
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Donnell White, III | -- | 2361 | 2022-06-22 21:46:57 | | | |
| 2 | Nora Tang | Meta information modification | 2361 | 2022-06-24 03:41:44 | | |
Video Upload Options
Measuring total ATP levels within cellular compartmental pools in real-time presents a newer and more innovative approach to qualitatively analyzing ATP. Although this method is not precisely quantitative, it can be helpful in determining changes in ATP concentrations in one region of a cell compared to another in a variety of disease states. The overall goal of these assays and imaging methodologies are to evaluate and visualize dynamic ATP trends, such as usage and depletion, between cells and their sub-compartments. However, semi-quantitative evaluation of the relative ATP signal is feasible. The currently developed technologies that utilize this approach to ATP quantification are mainly genetically encoded biosensors. In conjunction with a fluorescent or bioluminescent protein, most of these biosensors harness the folding capabilities of the ϵ subunit of the bacterial ATP synthase subunit. The bacterial ATP synthase protein comprises a β-barrel domain located at the N terminus and an α-helical domain with two α-helices located at the C terminus. Upon ATP binding, the two α-helices interact and refine their conformational structure of the ϵ subunit, leading to fluorescent/bioluminescent illumination, indicating that ATP is present. Overall, this subunit adopts two different conformations: open (ATP-free) or closed (ATP-bound). The uses and applicability of this technology are limitless.
1. Förster Resonance Energy Transfer (FRET)
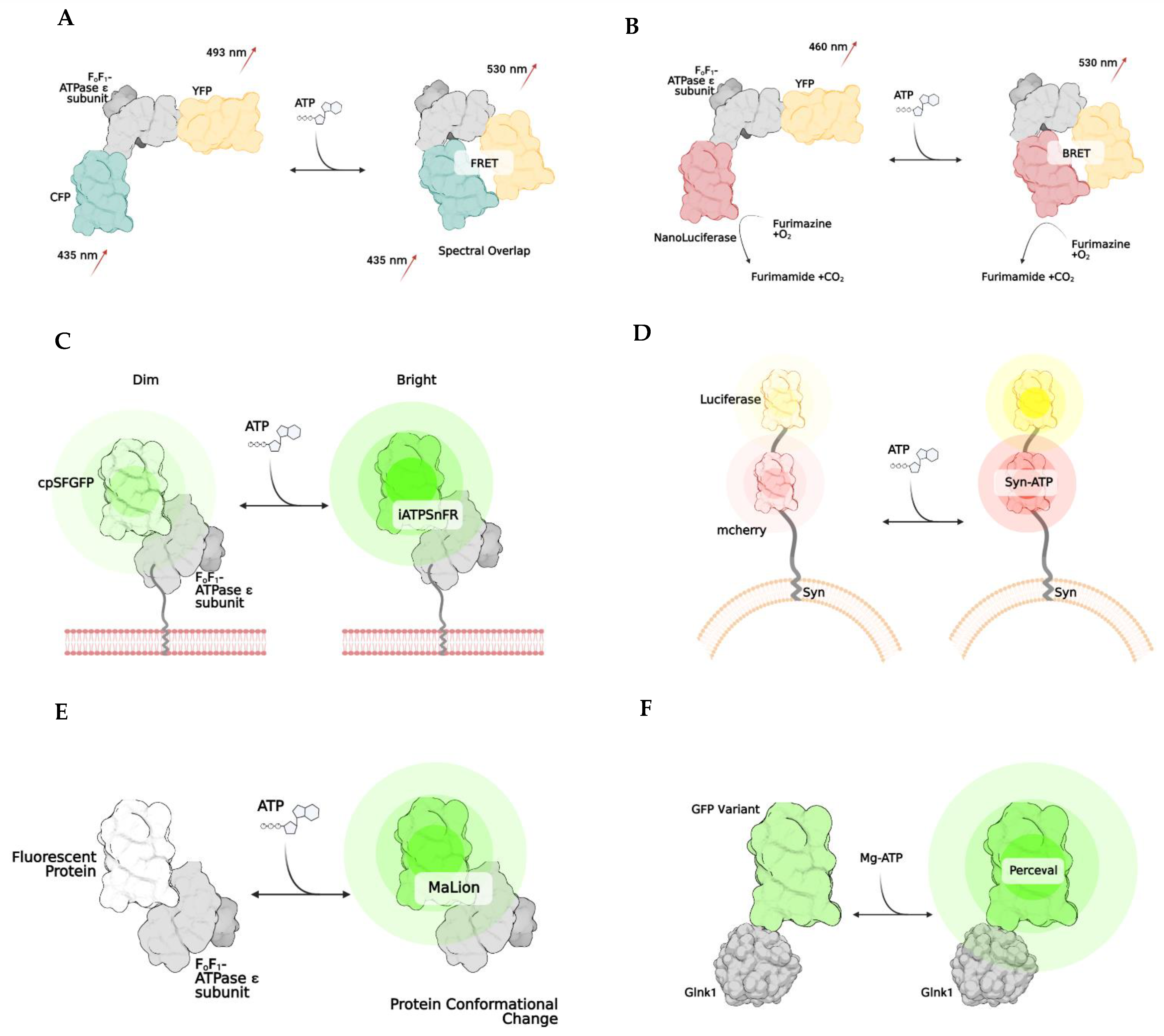
2. Bioluminescence and BRET
3. Single, Ratiometric, and Intensiometric Biosensors
| Genetically Encoded ATP Biosensors | ||||
|---|---|---|---|---|
| Biosensor | Technique | Mechanism | Advantage | Disadvantage |
| ATEAM [2] | FRET | Adenosine 5′-triphosphate indication based on ϵ subunit for analytical measurement; ATP binding causes an increase in Forster resonance energy transfer between a CFP and YFP and results in a higher wavelength release; comprised of bacterial ϵ subunit of bacterial ATP synthase with cyan and yellow donor/acceptor pairs at N and C terminals, respectively | Qualitative/quantitative; spatiotemporal resolution |
Sensitive to acidic pH, thus limiting which cellular subcomponent cell can use; can undergo glycosylation in ER and Golgi which inhibits its ability to bind to ATP |
| GO-ATEAM [4] | FRET | Similar to ATEAM but CFP and YFP are replaced by green (GFP) and orange (mKOk) fluorescent pair, respectively | ||
| BTEAM [13] | BRET | Composed of e subunit of bacterial ATP synthase flanked by Venus at the N terminal and Nanoluciferase at the C terminal; emitted light is produced by Nanoluciferase because oxidation of luciferin cases emission of photons; capacity of luciferin to emit light is directly correlated to amount of ATP available | Qualitative/quantitative; spatiotemporal resolution; no need for laser, as light emission come from enzymatic reaction after administration of luciferase substrate; avoid generation of autofluorescent and phototoxicity; very sensitive; simplicity of assay; can add localization signals to target cell subcompartments | Luciferin limitation due to inhibition of reaction from other drugs; limits potential with some drug development; enzymatic and substrate concentration limitations; transfection efficiency limitations; optimization required for maximal detection; some luciferases produce ATP from pools of ADP |
| ARSeNL [27] | BRET | ATP detection via ratiometric mScarlet-NanoLuc sensor, similar to BTEAM | ||
| QUEEN [28] | Ratiometric | Quantitative evaluator of cellular energy; cpFP is inserted between two a helices of ϵ subunit of ATP synthase with linkers | similar results to bioluminescence luciferase assays | Modest pH sensitivity |
| iATPSnFR [20] | Intensiometric | Intensity-based ATP-sensing fluorescent reporter consists of circularly superfolder GFP between 2 alpha helices of ϵ subunit of bacterial ATP synthase; when ATP binds, rapid increase in fluorescence occurs | spatiotemporal resolution | Modest pH sensitivity |
| Syn-ATP [11] | Bioluminescence | Luciferin-reaction based; synaptophysin targets synaptic vesicle proteins and mCherry helps to determine total amount of luciferase using a luminescence/fluorescent ratio | Qualitative/quantitative; only used for synaptic vesicles | No spatiotemporal resolution; some luciferases produce ATP from pools of ADP |
| MaLion [21] | Intensiometric | multiple constructs created to target subcellular compartments (cytosol, mitochondria, nucleus); consists of a fused ϵ subunit of bacterial ATP synthase to red, blue, or green | Qualitative/quantitative; spatiotemporal resolution; has organelle-targeted specific ATP estimations; the higher the ATP, the brighter the fluorescence; low pH sensitivity | Potential phototoxicity due to fluorescence emission in living cells; transfection efficiency in hard to transfect cells |
| Perceval [24] | Ratiometric | Based on estimation of ADP/ATP; composed of GlnK1 (a bacterial regulatory protein) linked to Venus; GlnK1 undergoes a conformational change when bound to ATP | Qualitative/quantitative; spatiotemporal resolution; no conformational change when bound to ADP | Some pH sensitivity |
References
- Padilla-Parra, S.; Tramier, M. FRET microscopy in the living cell: Different approaches, strengths and weaknesses. BioEssays 2012, 34, 369–376.
- Yoshida, T.; Alfaqaan, S.; Sasaoka, N.; Imamura, H. Application of FRET-Based Biosensor “ATeam” for Visualization of ATP Levels in the Mitochondrial Matrix of Living Mammalian Cells. Methods Mol. Biol. 2017, 1567, 231–243.
- Vishnu, N.; Jadoon Khan, M.; Karsten, F.; Groschner, L.N.; Waldeck-Weiermair, M.; Rost, R.; Hallstrom, S.; Imamura, H.; Graier, W.F.; Malli, R. ATP increases within the lumen of the endoplasmic reticulum upon intracellular Ca2+ release. Mol. Biol. Cell 2014, 25, 368–379.
- Imamura, H.; Huynh Nhat, K.P.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15651–15656.
- Zadran, S.; Standley, S.; Wong, K.; Otiniano, E.; Amighi, A.; Baudry, M. Fluorescence resonance energy transfer (FRET)-based biosensors: Visualizing cellular dynamics and bioenergetics. Appl. Microbiol. Biotechnol. 2012, 96, 895–902.
- Bilan, D.S.; Matlashov, M.E.; Gorokhovatsky, A.Y.; Schultz, C.; Enikolopov, G.; Belousov, V.V. Genetically encoded fluorescent indicator for imaging NAD+/NADH ratio changes in different cellular compartments. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 951–957.
- Depaoli, M.R.; Karsten, F.; Madreiter-Sokolowski, C.T.; Klec, C.; Gottschalk, B.; Bischof, H.; Eroglu, E.; Waldeck-Weiermair, M.; Simmen, T.; Graier, W.F.; et al. Real-Time Imaging of Mitochondrial ATP Dynamics Reveals the Metabolic Setting of Single Cells. Cell Rep. 2018, 25, 501–512.e3.
- Liemburg-Apers, D.C.; Imamura, H.; Forkink, M.; Nooteboom, M.; Swarts, H.G.; Brock, R.; Smeitink, J.A.M.; Willems, P.H.G.M.; Koopman, W.J.H. Quantitative Glucose and ATP Sensing in Mammalian Cells. Pharm. Res. 2011, 28, 2745–2757.
- Yamamoto, S.; Yamamoto, M.; Nakamura, J.; Mii, A.; Yamamoto, S.; Takahashi, M.; Kaneko, K.; Uchino, E.; Sato, Y.; Fukuma, S.; et al. Spatiotemporal ATP Dynamics during AKI Predict Renal Prognosis. J. Am. Soc. Nephrol. 2020, 31, 2855–2869.
- Inouye, S. Firefly luciferase: An adenylate-forming enzyme for multicatalytic functions. Cell. Mol. Life Sci. 2010, 67, 387–404.
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-Driven Local ATP Synthesis Is Required for Synaptic Function. Cell 2014, 156, 825–835.
- Brown, N.E.; Blumer, J.B.; Hepler, J.R. Bioluminescence Resonance Energy Transfer to Detect Protein-Protein Interactions in Live Cells. In Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 457–465.
- Yoshida, T.; Kakizuka, A.; Imamura, H. BTeam, a Novel BRET-based Biosensor for the Accurate Quantification of ATP Concentration within Living Cells. Sci. Rep. 2016, 6, 39618.
- Azad, T.; Janse Van Rensburg, H.J.; Morgan, J.; Rezaei, R.; Crupi, M.J.F.; Chen, R.; Ghahremani, M.; Jamalkhah, M.; Forbes, N.; Ilkow, C.; et al. Luciferase-Based Biosensors in the Era of the COVID-19 Pandemic. ACS Nanosci. Au 2021, 1, 15–37.
- Min, K.-L.; Steghens, J.-P. ADP is produced by firefly luciferase but its synthesis is independent of the light emitting properties. Biochimie 2001, 83, 523–528.
- Lemasters, J.J.; Hackenbrock, C.R. Firefly luciferase assay for ATP production by mitochondria. In Bioluminescence and Chemiluminescence; Elsevier: Amsterdam, The Netherlands, 1978; pp. 36–50.
- Deluca, M.; Mcelroy, W.D. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry 1974, 13, 921–925.
- Kostyuk, A.I.; Demidovich, A.D.; Kotova, D.A.; Belousov, V.V.; Bilan, D.S. Circularly Permuted Fluorescent Protein-Based Indicators: History, Principles, and Classification. Int. J. Mol. Sci. 2019, 20, 4200.
- Yaginuma, H.; Kawai, S.; Tabata, K.V.; Tomiyama, K.; Kakizuka, A.; Komatsuzaki, T.; Noji, H.; Imamura, H. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep. 2015, 4, 6522.
- Lobas, M.A.; Tao, R.; Nagai, J.; Kronschläger, M.T.; Borden, P.M.; Marvin, J.S.; Looger, L.L.; Khakh, B.S. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 2019, 10, 711.
- Arai, S.; Kriszt, R.; Harada, K.; Looi, L.-S.; Matsuda, S.; Wongso, D.; Suo, S.; Ishiura, S.; Tseng, Y.-H.; Raghunath, M.; et al. RGB-Color Intensiometric Indicators to Visualize Spatiotemporal Dynamics of ATP in Single Cells. Angew. Chem. Int. Ed. 2018, 57, 10873–10878.
- Nguyen, P.T.M.; Ishiwata-Kimata, Y.; Kimata, Y. Monitoring ADP/ATP ratio in yeast cells using the fluorescent-protein reporter PercevalHR. Biosci. Biotechnol. Biochem. 2019, 83, 824–828.
- Tantama, M.; Martínez-François, J.R.; Mongeon, R.; Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun. 2013, 4, 2550.
- Berg, J.; Hung, Y.P.; Yellen, G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods 2009, 6, 161–166.
- Atkinson, D.E. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034.
- Tarasov, A.I.; Rutter, G.A. Use of Genetically Encoded Sensors to Monitor Cytosolic ATP/ADP Ratio in Living Cells. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 289–311.
- Min, S.-H.; French, A.R.; Trull, K.J.; Tat, K.; Varney, S.A.; Tantama, M. Ratiometric BRET Measurements of ATP with a Genetically-Encoded Luminescent Sensor. Sensors 2019, 19, 3502.
- Yaginuma, H.; Okada, Y. Live cell imaging of metabolic heterogeneity by quantitative fluorescent ATP indicator protein, QUEEN-37C. bioRxiv 2021.




