Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabel M. Cabrita | -- | 4589 | 2022-06-21 02:48:12 | | | |
| 2 | Amina Yu | -37 word(s) | 4552 | 2022-06-21 04:35:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Teixeira, P.; Bacariza, C.; Correia, P.; Pinheiro, C.I.C.; Cabrita, I. Medium-Temperature CO2 Sorbents. Encyclopedia. Available online: https://encyclopedia.pub/entry/24240 (accessed on 07 February 2026).
Teixeira P, Bacariza C, Correia P, Pinheiro CIC, Cabrita I. Medium-Temperature CO2 Sorbents. Encyclopedia. Available at: https://encyclopedia.pub/entry/24240. Accessed February 07, 2026.
Teixeira, Paula, Carmen Bacariza, Patrícia Correia, Carla I. C. Pinheiro, Isabel Cabrita. "Medium-Temperature CO2 Sorbents" Encyclopedia, https://encyclopedia.pub/entry/24240 (accessed February 07, 2026).
Teixeira, P., Bacariza, C., Correia, P., Pinheiro, C.I.C., & Cabrita, I. (2022, June 21). Medium-Temperature CO2 Sorbents. In Encyclopedia. https://encyclopedia.pub/entry/24240
Teixeira, Paula, et al. "Medium-Temperature CO2 Sorbents." Encyclopedia. Web. 21 June, 2022.
Copy Citation
Hydrogen is a promising energy vector, with a high calorific value (122 kJ g−1), which is being considered as the cleanest energy option, with a zero-carbon footprint, since it burns cleanly, giving water as the only product. Therefore, if linked with renewable energy sources and CO2 capture, it allows for decarbonizing a wide range of final sectors of use, providing clean power and heat to transport and stationary applications. Hydrogen is an important raw material of some industrial processes, such as hydrocracking, ammonia synthesis, methanol production, and the manufacture of hydrochloric acid; it is also a reducing agent in the steel industry.
H purity
CO capture
Ca-based sorbents
1. Sorption-Enhanced Water–Gas Shift Reaction
The water–gas shift reaction using syngas feedstock derived from coal gasification or steam methane reforming is a very common means of generating pure H2. The syngas composition depends on several factors, such as the selected reforming or gasification process, the fuel composition, the S/C ratio, temperature and pressure conditions of the shift reaction. Usually, syngas that originated from natural gas has a higher H2 and a lower CO2 and CO content than coal-derived syngas (Table 1). Besides, it has very low levels of sulphur compounds because most of it is removed before the reformer to prevent catalyst poisoning, while coal-derived syngas typically contains more sulphur. As mentioned in the above sections, the WGS reaction can contribute to the syngas upgrade by converting CO into CO2, which can be easily separated, and contributes to an additional production of H2.
Table 1. Typical composition of syngas obtained by different processes and conditions.
| mol % (Dry Basis) |
SMR [1] |
Coal Gasification [2] |
Indian Coal Gasification [3] |
Wood Pellets Gasification [3][4] |
Rice Husk Gasification [3] |
|---|---|---|---|---|---|
| H2 | 71 | 13–18 | 9 | 7–34 | 25 |
| CO2 | 6 | 7–9 | 0.6 | 6–16 | 14 |
| CO | 16 | 55–62 | 42 | 16–31 | 20 |
| O2 | -- | -- | -- | 1–3 | -- |
| N2 | -- | ~7 | 32 | 48–58 | 40 |
| CH4 | 5 | 17 | 1–4 | 0.9 | |
| CxHy | -- | -- | 0.1–0.3 | -- |
At low temperatures, the water–gas shift (WGS) reaction is thermodynamically favored, since its equilibrium constant decreases as temperatures increases [5], but not kinetically, as it is an exothermic reaction. Since the WGS reaction proceeds without change in the number of moles, pressure does not affect equilibrium, but up to the equilibrium moment total pressure can positively affect CO conversion since it increases the reaction rate [6][7]. To overcome thermodynamic and kinetic aspects, WGS reaction is carried out industrially in two steps: the first, high-temperature shift (HTS; 350–500 °C), using Fe-Cr catalysts, and the second, low-temperature shift (LTS; 200–250 °C), using Cu-Zn-Al2O3, that allows for achieving CO concentrations near 3% and 0.1%, respectively [8]. However, these two steps increase the complexity and the energy requirements of the process. Besides, if O2 is present it acts as a poison for the HTS catalysts, due the oxidation of Fe3O4 into the inactive Fe2O3 [9]. In addition, the LTS catalysts require high-volume reactors and can lose activity easily, due to being susceptible to poisoning by S, and Cu sintering. If H2S is present, a cobalt-molybdenum catalyst should be used [10]. On account of the limitations of commercialized shift catalysts, some improvements have been developed, such as replacing part of the metals with modified materials or doping with some alkalis [5]. Pal et al. [11] considered three more catalysts’ categories, ceria and noble metal-based catalysts, carbon-based catalysts and nanostructured catalysts [12]. The WGS reaction requires a step for the CO2 removal, usually the pressure swing adsorption technique is used.
In recent years, the sorption-enhanced reaction (SER) has been studied, aiming to improve the performance of the WGS reaction and leading to sorption-enhanced water–gas shift (SEWGS) reactions. The SEWGS reaction consists of a WGS reaction with in situ CO2 capture occurring simultaneously in a single reactor. The implementation of in situ CO2 removal shifts the WGS reaction to its right side by capturing the CO2 and, thus, increasing the H2 yield. That is to say that in situ CO2 capture overcomes the limitations related to the equilibrium, resulting in both higher CO conversion and enhanced H2 production. The result is the production of a very-high-purity H2 without the elevated costs associated to a separation process and a much more compact and simple process, associated with a higher energy system efficiency and lower capital costs.
The success of the SEWGS is highly dependent on the sorbent selected for the in situ CO2 capture from the reaction medium. The adequate materials for this technology are medium-temperature solid sorbents. The hydrotalcite and modified hydrotalcite-based sorbents are largely studied [13][14][15][16], and exhibit low CO2 sorption capacity, i.e., less than 0.1 g CO2/g sorbent at medium temperatures, which in SEWGS will cause CO2 saturation and reduction in H2 production. Mg-based sorbents have also been applied in the enhanced CO2 removal from WGS reaction, having a high theoretical carrying capacity, which makes it very attractive. Moreover, the integration of Mg-based sorbents allows one to remove the CO2 at medium temperatures, that is, ranging from carbonation to calcination temperatures of 300 to 450 °C, respectively, without the need of low-temperature WGS reaction. The performance of SEWGS reaction in this range of temperatures can be greatly enhanced in the presence of suitable catalysts, i.e., with enhanced stability, applicability, and activity at medium temperatures [11]. The catalytic performance depends on the synthesis technique, the nature of the active site/phase, the type of support and the reaction environment [11][17][18][19]. At medium temperature, for single-stage WGS reaction, platinum-based catalysts and supports with oxygen storage capacity, such as CeO2, are particularly favorable, because they show very high activity [6][20]. In practical applications, Mg-based sorbents are promising; however, more attention need to be paid to approach the theoretical carrying capacity. This type of sorbents show an abrupt decrease in the CO2 carrying capacity under long-term cyclical operation, sintering, attrition, and potential competing sulphation reactions [21][22][23][24]. In fact, there is no record of pilot-scale projects in this area [24], but promising results have been obtained using Mg-based sorbents modified with alkali molten salts [22][23].
2. Enhancement of the H2 Production with Mg-Based Sorbents
In recent years, there has been a significant growing interest among scientific researchers in using Mg-based sorbents for CO2 capture [25]. There are various benefits associated to Mg-based compounds. Mg-based sorbent is nontoxic, noncorrosive and is largely available on nature, where it is abundant at a relatively low cost. It also offers a wide temperature range to work with, from room-to-medium temperatures. Its regeneration can occur below 500 °C, which is a moderately low temperature, when compared to the one used with high-temperature CO2 basic metal oxide sorbents, such as Ca-based or alkali-based sorbents. Economic benefits unfold from using lower temperatures, since energy consumption is reduced, which in turn impacts positively in the system efficiency and the use of energy resources. In addition, Mg-based sorbents have a moderate basicity, which leads to a high theoretical CO2 capture capacity of 1.09 g CO2/g MgO. One mole of MgO can absorb one mole of CO2, according to the reversible reaction described in following Equation[25].
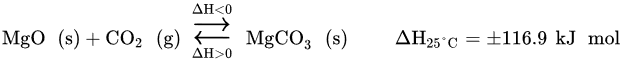
In practice, most of Mg-based sorbents do not exhibit the expected theoretical capture capacity. In fact, these materials are characterized with low CO2 capture capacity due to having slow kinetic reactivity [21][25]. As an example, commercial MgO powder presents a CO2 capture capacity of only 20 mg CO2/g MgO at 200 °C [26]. There are two main factors that are believed to explain both the poor capture capacity and the slow kinetics. Regarding the first one, this is related to the fact that MgO has a low surface area and, hence, does not expose its basic sites sufficiently well for CO2 sorption. Taking the same example of the commercially available MgO as above, its surface area is between 8 and 35 m2/g [21]. Moreover, MgO has a volume expansion of 2.49 times [21] caused by the formation of MgCO3. This product layer ends up covering with dense layers the adjacent basic active sites of the MgO sorbent, inhibiting the CO2 sorption to proceed. This evidence supports the fact that the poor sorption capacity is a surface phenomenon [25]. The other reason lies in MgO’s intrinsically high lattice enthalpy. Low porosity is also often related to low kinetics, since MgO’s strait pores obstruct the CO2 diffusion through them and, thus, delay the adsorption equilibrium [25]. In addition, MgO has a poor thermal and mechanical stability.
The circulation of the Mg-based sorbent between both the carbonation and the regeneration reactors is illustrated in Figure 1.

Figure 1. Carbonation–regeneration cycle of Mg-based sorbent.
In order to understand the thermodynamic limitations that lead to constraints of the operational conditions associated to the equilibrium described in Equation (22), the CO2 partial pressure, PCO2, in function of the dissociation or equilibrium temperature, Teq, is plotted in Figure 2; this is calculated using in following Equation[27].
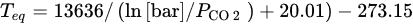

Figure 2. MgCO3 equilibrium curve relating the temperature to the CO2 partial pressure.
The thermodynamic equilibrium of MgCO3 decomposes itself in MgO and CO2, at 1 bar and above 300 °C; the CO2 pressure represents a limitation in what concerns increasing the working temperature [28]. Only if working at higher pressures would higher operating temperatures be possible to consider, but both kinetic and uptake could still be a concern and a limitation for practical applications [26][28]. The possibility of working at relatively low temperatures makes MgO compounds attractive to explore, especially for SEWGS reaction, but it is mandatory to overcome uncompetitive capacities and low sorption kinetics rates first. In this sense, researchers started to study different paths to enhance their performance, based on the sorbents’ dependence on intrinsic and extrinsic factors.
Most promising approaches aiming to improve the CO2 capture performance of Mg-based sorbents by upgrading their internal properties consist of the following: synthesizing mesoporous MgO, producing MgO from effective magnesium precursors, dispersing MgO on inert supports and doping MgO with alkali molten salts (AMS). The doping with alkali metal salts is the most widely recognized promising approach [25].
Most recent experimental works is to improve the CO2 uptake capacity of these materials up to 0.7–0.8 g CO2/g sorbent [29]. The alkali carbonates and the alkali nitrates/nitrites are the most commonly used [21]. In general, three categories of alkali doping are considered: alkali carbonate doping, alkali nitrate/nitrite doping and binary or ternary alkali doping.
Regarding alkali carbonate doping, the CO2 mechanism sorption is believed to happen in two steps. The first step consists in the quick generation of basic sites on the MgO surface, due to the established interactions between the sorbent and the alkali metal carbonate molecules. The nature of the AMS highly impacts the kinetics and the sorption capacity of the doped MgO sorbent at this stage because the basicity level of the produced active sites is influenced by the size of the ion salt. The second step is the slow formation of the double carbonate phase between the Mg and the AMS [21]. Concerning the alkali nitrate/nitrite, it was shown by Zhang et al. [30] that a MgO sorbent doped with NaNO3 exhibiting good CO2 sorption kinetics and a MgO conversion of 75% against of only 2% for an undoped MgO, both at 330 °C and ambient pressure. It was stated that molten NaNO3 provides an alternative reaction pathway to traditional gas–solid reactions, by acting as a phase transfer catalyst between bulk MgO and CO2 molecules which, in turn, facilitates the sorption reaction. It was described as the promoting effect of the molten nitrate. In addition, molten alkali metal nitrates are shown to prevent the formation of a rigid, CO2-impermeable, and monodentate carbonate layer on the surface of MgO as it occurs with bare MgO, but to promote the rapid generation of carbonate ions to allow a high rate of CO2 uptake. The binary doping with alkali nitrate/nitrite is also an interesting matter of study. Zhao et al. [31] compared the CO2 sorption capacities of the single NaNO3 and of the binary NaNO3/NaNO2 doped MgO sorbents. The latter showed higher CO2 sorption capacity than the former. This new evidence found explanation on the reduction in the melting temperature of the eutectic mixture. While single NaNO3 and NaNO2 present a theoretical melting point of 308 and 271 °C, respectively, the eutectic mixture of NaNO3/NaNO2 exhibits a melting temperature of 185 °C. Thus, the eutectic mixture facilitates the sorption process by providing a molten phase that works like a liquid channel. Ternary doping with NaNO3, lithium nitrate (LiNO3) and potassium nitrate (KNO3) registered an even more accentuated reduction in the eutectic mixture’s melting point and an enhanced CO2 sorption performance. In the case of the ternary doping with LiNO3, NaNO3 and Na2CO3, the former two form the molten phase in which Na2CO3 dissolves along with the bulk MgO to react with the CO2 molecules [21][31]. It is well accepted that the melting temperature of the eutectic mixture impacts greatly on the CO2 sorption performance.
The enhancement of Mg-based sorbents carrying capacity boosts its use for SEWGS processes, but current focus is on considering the simultaneous WGS reaction and CO2 capture are scarce, but promising.
To the best of our knowledge, the first experimental work conducted with Mg-based sorbent in a SEWGS reaction was performed by Abbasi et al. [32]. A partially calcined dolomite impregnated with K2CO3 was tested as sorbent/catalyst, at 20 atm, in a simulated syngas atmosphere. The sorbent was shown to be capable of achieving 95% of CO2 capture and 40% of conversion in the WGS reaction, but both activities decreased with increasing temperature. The results indicated that the pre-breakthrough WGS conversion diminishes as the sorbent is carbonated and CO2 concentration approaches the inlet concentration, leading to the conclusion that the catalytic activity of MgO is significantly greater than that of MgCO3. During the SEWGS at 400 °C, the H2 (dry basis) change from ~60 to ~45%, and CO2 from ~9 to ~25%, in the pre- and post-breakthrough phases, respectively.
Hu et al. [22] synthetized AMS-promoted MgO-CaCO3 sorbents and obtained a high CO2 carrying capacity and stability after 30 cycles, i.e., 0.55 g CO2/g sorbent (carbonation at 350 °C, 30 min, 1 atm, 50% CO2; and regeneration at 420 °C, 10 min, 1 atm, N2). The enhancement of sorbent performance resulted in a high H2 purity during the SEWGS process. For the optimized conditions, i.e., 12 atm, 300 °C, an initial ratio H2O/CO molar ratio of 1.5 and a three catalyst/sorbent layered configuration, the H2 purity was 99.4% for the 1st cycle and 98.2% after 10 cycles. Recently, Hu et al. [33] describes the preparation of K2CO3-promoted Cu/MgO-Al2O3 by sol-gel method to be used in a SEWGS reaction. Very much promising results were obtained for a sorbent with a K/(Mg + Al) ratio of 0.2 and a Mg/Al ratio of 9. A H2 yield of 99.9% was registered after 10 SEWGS/regeneration cycles at 300 and 380 °C for SEWGS and for regeneration, respectively.
In another one by Lee et al. [23] reported a Na-Mg double salt-based sorbent that was tested under SEWGS conditions using a commercial catalyst. A divided section packing concept of catalyst/sorbent was prepared and a high pure H2 was obtained (CO < 10 ppm). The carrying capacity of this Na-Mg double salt-based sorbent was ca. 0.15 g CO2/g sorbent, so the reactor column was divided into more sections (~10) and packed with increasing amounts of sorbent.
2.1. Effect of Temperature on Mg-Based Sorbents
As shown in Figure 2, the MgO carbonation is strongly dependent on temperature, but the sorbent synthesis and properties, such as the use and type of promotors, also had a relevant role on the CO2 uptake. Wang et al. [26] analyzed the effect of temperature on CO2 sorption by NaNO2 and NaNO3-promoted MgO. It was observed that at low temperatures (240–260 °C), the 0.2NaNO3/MgO sorbent exhibited relatively low CO2 uptake. On the other hand, the 0.2NaNO2/MgO demonstrated faster weight increases, which indicates that the formed MgCO3 product layer of 0.2NaNO2/MgO is thicker than that of 0.2NaNO3/MgO, which increases the CO2 diffusion resistance. Further increasing the temperature to 280–300 °C, the CO2 sorption of two sorbents was significantly enhanced since the diffusion process was activated, and values were attained near 0.55 g CO2/g sorbent after 60 and 120 min for 0.2NaNO2/MgO and 0.2NaNO3/MgO, respectively. With the temperature increasing to 320–340 °C, the sorption rates decreased during the initial period, whereas the final uptakes were slightly improved. It was justified by the increased CO2 equilibrium concentration in the molten salts and the enhanced diffusivity of CO2 in the product layer with increasing temperature. Hiremath et al. [34] synthetized KNO3-LiNO3/MgO-TiO2 sorbents and observed that the CO2 uptake initially increases with increasing temperature from 250 to 300 °C, and started to decrease for higher temperatures (325, 350 and 375 °C), which is in line with previous results. The kinetics of CO2 uptake showed an interesting behavior at a lower temperature (250 °C): the CO2 uptake was fast at the beginning (<10 min), but at 300 °C the initial 10 min showed a slight increase in CO2 uptake followed by a fast transition leading to a higher CO2 uptake, although the initial sorption kinetics was slower. Wang et al. [35] found similar behavior with the sorption temperature, i.e., during the initial stage of the CO2 absorption process, the lower the temperature (275 vs. 375 °C) the higher the rate, which was justified by the higher CO2 concentration in the molten salt at a lower temperature. With the progress of CO2 absorption, the disadvantage of slow kinetics at low temperatures is more prominent, and a low CO2 uptake after 120 min of absorption was observed. Pozzo et al. [29] analyzed the cyclic performance of MgO promoted by 10% of (Li,Na,K)NO3 for different carbonation temperatures, 250, 275 and 300 °C, with a partial pressure of CO2 of 0.2 atm. At 275 °C, the CO2 uptake was higher, which was explained by the higher thermodynamic driving force at lower carbonation temperatures. It was stated that the eutectic mixtures become particularly important, as the low melting point broadens the operating window of the material.
Then, the temperature affects the kinetics that is a relevant aspect of the in situ CO2 uptake. The Mg-based sorbent carbonation should be quick enough to produce high-purity H2 during the SEWGS process.
2.2. Effect of Steam on Mg-Based Sorbents
The SEWGS process requires the presence of high quantities of steam for the WGS reaction, which justifies the understanding of the steam effect on the Mg-based sorbents performance. Zarghami et al. [36] investigated the effect of the presence of H2O on the reactivity of Mg-based sorbents. The experimental results demonstrated that the existence of steam in the sorption step had a positive influence in the rate of the carbonation reaction. Several tests were carried out using reactant gas mixtures containing 50% CO2 and increasing concentrations of steam (10, 20 and 30%), in a pressurized system (20 bar) at 430 °C. A positive relationship was observed between the steam increase and the CO2 uptake, attaining values near 100% of CO2 uptake with 30% of steam, after 15 min.
Water is believed to work as a co-sorbent that boosts CO2 chemical reactivity, by creating a new carbonation pathway consisting of two mechanisms [25]. The primary mechanism forms an alternative transient compound, Mg(OH)2, with a larger molar volume than that of MgO (the following Equation). The secondary mechanism acts at the pore structural level, by expanding the inner pore volume and, thus, diminishing the resistance through diffusion in its inside (the following Equation) [21][25]. The overall result is the higher reactivity of the Mg-based sorbent toward CO2, that is, higher CO2 uptake capacity.
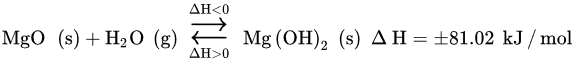
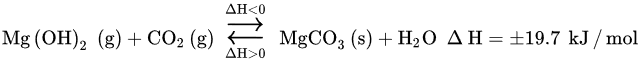
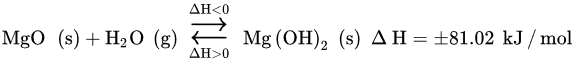
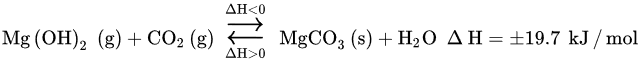
The kinetics of the reaction between CO2 and Mg(OH)2 described in above Equation is faster than that of the reaction between CO2 and MgO. The capture of CO2 with Mg(OH)2 is an exothermic reaction with a ∆H value much lower than that of the reaction of MgO with CO2, i.e., −19.7 vs. −100.9 kJ/mol. Moreover, the capture of CO2 with Mg(OH)2 at a high temperature is faster than with MgO. Researchers deduce that the presence of water provokes the rearrangement of surface oxide to hydroxide over MgO molecules, producing Mg(OH)2. This transient species have weaker lattice bonds when comparing to MgO, which smooths the transfer ability of OH− more than O2−.
At atmospheric pressure, the Mg(OH)2 registered an absorption capacity of 0.148 g CO2/g sorbent (1 bar), but its operation is limited to the temperature window of 200–315 °C and requires the rehydroxylation of MgO in the sorbent regeneration [26][28]. In addition, the existence of water decreases the operating temperature. Thus, the regeneration of MgCO3 into Mg(OH)2 can be achieved at lower temperatures [21][31][32].
Yang et al. [21] also found that the presence of H2O during the sorption step improved the kinetics of sorption rates. In addition, it is also reported that the introduction of H2O at the desorption step could have benefits in the improvement of both regeneration rate and efficiency of Mg-based sorbents. These results support the idea that the steam will be beneficial to the performance of SEWGS process when Mg-based sorbents are used for CO2 capture, especially at high pressures.
2.3. Effect of Pressure on Mg-Based Sorbents
Currently, SEWGS processes take place at high pressures [21]. Thus, a CO2 separation unit of a SEWGS process demands Mg-based sorbents to keep its cyclic CO2 uptake capacity stable at high pressures. Hwang et al. [37] investigated the effect of the operation pressure in the CO2 uptake capacity of a Mg-based sorbent impregnated with alkali metal nitrates under multiple cycles. The obtained experimental results showed a general upward profile of the CO2 uptake capacity with increasing operation pressure: the CO2 uptake capacity increased dramatically from 1 to 20 atm, while a more discreet increase was registered from 20 to 30 atm. This fact was attributed to gaseous diffusion being mainly controlled by Knudsen diffusion at higher pressures. A Mg-based sorbent impregnated with 5% of NaNO3 plus 5% of KNO3 was able to maintain its CO2 uptake capacity at 0.40 g CO2/g sorbent after five cycles at 300 °C and 20 atm. It was concluded it was an adequate sorbent to be used in a SEWGS process at high pressures.
Hu et al. [22] analyzed the pressure effect on the outlet gas composition at the pre-breakthrough stage of a SEWGS process, using an AMS-Mg95Ca5 sorbent and increasing the total pressure from ambient pressure to 12 atm. It was observed that the outlet concentration of CO2 during the pre-breakthrough period decreases, whereas those of H2 and CO increase, which is reasonable because the driving force for CO2 sorption increases with total pressure (Figure 2). For 1, 4, 8 and 12 atm, the measured CO2 concentrations during pre-breakthrough were 13.6%, 3.3%, 1.9% and 0.8%, respectively, while the equilibrium values were 5.2%, 1.3%, 0.6% and 0.4%, respectively. Therefore, the higher the pressure, the nearer the concentration approaches the equilibrium value. At the post-breakthrough stage, where the sorbent is completely saturated, no CO2 sorption occurs and only WGS takes place. At this stage, the outlet concentrations of H2, CO2 and CO at different pressures tend to the equilibrium values (46.3%, 46.3% and 7.4%, respectively)
Ryu et al. [38] examined the CO2 absorption properties of MgO-based sorbents loaded with K2CO3 according to the pressure (1, 10 and 20 atm). The MgO-based sorbent loaded with K2CO3 showed improved CO2 capture capacity at higher pressures, which was attributed also to the reaction of MgO and K2CO3 in the presence of water vapor at 20 atm, namely, due the formation of structures, such as MgCO3∙H2O and K2Mg(CO3)2. Hence, a positive effect of the high pressure on the CO2 uptake during the SEWGS process is expected.
In agreement with the MgCO3 thermodynamic equilibrium relative to CO2 partial pressure, it was reported [21] that working at a higher desorption pressure results in a higher desorption temperature needed for the regeneration of the sorbent. Hwang et al. [37] investigated the regeneration capacity of an AMS-promoted Mg-based sorbent at a high pressure and 100% CO2 condition. It was observed that the CO2 gas was desorbed at temperatures above 575 °C, with a peak at about 620 °C for CO2 desorption at 20 atm and 100% CO2. Based on this result, it was concluded that the optimum regeneration temperature was greatly shifted from 430 to 620 °C when regeneration conditions of 100% CO2 were used, compared to when N2 gas was used.
3. Medium-Temperature Catalyst–Sorbent: Hybrid/Mixed Materials and Sequential Arrangement
The application of WGS catalyst—Mg-based sorbents—during the SEWGS reaction is recent, and few experimental studies contemplate its use. Lee et al. [23] studied the influence of the catalyst packing method in the CO2 removal of a WGS reaction, using a Na-Mg double salt as sorbent and a commercial catalyst (Cu/ZnO/Al2O3). In a first attempt, it was successfully synthesized a one-body hybrid solid by ball-milling, consisting of both catalyst and sorbent. However, it exhibited low sorption capacity when compared to that of single materials. The XRD analysis shows that the characteristic peaks for NaNO3 in the one-body hybrid solid disappeared after the SEWGS reaction. Since the NaNO3 crystalline structure was not recovered after cooling, the formation of the molten phase was inhibited, resulting in poor CO2 sorption. It may be possible that the reduced Cu reacted with oxygen, converting NaNO3 to NaNO2. Subsequently, a multi-section concept was adopted for the reactor that minimized the contact between the catalyst and the sorbent. This attempt generated high-purity H2 by SEWGS. Moreover, higher production of high-purity H2 (>98%) was registered when using a higher ratio of sorbent-to-catalyst, as higher concentrations of CO2 were being captured. It was also observed that the SEWGS performance improved with the increasing number of the reactor sections. The effluent gas composition from the SEWGS reaction in a ten-section reactor and a total catalyst-to-sorbent ratio of 1 (1 g of catalyst or sorbent) were alternately loaded in each section. It showed that the pre-breakthrough of CO2 and CO was ∼25.5 min. Further investigations using a reactor packing method with different catalyst-to-sorbent ratios were proposed.
The SEWGS experiment performed in a fixed bed reactor using AMS-promoted MgO-CaCO3 as sorbent and Cu/Ce0.6Zr0.4O2 as catalyst is reported by Hu et al. [22]. Four catalyst/sorbent layered configurations were investigated: mode I with one layered configuration (2 g/2 g), mode II and III with two layered configurations (2 g/2 g–2g/2g and 2 g/2 g–0.5g/0.5g) and mode IV (2 g/2 g–0.5g/0.5g–0.125g/0.125g), catalyst/sorbent, respectively. The optimum condition was a reaction temperature of 300 °C, a total pressure of 12 atm and an initial H2O/CO molar ratio of 1.5 with a three catalyst/sorbent layered configuration. This optimum condition yielded a H2 purity as high as 99.4% (dry basis) at the first SEWGS cycle, which was stabilized at 98.2% after 10 consecutive cycles, demonstrating good cyclic stability. Recently, they [33] prepared a hybrid material, K2CO3-promoted Cu/MgO–Al2O3 by a sol–gel method. It was observed that the K/(Mg + Al) and Mg/Al atomic ratios affect the physicochemical properties of hybrid materials, especially in the morphology and the basicity distribution, which in turn affected the CO2 adsorption performance. In addition, it was found that the regeneration temperature of hybrid materials influences the SEWGS performance, 380 °C being the most favorable temperature since at higher temperatures the CO conversion at the post-breakthrough stage decreases with the number of cycles, but it does not happen for the material regenerated at 350 or 380 °C. The best performance was obtained for the hybrid material composed by a K/(Mg + Al) ratio of 0.2 and a Mg/Al ratio of 9, since the CO was completely converted and a yield >99.9% of H2 was attained in 10 consecutive SEWGS cycles at 300 °C, and regeneration at 380 °C.
The performance of hybrid materials is not consensual, further attention are needed. In relation to the sequential arrangement, both show an enhanced performance of SEWGS process with the increase in catalyst–sorbent layers. As mentioned above, also for sorbents at medium temperature, the preparation of catalysts and sorbents using wastes as precursors should be evaluated. In case of Mg-based materials, the potential of Mg recovered from magnesite mines sludges or from desalination reject brine [39] is an interesting alternative.
References
- Dubinin, A.M.; Tuponogov, V.G.; Ikonnikov, I.S. Modeling the process of producing hydrogen from methane. Theor. Found. Chem. Eng. 2013, 47, 697–701.
- Lu, X.; Wang, T. Investigation of low rank coal gasification in a two-stage downdraft entrained-flow gasifier. Int. J. Clean Coal Energy 2014, 3, 1–12.
- Raibhole, V.N.; Sapali, S.N. Simulation and parametric analysis of cryogenic oxygen plant for biomass gasification. Mech. Eng. Res. 2012, 2, 97–107.
- Plis, P.; Wilk, R.K. Theoretical and experimental investigation of biomass gasification process in a fixed bed gasifier. Energy 2011, 36, 3838–3845.
- Chen, W.H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078.
- Baraj, E.; Ciahotný, K.; Hlinčik, T.; Šnajdrová, V. Hydrogen production via water gas shift reaction on a nickel based catalyst. PALIVA 2016, 8, 138–142.
- Newsome, D.S. The water-gas shift reaction. Catal. Rev. Technol. 1980, 21, 275–318.
- Smith J, B.R.; Loganathan, M.; Shekhar Shantha, M. A Review of the water gas shift reaction kinetics. Int. J. Chem. React. Eng. 2010, 8, 49.
- Antzaras, A.N.; Lemonidou, A.A. Recent advances on materials and processes for intensified production of blue hydrogen. Renew. Sustain. Energy Rev. 2021, 155, 111917.
- Jin, S.; Ko, K.J.; Lee, C.H. Direct formation of hierarchically porous MgO-based sorbent bead for enhanced CO2 capture at intermediate temperatures. Chem. Eng. J. 2019, 371, 64–77.
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565.
- Oliveira, N.M.B.; Valença, G.P.; Vieira, R. Water gas shift reaction on copper catalysts supported on alumina and carbon nanofibers. Chem. Eng. Trans. 2015, 43, 931–936.
- Dewoolkar, K.D.; Vaidya, P.D. Tailored hydrotalcite-based hybrid materials for hydrogen production via sorption-enhanced steam reforming of ethanol. Int. J. Hydrogen Energy 2016, 41, 6094–6106.
- Soria, M.A.; Rocha, C.; Tosti, S.; Mendes, A.; Madeira, L.M. COx free hydrogen production through water-gas shift reaction in different hybrid multifunctional reactors. Chem. Eng. J. 2019, 356, 727–736.
- Halabi, M.H.; De Croon, M.H.J.M.; Van Der Schaaf, J.; Cobden, P.D.; Schouten, J.C. High capacity potassium-promoted hydrotalcite for CO2 capture in H2 production. Int. J. Hydrogen Energy 2012, 37, 4516–4525.
- Lee, C.H.; Kim, S.; Yoon, H.J.; Yoon, C.W.; Lee, K.B. Water gas shift and sorption-enhanced water gas shift reactions using hydrothermally synthesized novel Cu–Mg–Al hydrotalcite-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2021, 145, 111064.
- García-Moncada, N.; González-Castaño, M.; Ivanova, S.; Centeno, M.Á.; Romero-Sarria, F.; Odriozola, J.A. New concept for old reaction: Novel WGS catalyst design. Appl. Catal. B Environ. 2018, 238, 1–5.
- Vovchok, D.; Guild, C.J.; Llorca, J.; Xu, W.; Jafari, T.; Toloueinia, P.; Kriz, D.; Waluyo, I.; Palomino, R.M.; Rodriguez, J.A.; et al. Cu supported on mesoporous ceria: Water gas shift activity at low Cu loadings through metal-support interactions. Phys. Chem. Chem. Phys. 2017, 19, 17708–17717.
- Jeong, C.H.; Byeon, H.J.; Jang, W.J.; Jeon, K.W.; Jeong, D.W. The optimization of Nb loading amount over Cu–Nb–CeO2 catalysts for hydrogen production via the low-temperature water gas shift reaction. Int. J. Hydrogen Energy 2020, 45, 9648–9657.
- Petallidou, K.C.; Polychronopoulou, K.; Efstathiou, A.M. Novel catalytic systems for hydrogen production via the water-gas shift reaction. Conf. Pap. Energy 2013, 2013, 426980.
- Hu, Y.; Guo, Y.; Sun, J.; Li, H.; Liu, W. Progress in MgO sorbents for cyclic CO2 capture: A comprehensive review. J. Mater. Chem. A 2019, 7, 20103–20120.
- Hu, Y.; Cui, H.; Cheng, Z.; Zhou, Z. Sorption-enhanced water gas shift reaction by in situ CO2 capture on an alkali metal salt-promoted MgO-CaCO3 sorbent. Chem. Eng. J. 2019, 377, 119823.
- Lee, C.H.; Lee, K.B. Sorption-enhanced water gas shift reaction for high-purity hydrogen production: Application of a Na-Mg double salt-based sorbent and the divided section packing concept. Appl. Energy 2017, 205, 316–322.
- Gao, W.; Zhou, T.; Gao, Y.; Wang, Q. Enhanced water gas shift processes for carbon dioxide capture and hydrogen production. Appl. Energy 2019, 254, 113700.
- Wang, Q.; Gao, W. MgO-Based Intermediate-Temperature CO2 Adsorbents. In Pre-Combustion Carbon Dioxide Capture Materials; Wang, Q., Ed.; Royal Society of Chemistry: Beijing, China, 2018.
- Wang, K.; Zhao, Y.; Clough, P.T.; Zhao, P.; Anthony, E.J. Structural and kinetic analysis of CO2 sorption on NaNO2-promoted MgO at moderate temperatures. Chem. Eng. J. 2019, 372, 886–895.
- Fagerlund, J.; Highfield, J.; Zevenhoven, R. Kinetics studies on wet and dry gas-solid carbonation of MgO and Mg(OH)2 for CO2 sequestration. RSC Adv. 2012, 2, 10380–10393.
- Zhang, K. Development of Molten Salt Promoted Metal Oxide Based Absorbents for CO2 Separation. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2014.
- Dal Pozzo, A.; Armutlulu, A.; Rekhtina, M.; Abdala, P.M.; Müller, C.R. CO2 uptake and cyclic stability of MgO-based CO2 sorbents promoted with alkali metal nitrates and their eutectic mixtures. ACS Appl. Energy Mater. 2019, 2, 1295–1307.
- Zhang, K.; Li, X.S.; Li, W.Z.; Rohatgi, A.; Duan, Y.; Singh, P.; Li, L.; King, D.L. Phase transfer-catalyzed fast CO2 absorption by MgO-based absorbents with high cycling capacity. Adv. Mater. Interfaces 2014, 1, 1400030.
- Zhao, X.; Ji, G.; Liu, W.; He, X.; Anthony, E.J.; Zhao, M. Mesoporous MgO promoted with NaNO3/NaNO2 for rapid and high-capacity CO2 capture at moderate temperatures. Chem. Eng. J. 2018, 332, 216–226.
- Abbasi, E.; Hassanzadeh, A.; Zarghami, S.; Arastoopour, H.; Abbasian, J. Regenerable MgO-based sorbent for high temperature CO2 removal from syngas: 3. CO2 capture and sorbent enhanced water gas shift reaction. Fuel 2014, 137, 260–268.
- Hu, Y.; Cheng, Z.; Zhou, Z. High-purity H2 production by sorption-enhanced water gas shift on a K2CO3-promoted Cu/MgO-Al2O3 difunctional material. Sustain. Energy Fuels 2021, 5, 3340–3350.
- Hiremath, V.; Trivino, M.L.T.; Seo, J.G. Eutectic mixture promoted CO2 sorption on MgO-TiO2 composite at elevated temperature. J. Environ. Sci. (China) 2019, 76, 80–88.
- Zhou, Z.; Wang, L.; Hu, Y.; Cheng, Z. Nanosheet MgO-based CO2 sorbent promoted by mixed-alkali-metal nitrate and carbonate: Performance and mechanism. Ind. Eng. Chem. Res. 2017, 56, 5802–5812.
- Zarghami, S.; Hassanzadeh, A.; Arastoopour, H.; Abbasian, J. Effect of steam on the reactivity of MgO-based sorbents in precombustion CO2 capture processes. Ind. Eng. Chem. Res. 2015, 54, 8860–8866.
- Hwang, B.W.; Lim, J.H.; Chae, H.J.; Ryu, H.J.; Lee, D.; Lee, J.B.; Kim, H.; Lee, S.C.; Kim, J.C. CO2 capture and regeneration properties of MgO-based sorbents promoted with alkali metal nitrates at high pressure for the sorption enhanced water gas shift process. Process Saf. Environ. Prot. 2018, 116, 219–227.
- Ryu, D.Y.; Jo, S.; Kim, T.Y.; In, S.Y.; Kim, J.K.; Hwang, J.E.; Kim, J.C.; Lee, S.C. Influence of the sorption pressure and K2CO3 loading of a MgO-based sorbent for application to the SEWGS process. Korean J. Chem. Eng. 2022, 39, 1028–1035.
- Dong, H.; Yang, E.H.; Unluer, C.; Jin, F.; Al-Tabbaa, A. Investigation of the properties of MgO recovered from reject brine obtained from desalination plants. J. Clean. Prod. 2018, 196, 100–108.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
817
Revisions:
2 times
(View History)
Update Date:
21 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




