; Lightbody, E.; Hao, Y.; SenGupta, S.; Asselin, E.; Nicol, C.; Yang, X. PI3K Positively Regulates YAP and TAZ in Mammary Tumorigenesis Through Multiple Signaling Pathways. Mol. Cancer Res. 2018, 16, 1046–1058.
- Azad, T.; Nouri, K.; Janse van Rensburg, H.J.; Hao, Y.; Yang, X. Monitoring Hippo Signaling Pathway Activity Using a Luciferase-based Large Tumor Suppressor (LATS) Biosensor. J. Vis. Exp. 2018, 139, 58416.
- Azad, T.; Janse van Rensburg, H.J.; Lightbody, E.D.; Neveu, B.; Champagne, A.; Ghaffari, A.; Kay, V.R.; Hao, Y.; Shen, H.; Yeung, B.; et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun. 2018, 9, 1061.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
882
Revisions:
2 times
(View History)
Update Date:
28 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




