| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silvia Canaider | -- | 2353 | 2022-06-27 16:08:01 | | | |
| 2 | Catherine Yang | -111 word(s) | 2242 | 2022-06-28 04:08:13 | | |
Video Upload Options
Opioids are considered the oldest drugs known by humans and have been used for sedation and pain relief for several centuries. Nowadays, endogenous opioid peptides are divided into four families: enkephalins, dynorphins, endorphins, and nociceptin/orphanin FQ. They exert their action through the opioid receptors (ORs), transmembrane proteins belonging to the su-per-family of G-protein-coupled receptors, and are expressed throughout the body; the receptors are the δ opioid receptor (DOR), μ opioid receptor (MOR), κ opioid receptor (KOR), and nociceptin/orphanin FQ receptor (NOP). Endogenous opioids are mainly studied in the central nervous system (CNS), but their role has been investigated in other organs, both in physiological and in pathological conditions. Here, it is presented a revision of their role in stem cell (SC) biology, since these cells are a subject of great scientific interest due to their peculiar features and their involvement in cell-based therapies in regenerative medicine. In particular, it will be focused on the endogenous opioids’ ability to modulate SC proliferation, stress response (to oxidative stress, starvation, or damage following ischemia–reperfusion), and differentiation towards different lineages, such as neuro-genesis, vasculogenesis, and cardiogenesis.
1. Introduction
Opioids are considered the oldest drugs known by humans and have been used for pain relief and sedation for several centuries. They are a class of compounds related in structure to the natural plant alkaloids which are extracted from the resin of the poppy plant (Papaver somniferum) [1]. Among them, morphine is the most common, active compound, which exerts its action in the central and peripheral nervous systems (CNS and PNS, respectively) through binding to the opioid receptors (ORs) [2].
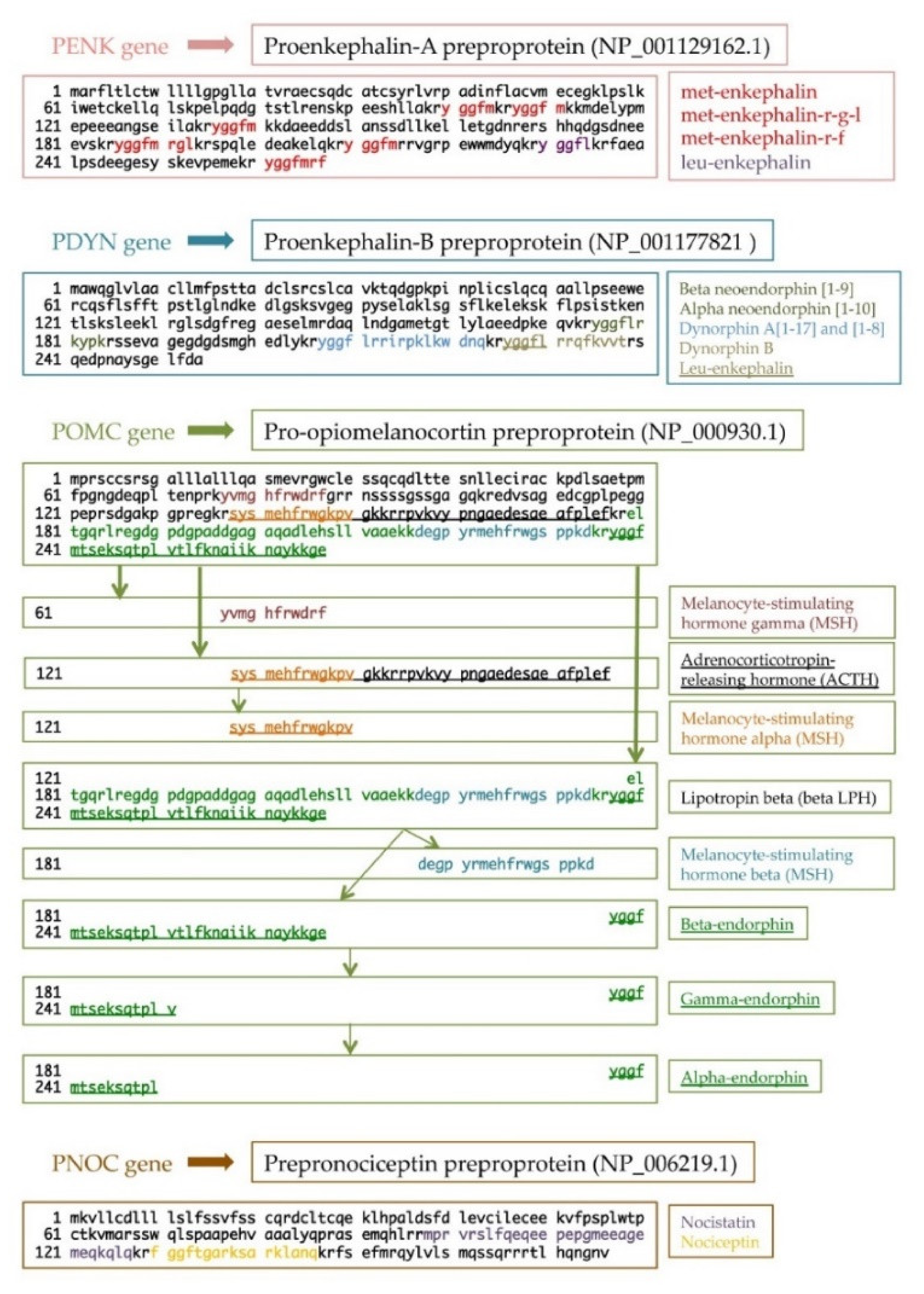
Nowadays, endogenous opioid peptides are divided into four families: enkephalins, dynorphins, endorphins, and nociceptin/orphanin FQ [3]. From a molecular point of view, each opioid peptide is synthesized as a prepro and a proform, creating functional peptides after precursor processing. All peptides share a common aminoterminal sequence, Tyr-Gly-Gly-Phe-(Met/Leu), namely, the opioid motif. For this reason, the same precursor may result in different opioid peptides (Figure 1) [4][5].
Endogenous opioid peptides (and exogenous opioids) exert their action through the opioid receptors. ORs are transmembrane proteins belonging to the super-family of G-protein-coupled receptors (GPCRs), which are widely studied due to their key role in mood disorders, drug abuse/addiction, and pain management [6][7][8]. They are expressed not only in the CNS but also in many other districts. There are four subtypes of OR: δ opioid receptor (DOR), μ opioid receptor (MOR), κ opioid receptor (KOR), and nociception/orphanin FQ (NOP) receptor.
Here it will be presented the effect od endogenous opioids on stem cells. Among all the cell types forming the body’s tissues, stem cells (SCs) are the subject of great scientific interest due to their peculiar features. In fact, they are characterized by two important properties: the ability to self-renew and the ability to differentiate into different cell types. Although the mechanisms orchestrating the biology of SCs are not completely understood, it is suggested that their fate strongly depends on the interactions with their microenvironment, called the niche. Increasing evidence states that the niche, consisting of other non-SCs, the extracellular matrix, and signaling factors, in combination with the intrinsic characteristics of SCs, consistently defines their properties and potential. Within this frame, SCs represent a particularly attractive tool for therapeutic applications and regenerative medicine.
2. Endogenous Opioids Modulate Stem Cell Proliferation and Cell Stress Response
The opportunity to modulate SC proliferation and stress response represents one of the main goals of biological SC research aimed at improving the efficiency of SC transplantation. The following Table shows the major outcomes of studies committed to evaluating the role of endogenous opioid peptides on these SC features (Table 1)
| Opioids/Agonists | Pre-Treatment | Antagonists | Opioid Receptor | Cell Type | Biological Effects | Ref. |
|---|---|---|---|---|---|---|
| Met-enkephalin Morphine (10−6 M) |
Naloxone (3 × 10−6 M) |
DOR MOR |
NPCs (from EGL of postnatal 5- and 6-day-old mice) |
Morphine significantly reduced DNA content; this effect was attenuated by naloxone co-administration. Met-enkephalin did not alter DNA synthesis. Opioids did not affect cell viability. |
[9] | |
| Met-enkephalin (10−6 or 10−5 M) |
MOR | hCB-CD34+ and hPB-CD34+ cells |
hCB-CD34+ expressed MOR more than hPB-CD34+ cells. In treated hCB-CD34+ cells, phospho-MAPK was increased by 4.7- to 6.1-fold compared to the untreated cells; the increase of phospho-p38 was moderate. In hCB-CD34+, met-enkephalin did not reducethe apoptosis induced by irradiation. |
[10] | ||
| Dynorphin-A[1–17] Dynorphin-A[2–17] U50,488 (10−14 to 10−8 M) |
Nor-BNI (10−6 M) |
KOR | NPCs (from 7- to 9-week-old human fetal brain tissue) |
Dynorphin-A[1–17] and U50,488 stimulated cell proliferation and migration in a dose-dependent manner. |
[11] | |
| Morphine | MOR | NSCs | Theoretical hypothesis: since morphine reduces testosterone levels, increases DHT levels, andover-expresses p53 gene, it might prevent NSC proliferation. |
[12] | ||
| Morphine sulfate (10−6 to 1.3 × 10−5 M) |
Naloxone | MOR | NPCs (from 14-day-oldmouse embryos) |
Morphine decreased proliferation of NPCs and induced the caspase-3 activity in a dose-dependent manner. Morphine induced neuronal differentiation of NPCs. |
[13] | |
| Nociceptin | NOP | Mouse SSCs and spermatocytes |
Nociceptin is an upstream Sertoli cell transcription factor that regulates SSC self-renewal and spermatocyte meiosis. |
[14] | ||
| Morphine (10−4 M) |
Naloxone (5 × 10−5 M) |
MOR | Rat NSCs | Morphine decreased NSC growth and increased apoptosis. Morphine reduced the secretion of insulin and insulin-like growth factors and downregulated insulin receptor expression. |
[15] | |
| DADLE (10−7 M) |
Serum deprivation |
Naltrindole | DOR | hUCB-MSCs | DADLE increased anti-apoptotic Bcl-2, decreased pro-apoptotic Bax/Bad, decreased the activated caspase-3, upregulated PI3K subunit p110γ, and activated Akt. DADLE upregulated the release of anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) and downregulated the secretion of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1). |
[16] |
| DADLE (10−7 M) |
H2O2 (6 × 10−4 M) |
DOR | hUCB-MSCs | DADLE increased cell viability, upregulated the anti-apoptotic protein Bcl-2,and suppressed the pro-apoptotic proteins Bax/Bad. DADLE reduced intracellular ROS levels and AP sites. DADLE downregulated UPR genes: IRE-1α, BiP, PERK, ATF-4, and CHOP. |
[17] | |
| DADLE (10−7 M) |
H/R induced by CoCl2 (7.5 × 10−4 M) |
Naltrindole | DOR | hUCB-MSCs | DADLE increased cell viability and reduced intracellular ROS levels. DADLE suppressed mitochondrial complex 1 activity. DADLE upregulated the anti-apoptotic gene Bcl-2 while downregulating the pro-apoptotic gene Bax and UPR genes PERK, IRE-1α, BiP, PERK, and ATF-6. DADLE upregulated the release of anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) and downregulated the secretion of pro-inflammatory cytokines (TNF-α, IL-6, IFN-γ, and IL-1β). |
[18] |
2. Endogenous Opioids modulate Stem Cell Differentiation
The ability to differentiate is one of the most important properties of SCs. The following Table summarizes the results obtained from the studies demonstrating the involvement of endogenous or synthetic opioids in SC commitment and/or differentiation, in particolar neural, hematopoietic, vascular and cardiac stem cell differentiation (Table 2).
Table 1. Effects of endogenous opioids on stem cell proliferation and stress response.
| Opioids/Agonists | Pre-Treatment | Antagonists | Opioid Receptor | Cell Type | Biological Effects | Ref. |
|---|---|---|---|---|---|---|
| Neural Differentiation | ||||||
| DAMGO U69,593 (10−7–10−6 M) |
RA neuralinduction | KOR-1 MOR-1 |
ESCs (from mouse blastocyst) ESCs (from ICM of 3.5-day-old mouse) |
MOR-1 and KOR-1 were expressed in undifferentiated ESCs and in RA-induced ESC-derived NPCs. Both opioids induced ESC neuronal differentiation activating ERK pathway. |
[19] | |
| DAMGO U69,593 (10−6 M) |
RA neural induction |
KOR MOR |
ESCs (from mouse blastocyst) |
Opioids reduced neurogenesis and astrogenesis in RA-induced ESC-NPCs through p38 MAPK and ERK pathways, respectively. Opioids stimulated oligodendrogenesis via both ERK and p38 signaling pathways. |
[20] | |
| DAMGO SNC80 U50,488H (10−7–3 × 10−5 M) |
DOR KOR MOR |
MEB5 (from 14.5-day-old mouse forebrains) |
Only the DOR agonist SNC80 promoted neural differentiation. | [21] | ||
| Neural induction |
Human USSCs and BM-MSCs |
Neural induction increased enkephalinergic markers (Ikaros, CREBZF, and PENK), especially in USSC-derived neuron-like cells. PDYN expression was enhanced in USSC-derived neuron-like cells. |
[22] | |||
| Dynorphin-A U50,488H (10−6 M) |
Neural induction with opioid/ agonist |
Nor-BNI (10−5 M) |
KOR | NSCs (from 8-week-old mouse hippocampus) |
NSCs expressed high levels of KOR. Opioid treatment decreased neurogenesis by modulating Pax6/Neurog2/NeuroD1 activities via upregulation of miR-7a expression. Opioid treatment did not alter astrogenesis and oligodendrogenesis. Opioid treatment did not affect proliferation and apoptosis. |
[23] |
| Morphine (10−5 M) |
Neural induction with opioid |
NSCs (from postnatal p0 mouse hippocampus) |
Morphine promoted neurogenesis, increased apoptosis, and decreased total cell number during the later stages of differentiation. Morphine increased glutathione/glutathione disulfide ratio and decreased S-adenosylmethionine/S-adenosylhomocysteine ratio. |
[24] | ||
| Hematopoietic and Vascular Differentiation | ||||||
| Beta-endorphin (1 to 1000 ng/mL) Dynorphin (1 ng/mL) Leu-enkephalin Met-enkephalin (100 ng/mL) |
EP (0.4 U/mL) induced erythropoiesis with opioid |
Mouse BM progenitor cells | In the presence of EP, opioids enhanced BM progenitor differentiation into CFU-e. |
[25] | ||
| TRK820 U50,488H (10−5 M) |
Vascular induction | KOR | ESstA-ROSA (engineered mouse ESCs) |
KOR agonists inhibited EC differentiation and 3D vascular formation in ESC-derived vascular progenitor cells. KOR agonists decreased the expression of Flk1 and NRP1 through inhibition of cAMP/PKA signaling in vascular progenitor cells. |
[26] | |
| Met-enkephalin (10−14 to 10−8 M) |
KOR DOR |
Mouse BM progenitor cells |
Met-enk upregulated the expression of KOR and DOR in BM-derived DCs. Met-enk induced BM-derived DCs to differentiate mainly towards the mDC subtype. Met-enk increased the expression of MHC class II molecules and the release of pro-inflammatory cytokines (IL-12p70, TNF-α). |
[27] | ||
| Hematopoietic and Vascular Differentiation | ||||||
| Morphine (10−4 M) |
Naloxone (10−4 M) |
Rat NSCs | Morphine reduced survival and clonogenicity, negatively affecting tubulogenesis properties of NSCs by the inhibition of neuro-angiogenesis trans-differentiation. |
[28] | ||
| Cardiac Differentiation | ||||||
| Dynorphin-B (10−9 to 10−6 M) |
DMSO 1% | KOR | Mouse ESCs | DMSO increased PDYN gene expression and dynorphin-B synthesis and secretion. Dynorphin-B elicited GATA-4 and Nkx-2.5 gene transcription and enhanced gene and protein expression of α-MHC and MLC-2V. |
[29] | |
| Dynorphin-B (10−8 to 10−6 M) |
Cardiac induction |
KOR | GTR1-ESCs (engineered mouse ESCs) |
ESC plasma membranes and nuclei expressed KOR-specific opioid binding sites. ESC-derived cardiomyocytes showed an increase in dynorphin-B around the nucleus. Dynorphin-B induced an increase of GATA-4, Nkx-2.5, and PDYN gene expressions and promoted cardiogenesis by PKC signaling. |
[30][31] | |
| HBR cardiac induction (0.75 mg/mL) |
GTR1-ESCs (engineered mouse ESCs) |
HBR-induced ESC-derived cardiomyocytes enhanced GATA-4, Nkx-2.5, and PDYN gene transcriptions and the intracellular level of dynorphin-B. |
[32] | |||
| ELF-MF exposition during cardiac induction (50 Hz, 0.8 m Trms) |
GTR1-ESCs (engineered mouse ESCs) |
ELF-MF spontaneously induced cardiogenesis, upregulating GATA-4, Nkx-2.5, and PDYN gene expression and enhancing intracellular levels and secretion of dynorphin-B. |
[33] | |||
| Cardiac Differentiation | ||||||
| REAC exposition during cardiac induction (MF of 2.4 and 5.5 GHz) |
Mouse ESCs and human ASCs |
Both SCs committed to cardiac lineage and exposed to REAC increased the expression of GATA-4, Nkx-2.5, and PDYN gene. |
[34][35] | |||
| Dynorphin-B (10−7 M) |
Cardiac induction |
CPCs (from 11.5-day-oldembryonic mouseventricles) |
Dynorphin B promoted CPC differentiation into cardiomyocytes. | [36] | ||
| Dynorphin-A Dynorphin-B Met-enkephalins Leu-enkephalins (10−5 M) |
Cardiac induction |
DOR KOR |
Mouse ESCs | Both DOR and KOR increased during ESC differentiation. Dynorphin-B inhibited Oct-4 and increased Nkx-2.5 gene expression. Dynorphin-A, met-enkephalins, and leu-enkephalinsdid not affect ESC differentiation. |
[37] | |
DAMGO, [D-Ala2,MePhe4,Glyol5]-enkephalin; U69,593, N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide; RA, retinoic ac-id; KOR-1, κ opioid receptor isoform 1; MOR-1, μ opioid receptor isoform 1; ESCs, embryonic stem cells; ICM, inner cell mass; NPCs, neural progenitor cells; ERK, extracellular signal-regulated kinase; p38 MAPK, p38 mitogen-activated protein kinase; SNC80, [(+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide]; U50,488H, (–)-trans-(1S,2S)-U-50488 hydrochlo-ride; Nor-BNI, nor-binaltorphimine; DOR, δ opioid receptor; MEB5, multipotent neural stem cells; USSCs, unrestricted somatic stem cells; BM-MSCs, bone mar-row mesenchymal stem cells; Ikaros, IKAROS family zinc finger 1; CREBZF, CREB/ATF bZIP transcription factor; PENK, proenkephalin; PDYN, prodynorphin; NSCs, neural stem cells; Pax6, paired box 6; Neurog2, neurogenin 2; NeuroD1, neuronal differentiation 1; leu-enkephalin, leucine-enkephalin; met-enkephalin, methionine-enkephalin; EP, erythropoietin; CFU-e, colony-forming unit-erythroid; TRK820, 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3-furyl) acrylamido]morphinan hydrochloride; EC, endothelial cell; Flk1, fetal liver kinase 1/VEGF receptor 2; NRP1, neuropilin 1; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; DCs, dendritic cells; mDCs, myeloid dendritic cells; MHC, major histocompatibility complex; TNF-α, tumor necrosis factor alpha; IL-12p70, active heteodimer of interleukin 12. p53, tumor protein p53; DMSO, dimethyl sulfoxide; GATA-4, GATA binding protein 4; Nkx-2.5, Nkx homeobox 5; α-MHC, α-myosin heavy chain; MLC-2V, myosin light chain; PKC, protein kinase C; HBR, hyaluronan mixed esters of butyric and retinoic acids; ELF-MF, extremely low frequency magnetic fields; REAC, radio electric asymmet-ric conveyer; ASCs, adipose-derived mesenchymal stem cells; SCs, stem cells; CPCs, cardiac progenitor cells; Oct-4, octamer-binding transcription factor 4.
3. Conclusion
Overall, opioidergic systems encompass a wide-ranging variety of bioactive peptides, providing multi-layered control of major determinants in cell and SC biology. Compounding their biological complexity, opioid peptides were found to act as “one component–multiple target conductors”, which often led to the observation of opposite effects on the same outcome (i.e., proliferation or differentiation) depending on the spe-cific SC target towards which activity was probed.
Nevertheless, deciphering the complexity of the informational cues associated with opioid peptide-mediated responses may hold promise for intriguing future developments. These future perspectives involve the potential for the timely and synergistic use of naturally occurring and synthetic opioids for the fine tuning of remarkable develop-ments in regenerative medicine, including differentiation, proliferation, multicellular cross talk, inflammation, and tissue remodelling.
References
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Phys. 2008, 11, S133–S153.
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharmacol. Rev. 2013, 65, 1257–1317.
- Kibaly, C.; Xu, C.; Cahill, C.M.; Evans, C.J.; Law, P.Y. Non-nociceptive roles of opioids in the CNS: Opioids’ effects on neurogenesis, learning, memory and affect. Nat. Rev. Neurosci. 2019, 20, 5–18.
- Abrimian, A.; Kraft, T.; Pan, Y.X. Endogenous Opioid Peptides and Alternatively Spliced Mu Opioid Receptor Seven Transmembrane Carboxyl-Terminal Variants. Int. J. Mol. Sci. 2021, 22, 3779.
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473.
- Lutz, P.E.; Kieffer, B.L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013, 36, 195–206.
- Kreek, M.J.; Levran, O.; Reed, B.; Schlussman, S.D.; Zhou, Y.; Butelman, E.R. Opiate addiction and cocaine addiction: Underlying molecular neurobiology and genetics. J. Clin. Investig. 2012, 122, 3387–3393.
- Pasternak, G.W. Opiate pharmacology and relief of pain. J. Clin. Oncol. 2014, 32, 1655–1661.
- Hauser, K.F.; Houdi, A.A.; Turbek, C.S.; Elde, R.P.; Maxson, W., 3rd. Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: Differential impact of mu and delta receptor activation on proliferation and neurite elongation. Eur. J. Neurosci. 2000, 12, 1281–1293.
- Rozenfeld-Granot, G.; Toren, A.; Amariglio, N.; Nagler, A.; Rosenthal, E.; Biniaminov, M.; Brok-Simoni, F.; Rechavi, G. MAP kinase activation by mu opioid receptor in cord blood CD34(+)CD38(-) cells. Exp. Hematol. 2002, 30, 473–480.
- Sheng, W.S.; Hu, S.; Herr, G.; Ni, H.T.; Rock, R.B.; Gekker, G.; Lokensgard, J.R.; Peterson, P.K. Human neural precursor cells express functional kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2007, 322, 957–963.
- Shoae-Hassani, A.; Sharif, S.; Tabatabaei, S.A.; Verdi, J. Could the endogenous opioid, morphine, prevent neural stem cell proliferation? Med. Hypotheses 2011, 76, 225–229.
- Willner, D.; Cohen-Yeshurun, A.; Avidan, A.; Ozersky, V.; Shohami, E.; Leker, R.R. Short term morphine exposure in vitro alters proliferation and differentiation of neural progenitor cells and promotes apoptosis via mu receptors. PLoS ONE 2014, 9, e103043.
- Chen, S.R.; Liu, Y.X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167.
- Salarinasab, S.; Nourazarian, A.; Nikanfar, M.; Abdyazdani, N.; Kazemi, M.; Feizy, N.; Rahbarghazi, R. Impact of morphine on the expression of insulin receptor and protein levels of insulin/IGFs in rat neural stem cells. Neurosci. Lett. 2017, 660, 147–154.
- Reddy, L.V.K.; Sen, D. DADLE enhances viability and anti-inflammatory effect of human MSCs subjected to ‘serum free’ apoptotic condition in part via the DOR/PI3K/AKT pathway. Life Sci. 2017, 191, 195–204.
- Mullick, M.; Venkatesh, K.; Sen, D. d-Alanine 2, Leucine 5 Enkephaline (DADLE)-mediated DOR activation augments human hUCB-BFs viability subjected to oxidative stress via attenuation of the UPR. Stem. Cell Res. 2017, 22, 20–28.
- Mullick, M.; Sen, D. The Delta Opioid Peptide DADLE Represses Hypoxia-Reperfusion Mimicked Stress Mediated Apoptotic Cell Death in Human Mesenchymal Stem Cells in Part by Downregulating the Unfolded Protein Response and ROS along with Enhanced Anti-Inflammatory Effect. Stem. Cell Rev. Rep. 2018, 14, 558–573.
- Kim, E.; Clark, A.L.; Kiss, A.; Hahn, J.W.; Wesselschmidt, R.; Coscia, C.J.; Belcheva, M.M. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J. Biol. Chem. 2006, 281, 33749–33760.
- Hahn, J.W.; Jagwani, S.; Kim, E.; Rendell, V.R.; He, J.; Ezerskiy, L.A.; Wesselschmidt, R.; Coscia, C.J.; Belcheva, M.M. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J. Neurochem. 2010, 112, 1431–1441.
- Narita, M.; Kuzumaki, N.; Miyatake, M.; Sato, F.; Wachi, H.; Seyama, Y.; Suzuki, T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem. 2006, 97, 1494–14505.
- Hafizi, M.; Bakhshandeh, B.; Soleimani, M.; Atashi, A. Exploring the enkephalinergic differentiation potential in adult stem cells for cell therapy and drug screening implications. Vitr. Cell Dev. Biol. Anim. 2012, 48, 562–569.
- Xu, C.; Fan, W.; Zhang, Y.; Loh, H.H.; Law, P.Y. Kappa opioid receptor controls neural stem cell differentiation via a miR-7a/Pax6 dependent pathway. Stem. Cells 2021, 39, 600–616.
- Trivedi, M.; Zhang, Y.; Lopez-Toledano, M.; Clarke, A.; Deth, R. Differential neurogenic effects of casein-derived opioid peptides on neuronal stem cells: Implications for redox-based epigenetic changes. J. Nutr. Biochem. 2016, 37, 39–46.
- Skelly, R.R.; Fata, J.; Sharkis, S.J.; Sensenbrenner, L.; Ansari, A.A. Neuropeptide modulation of murine erythropoiesis. Ann. Clin. Lab. Sci. 1987, 17, 324–330.
- Yamamizu, K.; Furuta, S.; Katayama, S.; Narita, M.; Kuzumaki, N.; Imai, S.; Nagase, H.; Suzuki, T.; Narita, M.; Yamashita, J.K. The κ opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood 2011, 118, 775–785.
- Liu, J.; Chen, W.; Meng, J.; Lu, C.; Wang, E.; Shan, F. Induction on differentiation and modulation of bone marrow progenitor of dendritic cell by methionine enkephalin (MENK). Cancer Immunol. Immunother. 2012, 61, 1699–1711.
- Abdyazdani, N.; Nourazarian, A.; Nozad Charoudeh, H.; Kazemi, M.; Feizy, N.; Akbarzade, M.; Mehdizadeh, A.; Rezaie, J.; Rahbarghazi, R. The role of morphine on rat neural stem cells viability, neuro-angiogenesis and neuro-steroidgenesis properties. Neurosci. Lett. 2017, 636, 205–212.
- Ventura, C.; Maioli, M. Opioid peptide gene expression primes cardiogenesis in embryonal pluripotent stem cells. Circ. Res. 2000, 87, 189–194.
- Ventura, C.; Zinellu, E.; Maninchedda, E.; Fadda, M.; Maioli, M. Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells. Circ Res. 2003, 92, 617–622.
- Ventura, C.; Zinellu, E.; Maninchedda, E.; Maioli, M. Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells. Circ. Res. 2003, 92, 623–629.
- Ventura, C.; Maioli, M.; Asara, Y.; Santoni, D.; Scarlata, I.; Cantoni, S.; Perbellini, A. Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J. Biol. Chem. 2004, 279, 23574–23579.
- Ventura, C.; Maioli, M.; Asara, Y.; Santoni, D.; Mesirca, P.; Remondini, D.; Bersani, F. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J. 2005, 19, 155–157.
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Gualini, S.; Fontani, V.; Ventura, C. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: A new tool for improving tissue regeneration. Cell Transplant. 2012, 21, 1225–1233.
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Bianchi, F.; Tremolada, C.; Fontani, V.; Ventura, C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transpl. 2014, 23, 1489–1500.
- Feridooni, T.; Pasumarthi, K.B.S. Fractionation of embryonic cardiac progenitor cells and evaluation of their differentiation potential. Differentiation 2019, 105, 1489–1500.
- Šínová, R.; Kudová, J.; Nešporová, K.; Karel, S.; Šuláková, R.; Velebný, V.; Kubala, L. Opioid receptors and opioid peptides in the cardiomyogenesis of mouse embryonic stem cells. J. Cell Physiol. 2019, 234, 13209–13219.