
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiumei Mo | -- | 2737 | 2022-06-23 02:14:05 | | | |
| 2 | Lindsay Dong | Meta information modification | 2737 | 2022-06-23 11:00:20 | | |
Video Upload Options
Electrospun techniques are promising and flexible technologies to fabricate ultrafine fiber/nanofiber materials from diverse materials with unique characteristics under optimum conditions. These fabricated fibers/nanofibers via electrospinning can be easily assembled into several shapes of three-dimensional (3D) structures and can be combined with other nanomaterials. Therefore, electrospun nanofibers, with their structural and functional advantages, have gained considerable attention from scientific communities as suitable candidates in biomedical fields, such as the regeneration of tissues and organs, where they can mimic the network structure of collagen fiber in its natural extracellular matrix(es). Due to these special features, electrospinning has been revolutionized as a successful technique to fabricate such nanomaterials from polymer media.
1. Introduction
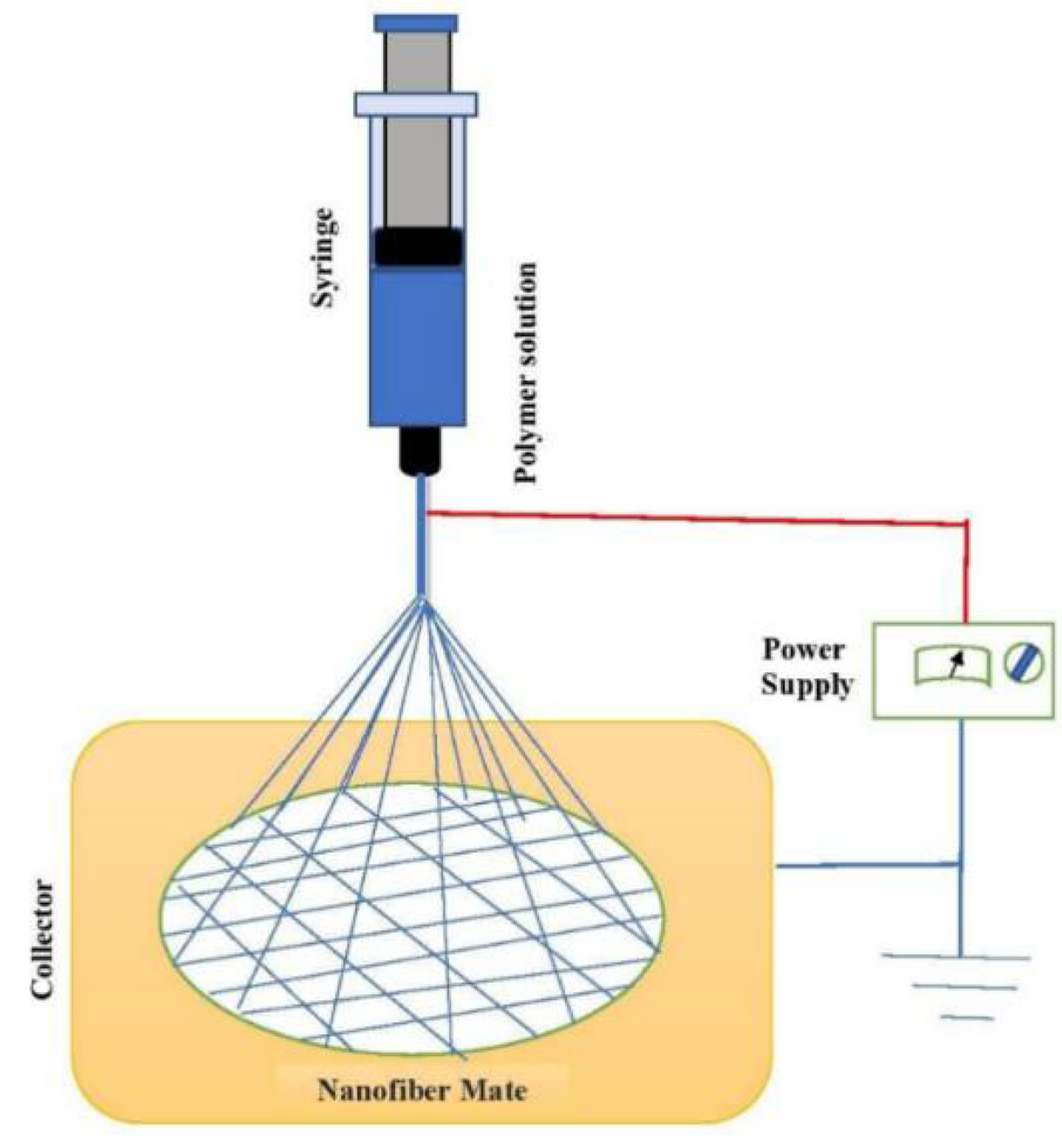
2. Fabrication of Nanofibers via Electrospinning
3. Applications of Nanofibers
3.1. Biomedical Applications
3.1.1. Bone Cell Proliferation
3.1.2. Nerve Regeneration
3.1.3. Vascular Tissue
3.1.4. Skin Tissue Engineering
4. Conclusions
References
- Anstey, A.; Chang, E.; Kim, E.S.; Rizvi, A.; Kakroodi, A.C.; Park, B.; Lee, P.C. Nanofibrillated polymer systems: Design, application, and current state of the art. Prog. Polym. Sci. 2021, 113, 101346.
- Guerrero-Pérez, M.O. Research Progress on the Applications of Electrospun Nanofibers in Catalysis. Catalysts 2022, 12, 9.
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175.
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746.
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2022, 14, 1.
- Yu, Y.S.; Ahn, C.B.; Son, K.H.; Lee, J.W. Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology. Polymers 2021, 13, 971.
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021, 11, 13877.
- Ikegami, Y.; Ijima, H. Development of heparin-conjugated nanofibers and a novel biological signal by immobilized growth factors for peripheral nerve regeneration. J. Biosci. Bioeng. 2020, 129, 354–362.
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721.
- Lee, K.S.; Kayumov, M.; Emechebe, G.A.; Kim, D.-W.; Cho, H.-J.; Jeong, Y.-J.; Lee, D.-W.; Park, J.-K.; Park, C.-H.; Kim, C.-S.; et al. A Comparative Study of an Anti-Thrombotic Small-Diameter Vascular Graft with Commercially Available e-PTFE Graft in a Porcine Carotid Model. Tissue Eng. Regen. Med. 2022.
- Jiang, J.; Chen, S.; Wanga, H.; Carlson, M.A.; Gombart, A.F.; Xie, J. CO2-expanded nanofiber scaffolds maintain activity of encapsulated bioactive materials and promote cellular infiltration and positive host response. Acta Biomater. 2018, 68, 237–248.
- Cojocaru, E.; Ghitman, J.; Biru, E.I.; Pircalabioru, G.G.; Vasile, E.; Iovu, H. Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications. Materials 2021, 14, 2535.
- El-Ghazali, S.; Kobayashi, H.; Khatri, M.; Phan, D.-N.; Khatri, Z.; Mahar, S.K.; Kobayashi, S.; Kim, I.-S. Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications. Polymers 2021, 13, 3458.
- Mehrani, Z.; Ebrahimzadeh, H.; Moradi, E.; Yamini, Y. Using three-dimensional poly (vinyl alcohol)/sodium hexametaphosphate nanofiber as a non-toxic and efficient nanosorbent for extraction and recovery of Lanthanide ions from aqueous solutions. J. Mol. Liq. 2020, 307, 112925.
- Kumar, P.S.; Venkatesh, K.; Gui, E.L.; Sundaramurthy, J.; Singh, G.; Arthanareeswaran, G. Electrospun carbon nanofibers/TiO2-PAN hybrid membranes for effective removal of metal ions and cationic dye, Environmental Nanotechnology. Monit. Manag. 2018, 10, 366–376.
- Yin, J.; Zhan, F.; Jiaoa, T.; Deng, H.; Zou, G.; Bai, Z.; Zhang, Q.; Peng, Q. Highly efficient catalytic performances of nitro compounds via hierarchical PdNPs-loaded MXene/polymer nanocomposites synthesized through electrospinning strategy for wastewater treatment. Chin. Chem. Lett. 2020, 31, 992–995.
- Zhang, Z.; Wu, X.; Kou, Z.; Song, N.; Nie, G.; Wang, C.; Verpoort, F.; Mu, S. Rational design of electrospun nanofiber-typed electrocatalysts for water splitting: A review. Chem. Eng. J. 2022, 428, 131133.
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Weia, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189.
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Kim, H.J.; Park, C.H.; Kim, C.S. Multi-layered macroporous three-dimensional nanofibrous scaffold via a novel gas foaming technique. Chem. Eng. J. 2015, 275, 79–88.
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704.
- Shikhi-Abadi, P.G.; Irani, M. A review on the applications of electrospun chitosan nanofibers for the cancer treatment. Int. J. Biol. Macromol. 2021, 183, 790–810.
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908.
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726.
- Ali, M.G.; Mousa, H.M.; Blaudez, F.; Abd El-sadek, M.S.; Mohamed, M.A.; Abdel-Jaber, G.T.; Abdal-hay, A.; Ivanovski, S. Dual nanofiber scaffolds composed of polyurethane-gelatin/nylon 6-gelatin for bone tissue engineering. Colloids Surf. A 2020, 597, 124817.
- Deng, L.; Li, Y.; Zhang, H. In vitro and in vivo assessment of glucose cross-linked gelatin/zein nanofibrous scaffolds for cranial bone defects regeneration. J. Biomed. Mater. Res. 2020, 108B, 1505–1517.
- Singh, Y.P.; Dasgupta, S.; Nayar, S.; Bhaskar, R. Optimization of electrospinning process & parameters for producing defect-free chitosan/polyethylene oxide nanofibers for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 781–803.
- Zhu, J.; Ye, H.; Deng, D.; Li, J.; Wu, Y. Electrospun metformin-loaded polycaprolactone/chitosan nanofibrous membranes as promoting guided bone regeneration membranes: Preparation and characterization of fibers, drug release, and osteogenic activity in vitro. J. Biomater. Appl. 2020, 34, 1282–1293.
- Sharifi, F.; Atyabi, S.M.; Irani, S.; Bakhshi, H. Bone morphogenic protein-2 immobilization by cold atmospheric plasma to enhance the osteoinductivity of carboxymethyl chitosan-based nanofibers. Carbohydr. Polym. 2020, 231, 115681.
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168.
- Yang, S.; Zhu, J.; Lu, C.; Chai, Y.; Cao, Z.; Lu, J.; Zhang, Z.; Zhao, H.; Huang, Y.; Yao, S.; et al. Aligned fibrin/functionalized self-assembling peptide interpenetrating nanofiber hydrogel presenting multi-cues promotes peripheral nerve functional recovery. Bioact. Mater. 2022, 8, 529–544.
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603.
- Liu, F.; Liao, X.; Liu, C.; Li, M.; Chen, Y.; Shao, W.; Weng, K.; Li, F.; Ou, K.; He, J. Poly(L-lactide-co-caprolactone)/tussah silk fibroin nanofiber vascular scaffolds with small diameter fabricated by core-spun electrospinning technology. J. Mater. Sci. 2020, 55, 7106–7119.
- Li, X.; Huang, L.; Li, L.; Tang, Y.; Liu, Q.; Xie, H.; Tian, J.; Zhou, S.; Tang, G. Biomimetic dual-oriented/bilayered electrospun scaffold for vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 439–455.
- Chen, M.; Li, L.; Xia, L.; Zhang, F.; Jiang, S.; Hu, H.; Li, X.; Wang, H. Temperature Responsive Shape-Memory Scaffolds with Circumferentially Aligned Nanofibers for Guiding Smooth Muscle Cell Behavior. Masthead Macromol. Biosci. 2020, 20, 1900312.
- Liua, G.; Fu, M.; Li, F.; Fu, W.; Zhao, Z.; Xia, H.; Niu, Y. Tissue-engineered PLLA/gelatine nanofibrous scaffold promoting the phenotypic expression of epithelial and smooth muscle cells for urethral reconstruction. Mater. Sci. Eng. C 2020, 111, 110810.
- Liu, Y.; Huang, L.; Yuan, W.; Zhang, D.; Gu, Y.; Huang, J.; Murphy, S.; Ali, M.; Zhang, Y.; Song, L. Sustained release of stromal cell–derived factor-1 alpha from silk fibroin microfiber promotes urethral reconstruction in rabbits. J. Biomed. Mater. Res. 2020, 108, 1591–1786.
- Jia, W.; Li, M.; Weng, H.; Gu, G.; Chen, Z. Design and comprehensive assessment of a biomimetic tri-layer tubular scaffold via biodegradable polymers for vascular tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110717.
- Eilenberg, M.; Enayati, M.; Ehebruster, D.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Potzmann, P.; Kaun, C.; Podesser, B.K.; et al. Long Term Evaluation of Nanofibrous, Bioabsorbable Polycarbonate Urethane Grafts for Small Diameter Vessel Replacement in Rodents. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 643–652.
- Narayanana, K.B.; Park, G.T.; Han, S.S. Electrospun poly(vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering. Colloids Surf. B Biointerfaces 2020, 191, 110994.
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427.
- Asadi, H.; Ghaee, A.; Nourmohammadi, J.; Mashak, A. Electrospun zein/graphene oxide nanosheet composite nanofibers with controlled drug release as antibacterial wound dressing. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 173–185.
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521.




