
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Romchat Chutoprapat | -- | 2049 | 2022-06-23 12:15:08 | | | |
| 2 | Lindsay Dong | Meta information modification | 2049 | 2022-06-24 02:35:35 | | |
Video Upload Options
Acne vulgaris (acne) is one of the most common dermatological problems affecting adolescents and young adults. Although acne may not lead to serious medical complications, its psychosocial effects are tremendous and scientifically proven. The first-line treatment for acne is topical medications composed of synthetic compounds, which usually cause skin irritation, dryness and itch. Therefore, naturally occurring constituents from plants (phytochemicals), which are generally regarded as safe, have received much attention as an alternative source of treatment. However, the degradation of phytochemicals under high temperature, light and oxygen, and their poor penetration across the skin barrier limit their application in dermatology. Encapsulation in lipid nanoparticles is one of the strategies commonly used to deliver drugs and phytochemicals because it allows appropriate concentrations of these substances to be delivered to the site of action with minimal side effects. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are promising delivery systems developed from the combination of lipid and emulsifier. They have numerous advantages that include biocompatibility and biodegradability of lipid materials, enhancement of drug solubility and stability, ease of modulation of drug release, ease of scale-up, feasibility of incorporation of both hydrophilic and lipophilic drugs and occlusive moisturization, which make them very attractive carriers for delivery of bioactive compounds for treating skin ailments such as acne.
1. Introduction
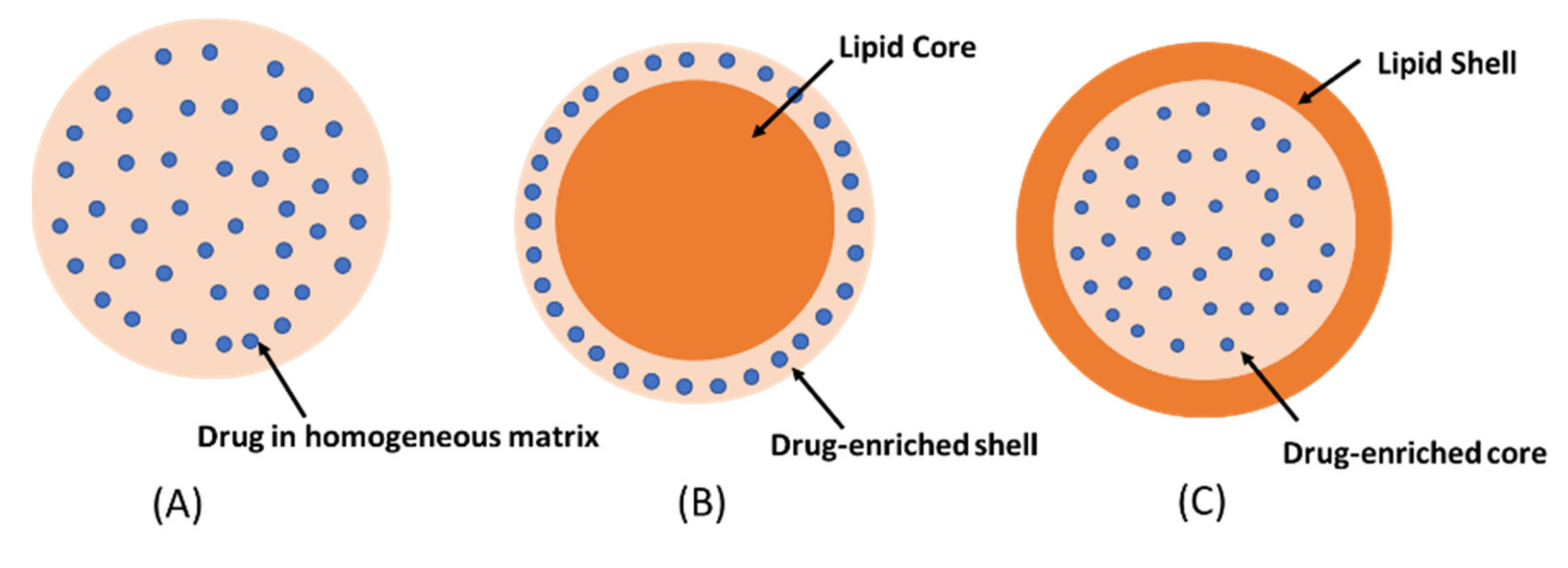
2. Solid Lipid Nanoparticles (SLNs)
-
Homogeneous matrix model: the drug is dispersed in lipid matrix without the use of solubilizers or surfactants. This model is usually prepared by the cold homogenization technique [10].
-
The drug-enriched shell model: a mixture of lipid and drug is heated at temperature above the melting point of the lipid. On rapid cooling, lipid precipitates at the core whereas the drug is concentrated at the outer melted lipid. The drug-enriched shell is completely formed when the melted mixture is cooled to room temperature [11].
-
The drug-enriched core model: the concentration of drug in the melted lipid is close to its saturation solubility. The cooling process creates supersaturation of the drug in the melted lipid, resulting in drug precipitation at the core prior to lipid crystallization. Further cooling will lead to the crystallization of the lipid surrounding the drug core as a shell [12]. The drug-enriched shell and the drug-enriched core models are usually produced by hot homogenization technique.
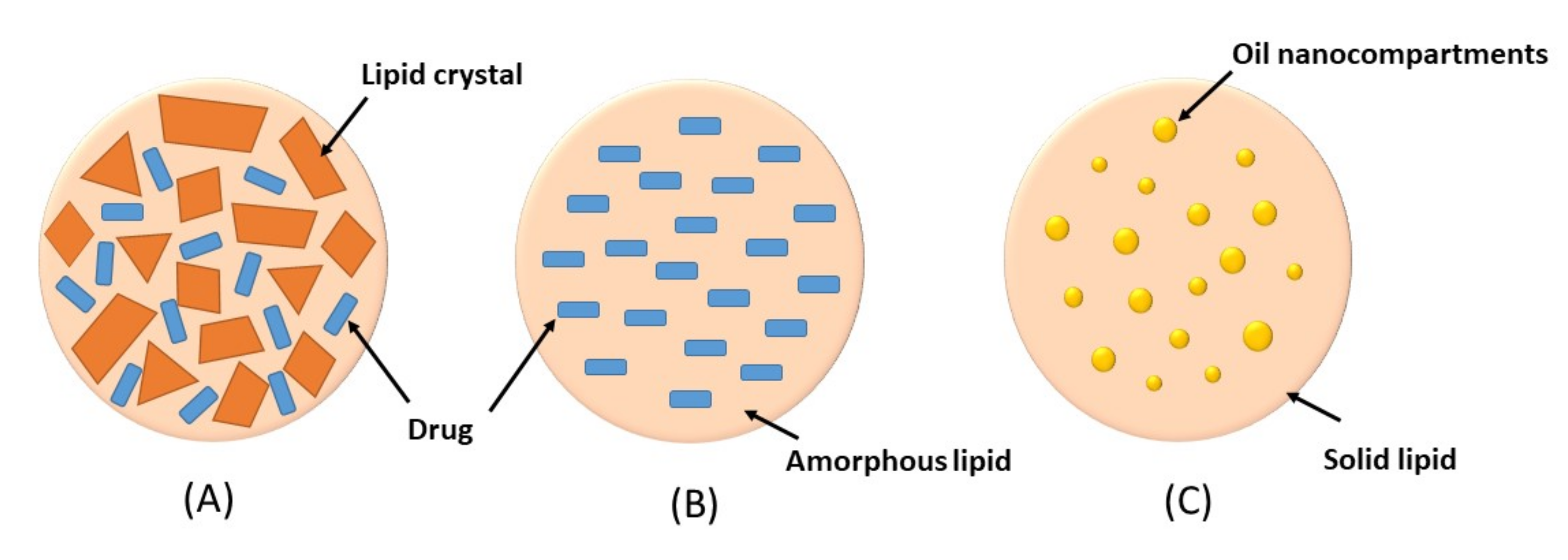
3. Nanostructured Lipid Carriers (NLCs)
-
Imperfect crystal type: this type involves mixing of spatially different liquid lipids such as glycerides and solid lipids which introduce imperfections in the crystal order leading to more space for drug loading. The imperfection can be increased by using a mixture of various glycerides which vary in saturation and length of carbon chains.
-
Amorphous type: this type is formed by incorporating special liquid oils such as isopropyl myristate or hydroxyoctacosanyl hydroxystearate in a lipid matrix. The matrix will solidify in an amorphous form that potentially reduces the expulsion of the loaded drug by delaying the crystallization of lipids during the preparation and storage of the NLCs
-
Multiple O/F/W type: this type is formed by adding high amount of liquid lipid beyond its solubility in the lipid matrix. This will create oil nanocompartments distributed in the solid matrix. Drug solubility in oil nanocompartment is higher than in solid matrix which enables higher drug loading. Moreover, a solid lipid matrix around the oil nanocompartments acts as a barrier that prevents drug leakage and provides controlled drug release.
4. SLNs and NLCs as Topical Carriers for Anti-Acne Phytochemicals
4.1. SLNs as a Promising Carrier System for the Topical Delivery of Anti-Acne Phytochemicals
4.2. NLCs as a Promising Carrier System for the Topical Delivery of Anti-Acne Phytochemicals
5. Conclusions
References
- Garg, T. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif. Cells Nanomed. Biotechnol. 2016, 44, 98–105.
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508.
- Beloqui, A.; Solinís, M.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161.
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J. Pharm. Sci. 2017, 106, 423–445.
- Verma, S.; Utreja, P.; Duvedi, D.R.; Prasad, D.; Kumar, L. Nanotechnological carriers for treatment of acne. Recent Pat. Antiinfect Drug Discov. 2018, 13, 105–126.
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358.
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191.
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 16989.
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Khosroshahi, A.Y.; Hamishehkar, H. The effect of particle size on the deposition of solid lipid nanoparticles in different skin layers: A histological study. Adv. Pharm. Bull. 2016, 6, 31–36.
- Zur Mühlen, A.; Mehnert, W. Drug release and release mechanisms of prednisolone loaded solid lipid nanoparticles. Pharmazie 1998, 53, 552–555.
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155.
- Wissing, S.A.; Muller, R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379.
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830.
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Coll. Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156.
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613.
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291.
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440.
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro- and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A Literature Review. Pharmaceutics 2021, 13, 451.
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482.
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712.
- Hamishehkar, H.; Same, S.; Adibkia, K.; Zarza, K.; Shokri, J.; Taghaee, M.; Kouhsoltani, M. A comparative histological study on the skin occlusion performance of a cream made of solid lipid nanoparticles and Vaseline. Res. Pharm. Sci. 2015, 10, 378–387.
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid lipid nanoparticles produced via a coacervation method as promising carriers for controlled release of Quercetin. Molecules 2021, 26, 2694.
- Tsai, T.H.; Huang, W.C.; Lien, T.J.; Huang, Y.H.; Chang, H.; Yu, C.H.; Tsai, P.J. Clove extract and eugenol suppress inflammatory responses elicited by Propionibacterium acnes in vitro and in vivo. Food Agric. Immunol. 2017, 28, 916–931.
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438.
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Panteli, M.I.; Rasit, I.; Badea, N. Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind. Crops Prod. 2017, 109, 141–150.
- Lacatusu, I.; Istrati, D.; Bordei, N.; Popescu, M.; Seciu, A.M.; Panteli, L.M.; Badea, N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: Emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C 2020, 108, 110412.
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259.
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid-willow bark extract-panthenol—Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020, 154, 112658.
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032.
- Da Rocha, P.B.R.; Souza, B.S.; Andrade, L.M.; Dos Anjos, J.L.V.; Mendanha, S.A.; Alonso, A.; Marreto, R.N.; Taveira, S.F. Enhanced asiaticoside skin permeation by Centella asiatica-loaded lipid nanoparticles: Effects of extract type and study of stratum corneum lipid dynamics. J. Drug Deliv. Sci. Technol. 2019, 50, 305–312.




