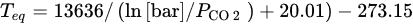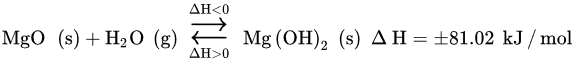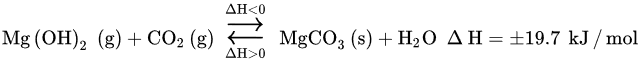The European Green Deal has the overarching aim of making Europe climate neutral in 2050, but global energy consumption is expected to continue growing, requiring decarbonized energy vectors for end use applications. Hydrogen is a promising energy vector, with a high calorific value (122 kJ g−1), which is being considered as the cleanest energy option, with a zero-carbon footprint, since it burns cleanly, giving water as the only product. Therefore, if linked with renewable energy sources and CO2 capture, it allows for decarbonizing a wide range of final sectors of use, providing clean power and heat to transport and stationary applications. Hydrogen is an important raw material of some industrial processes, such as hydrocracking, ammonia synthesis, methanol production, and the manufacture of hydrochloric acid; it is also a reducing agent in the steel industry.
1. Sorption-Enhanced Water–Gas Shift Reaction
The water–gas shift reaction (Equation (1)) using syngas feedstock derived from coal gasification or steam methane reforming is a very common means of generating pure H2. The syngas composition depends on several factors, such as the selected reforming or gasification process, the fuel composition, the S/C ratio, temperature and pressure conditions of the shift reaction. Usually, syngas that originated from natural gas has a higher H2 and a lower CO2 and CO content than coal-derived syngas (Table 12). Besides, it has very low levels of sulphur compounds because most of it is removed before the reformer to prevent catalyst poisoning, while coal-derived syngas typically contains more sulphur. As mentioned in the above sections, the WGS reaction can contribute to the syngas upgrade by converting CO into CO2, which can be easily separated, and contributes to an additional production of H2.
Table 12.
Typical composition of syngas obtained by different processes and conditions.
mol %
(Dry Basis) |
SMR
[1][30] |
Coal
Gasification [2][31] |
Indian Coal
Gasification
[3][32] |
Wood Pellets Gasification
[3][4][32,33] |
Rice Husk
Gasification
[3][32] |
| H2 |
71 |
13–18 |
9 |
7–34 |
25 |
| CO2 |
6 |
7–9 |
0.6 |
6–16 |
14 |
| CO |
16 |
55–62 |
42 |
16–31 |
20 |
| O2 |
-- |
-- |
-- |
1–3 |
-- |
| N2 |
-- |
~7 |
32 |
48–58 |
40 |
| CH4 |
5 |
17 |
1–4 |
0.9 |
| CxHy |
-- |
-- |
0.1–0.3 |
-- |
At low temperatures, the
water–gas shift (WGS
) reaction is thermodynamically favored, since its equilibrium constant decreases as temperatures increases
[5][158], but not kinetically, as it is an exothermic reaction
(Equation (1)). Since the WGS reaction proceeds without change in the number of moles, pressure does not affect equilibrium, but up to the equilibrium moment total pressure can positively affect CO conversion since it increases the reaction rate
[6][7][159,160]. To overcome thermodynamic and kinetic aspects, WGS reaction is carried out industrially in two steps: the first, high-temperature shift (HTS; 350–500 °C), using Fe-Cr catalysts, and the second, low-temperature shift (LTS; 200–250 °C), using Cu-Zn-Al
2O
3, that allows for achieving CO concentrations near 3% and 0.1%, respectively
[8][161]. However, these two steps increase the complexity and the energy requirements of the process. Besides, if O
2 is present it acts as a poison for the HTS catalysts, due the oxidation of Fe
3O
4 into the inactive Fe
2O
3 [9][37]. In addition, the LTS catalysts require high-volume reactors and can lose activity easily, due to being susceptible to poisoning by S, and Cu sintering. If H
2S is present, a cobalt-molybdenum catalyst should be used
[10][162]. On account of the limitations of commercialized shift catalysts, some improvements have been developed, such as replacing part of the metals with modified materials or doping with some alkalis
[5][158]. Pal et al.
[11][24] considered three more catalysts’ categories, ceria and noble metal-based catalysts, carbon-based catalysts and nanostructured catalysts
[12][163]. The WGS reaction requires a step for the CO
2 removal, usually the pressure swing adsorption technique is used.
In recent years, the sorption-enhanced reaction (SER) has been studied, aiming to improve the performance of the WGS reaction and leading to sorption-enhanced water–gas shift (SEWGS) reactionsSEWGS reaction. The SEWGS reaction consists of a WGS reaction with in situ CO2 capture occurring simultaneously in a single reactor. TAccording to Equation (1), the implementation of in situ CO2 removal shifts the WGS reaction to its right side by capturing the CO2 and, thus, increasing the H2 yield. That is to say that in situ CO2 capture overcomes the limitations related to the equilibrium, resulting in both higher CO conversion and enhanced H2 production. The result is the production of a very-high-purity H2 without the elevated costs associated to a separation process and a much more compact and simple process, associated with a higher energy system efficiency and lower capital costs.
The success of the SEWGS is highly dependent on the sorbent selected for the in situ CO
2 capture from the reaction medium. The adequate materials for this technology are medium-temperature solid sorbents. The hydrotalcite and modified hydrotalcite-based sorbents are largely studied
[13][14][15][16][12,168,169,170], and exhibit low CO
2 sorption capacity, i.e., less than 0.1 g CO
2/g sorbent at medium temperatures, which in SEWGS will cause CO
2 saturation and reduction in H
2 production. Mg-based sorbents have also been applied in the enhanced CO
2 removal from WGS reaction, having a high theoretical carrying capacity, which makes it very attractive. Moreover, the integration of Mg-based sorbents allows one to remove the CO
2 at medium temperatures, that is, ranging from carbonation to calcination temperatures of 300 to 450 °C, respectively, without the need of low-temperature WGS reaction. The performance of SEWGS reaction in this range of temperatures can be greatly enhanced in the presence of suitable catalysts, i.e., with enhanced stability, applicability, and activity at medium temperatures
[11][24]. The catalytic performance depends on the synthesis technique, the nature of the active site/phase, the type of support and the reaction environment
[11][17][18][19][24,171,172,173]. At medium temperature, for single-stage WGS reaction, platinum-based catalysts and supports with oxygen storage capacity, such as CeO
2, are particularly favorable, because they show very high activity
[6][20][159,174]. In practical applications, Mg-based sorbents are promising; however, more
ast
tentionudies need to be
paiconducted to approach the theoretical carrying capacity. This type of sorbents show an abrupt decrease in the CO
2 carrying capacity under long-term cyclical operation, sintering, attrition, and potential competing sulphation reactions
[21][22][23][24][27,165,166,167]. In fact, there is no record of pilot-scale projects in this area
[24][167], but promising results have been obtained using Mg-based sorbents modified with alkali molten salts
[22][23][165,166].
2. Enhancement of the H2 Production with Mg-Based Sorbents
In recent years, there has been a significant growing interest among scientific researchers in using Mg-based sorbents for CO
2 capture
[25][175]. There are various benefits associated to Mg-based compounds. Mg-based sorbent is nontoxic, noncorrosive and is largely available on nature, where it is abundant at a relatively low cost. It also offers a wide temperature range to work with, from room-to-medium temperatures. Its regeneration can occur below 500 °C, which is a moderately low temperature, when compared to the one used with high-temperature CO
2 basic metal oxide sorbents, such as Ca-based or alkali-based sorbents. Economic benefits unfold from using lower temperatures, since energy consumption is reduced, which in turn impacts positively in the system efficiency and the use of energy resources. In addition, Mg-based sorbents have a moderate basicity, which leads to a high theoretical CO
2 capture capacity of 1.09 g CO
2/g MgO. One mole of MgO can absorb one mole of CO
2, according to the reversible reaction described in
following Equation
[25] (22) [175].
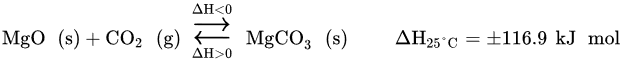
In practice, most of Mg-based sorbents do not exhibit the expected theoretical capture capacity. In fact, these materials are characterized with low CO
2 capture capacity due to having slow kinetic reactivity
[21][25][27,175]. As an example, commercial MgO powder presents a CO
2 capture capacity of only 20 mg CO
2/g MgO at 200 °C
[26][176]. There are two main factors that are believed to explain both the poor capture capacity and the slow kinetics. Regarding the first one, this is related to the fact that MgO has a low surface area and, hence, does not expose its basic sites sufficiently well for CO
2 sorption. Taking the same example of the commercially available MgO as above, its surface area is between 8 and 35 m
2/g
[21][27]. Moreover, MgO has a volume expansion of 2.49 times
[21][27] caused by the formation of MgCO
3. This product layer ends up covering with dense layers the adjacent basic active sites of the MgO sorbent, inhibiting the CO
2 sorption to proceed. This evidence supports the fact that the poor sorption capacity is a surface phenomenon
[25][175]. The other reason lies in MgO’s intrinsically high lattice enthalpy. Low porosity is also often related to low kinetics, since MgO’s strait pores obstruct the CO
2 diffusion through them and, thus, delay the adsorption equilibrium
[25][175]. In addition, MgO has a poor thermal and mechanical stability.
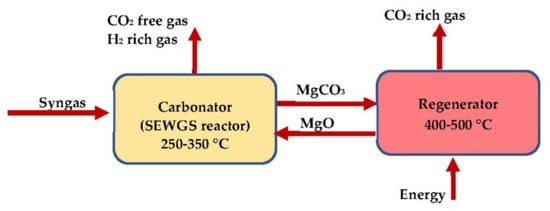
The circulation of the Mg-based sorbent between both the carbonation and the regeneration reactors is illustrated in
Figure 17.
Figure 17.
Carbonation–regeneration cycle of Mg-based sorbent.
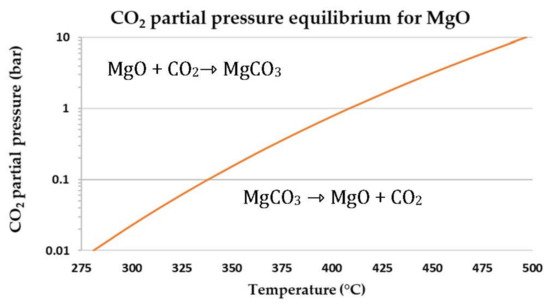
In order to understand the thermodynamic limitations that lead to constraints of the operational conditions associated to the equilibrium described in Equation (22), the CO
2 partial pressure,
PCO2, in function of the dissociation or equilibrium temperature,
Teq, is plotted in
Figure 218; this is calculated using
in following Equation
[27] (23) [177].
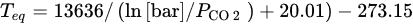
Figure 218.
MgCO3
equilibrium curve relating the temperature to the CO2
partial pressure.
The thermodynamic equilibrium of MgCO
3 decomposes itself in MgO and CO
2, at 1 bar and above 300 °C; the CO
2 pressure represents a limitation in what concerns increasing the working temperature
[28][178]. Only if working at higher pressures would higher operating temperatures be possible to consider, but both kinetic and uptake could still be a concern and a limitation for practical applications
[26][28][176,178]. The possibility of working at relatively low temperatures makes MgO compounds attractive to explore, especially for SEWGS reaction, but it is mandatory to overcome uncompetitive capacities and low sorption kinetics rates first. In this sense, researchers started to study different paths to enhance their performance, based on the sorbents’ dependence on intrinsic and extrinsic factors.
Most promising approaches aiming to improve the CO
2 capture performance of Mg-based sorbents by upgrading their internal properties consist of the following: synthesizing mesoporous MgO, producing MgO from effective magnesium precursors, dispersing MgO on inert supports and doping MgO with alkali molten salts (AMS). The doping with alkali metal salts is the most widely recognized promising approach
[25][175].
MThe aim of the most recent experimental works is to improve the CO
2 uptake capacity of these materials up to 0.7–0.8 g CO
2/g sorbent
[29][179]. The alkali carbonates and the alkali nitrates/nitrites are the most commonly used
[21][27]. In general, three categories of alkali doping are considered: alkali carbonate doping, alkali nitrate/nitrite doping and binary or ternary alkali doping.
Regarding alkali carbonate doping, the CO
2 mechanism sorption is believed to happen in two steps. The first step consists in the quick generation of basic sites on the MgO surface, due to the established interactions between the sorbent and the alkali metal carbonate molecules. The nature of the AMS highly impacts the kinetics and the sorption capacity of the doped MgO sorbent at this stage because the basicity level of the produced active sites is influenced by the size of the ion salt. The second step is the slow formation of the double carbonate phase between the Mg and the AMS
[21][27]. Concerning the alkali nitrate/nitrite, it was shown by Zhang et al.
[30][180] that a MgO sorbent doped with NaNO
3 exhibiting good CO
2 sorption kinetics and a MgO conversion of 75% against of only 2% for an undoped MgO, both at 330 °C and ambient pressure. It was stated that molten NaNO
3 provides an alternative reaction pathway to traditional gas–solid reactions, by acting as a phase transfer catalyst between bulk MgO and CO
2 molecules which, in turn, facilitates the sorption reaction. It was described as the promoting effect of the molten nitrate. In addition, molten alkali metal nitrates are shown to prevent the formation of a rigid, CO
2-impermeable, and monodentate carbonate layer on the surface of MgO as it occurs with bare MgO, but to promote the rapid generation of carbonate ions to allow a high rate of CO
2 uptake. The binary doping with alkali nitrate/nitrite is also an interesting matter of study. Zhao et al.
[31][181] compared the CO
2 sorption capacities of the single NaNO
3 and of the binary NaNO
3/NaNO
2 doped MgO sorbents. The latter showed higher CO
2 sorption capacity than the former. This new evidence found explanation on the reduction in the melting temperature of the eutectic mixture. While single NaNO
3 and NaNO
2 present a theoretical melting point of 308 and 271 °C, respectively, the eutectic mixture of NaNO
3/NaNO
2 exhibits a melting temperature of 185 °C. Thus, the eutectic mixture facilitates the sorption process by providing a molten phase that works like a liquid channel. Ternary doping with NaNO
3, lithium nitrate (LiNO
3) and potassium nitrate (KNO
3) registered an even more accentuated reduction in the eutectic mixture’s melting point and an enhanced CO
2 sorption performance. In the case of the ternary doping with LiNO
3, NaNO
3 and Na
2CO
3, the former two form the molten phase in which Na
2CO
3 dissolves along with the bulk MgO to react with the CO
2 molecules
[21][31][27,181]. It is well accepted that the melting temperature of the eutectic mixture impacts greatly on the CO
2 sorption performance.
The enhancement of Mg-based sorbents carrying capacity boosts its use for SEWGS processes, but
currthe
nt focus is on studies available in literature, considering the simultaneous WGS reaction and CO
2 capture are scarce, but promising.
To the best of our knowledge, the first experimental work conducted with Mg-based sorbent in a SEWGS reaction was performed by Abbasi et al.
[32][182].
The authors Atested a partially calcined dolomite impregnated with K
2CO
3 was
tested as ssorbent/catalyst, at 20 atm, in a simulated syngas atmosphere. The sorbent was shown to be capable of achieving 95% of CO
2 capture and 40% of conversion in the WGS reaction, but both activities decreased with increasing temperature. The results indicated that the pre-breakthrough WGS conversion diminishes as the sorbent is carbonated and CO
2 concentration approaches the inlet concentration, leading to the conclusion that the catalytic activity of MgO is significantly greater than that of MgCO
3. During the SEWGS at 400 °C, the H
2 (dry basis) change from ~60 to ~45%, and CO
2 from ~9 to ~25%, in the pre- and post-breakthrough phases, respectively.
Hu et al.
[22][165] synthetized AMS-promoted MgO-CaCO
3 sorbents and obtained a high CO
2 carrying capacity and stability after 30 cycles, i.e., 0.55 g CO
2/g sorbent (carbonation at 350 °C, 30 min, 1 atm, 50% CO
2; and regeneration at 420 °C, 10 min, 1 atm, N
2). The enhancement of sorbent performance resulted in a high H
2 purity during the SEWGS process. For the optimized conditions, i.e., 12 atm, 300 °C, an initial ratio H
2O/CO molar ratio of 1.5 and a three catalyst/sorbent layered configuration, the H
2 purity was 99.4% for the 1st cycle and 98.2% after 10 cycles.
RIn a more recent
ly work, Hu et al.
[33][183] describes the preparation of K
2CO
3-promoted Cu/MgO-Al
2O
3 by sol-gel method to be used in a SEWGS reaction. Very much promising results were obtained for a sorbent with a K/(Mg + Al) ratio of 0.2 and a Mg/Al ratio of 9. A H
2 yield of 99.9% was registered after 10 SEWGS/regeneration cycles at 300 and 380 °C for SEWGS and for regeneration, respectively.
In another
wo
ne by rk, Lee et al.
[23][166] reported a Na-Mg double salt-based sorbent that was tested under SEWGS conditions using a commercial catalyst. A divided section packing concept of catalyst/sorbent was prepared and a high pure H
2 was obtained (CO < 10 ppm). The carrying capacity of this Na-Mg double salt-based sorbent was ca. 0.15 g CO
2/g sorbent, so the reactor column was divided into more sections (~10) and packed with increasing amounts of sorbent.
2.1. Effect of Temperature on Mg-Based Sorbents
As shown in
Figure 218, the MgO carbonation is strongly dependent on temperature, but the sorbent synthesis and properties, such as the use and type of promotors, also had a relevant role on the CO
2 uptake. Wang et al.
[26][176] analyzed the effect of temperature on CO
2 sorption by NaNO
2 and NaNO
3-promoted MgO. It was observed that at low temperatures (240–260 °C), the 0.2NaNO
3/MgO sorbent exhibited relatively low CO
2 uptake. On the other hand, the 0.2NaNO
2/MgO demonstrated faster weight increases, which indicates that the formed MgCO
3 product layer of 0.2NaNO
2/MgO is thicker than that of 0.2NaNO
3/MgO, which increases the CO
2 diffusion resistance. Further increasing the temperature to 280–300 °C, the CO
2 sorption of two sorbents was significantly enhanced since the diffusion process was activated, and values were attained near 0.55 g CO
2/g sorbent after 60 and 120 min for 0.2NaNO
2/MgO and 0.2NaNO
3/MgO, respectively. With the temperature increasing to 320–340 °C, the sorption rates decreased during the initial period, whereas the final uptakes were slightly improved. It was justified by the increased CO
2 equilibrium concentration in the molten salts and the enhanced diffusivity of CO
2 in the product layer with increasing temperature. Hiremath et al.
[34][184] synthetized KNO
3-LiNO
3/MgO-TiO
2 sorbents and observed that the CO
2 uptake initially increases with increasing temperature from 250 to 300 °C, and started to decrease for higher temperatures (325, 350 and 375 °C), which is in line with previous results. The kinetics of CO
2 uptake showed an interesting behavior at a lower temperature (250 °C): the CO
2 uptake was fast at the beginning (<10 min), but at 300 °C the initial 10 min showed a slight increase in CO
2 uptake followed by a fast transition leading to a higher CO
2 uptake, although the initial sorption kinetics was slower. Wang et al.
[35][185] found similar behavior with the sorption temperature, i.e., during the initial stage of the CO
2 absorption process, the lower the temperature (275 vs. 375 °C) the higher the rate, which was justified by the higher CO
2 concentration in the molten salt at a lower temperature. With the progress of CO
2 absorption, the disadvantage of slow kinetics at low temperatures is more prominent, and a low CO
2 uptake after 120 min of absorption was observed. Pozzo et al.
[29][179] analyzed the cyclic performance of MgO promoted by 10% of (Li,Na,K)NO
3 for different carbonation temperatures, 250, 275 and 300 °C, with a partial pressure of CO
2 of 0.2 atm. At 275 °C, the CO
2 uptake was higher, which was explained by the higher thermodynamic driving force at lower carbonation temperatures.
ItThe waauthors state
d that the eutectic mixtures become particularly important, as the low melting point broadens the operating window of the material.
Then, the temperature affects the kinetics that is a relevant aspect of the in situ CO
2 uptake. The Mg-based sorbent carbonation should be quick enough to produce high-purity H
2 during the SEWGS process.
2.2. Effect of Steam on Mg-Based Sorbents
The SEWGS process requires the presence of high quantities of steam for the WGS reaction, which justifies the understanding of the steam effect on the Mg-based sorbents performance. Zarghami et al.
[36][186] investigated the effect of the presence of H
2O on the reactivity of Mg-based sorbents. The experimental results demonstrated that the existence of steam in the sorption step had a positive influence in the rate of the carbonation reaction.
SThe
veral tests were authors carried out
several tests using reactant gas mixtures containing 50% CO
2 and increasing concentrations of steam (10, 20 and 30%), in a pressurized system (20 bar) at 430 °C. A positive relationship was observed between the steam increase and the CO
2 uptake, attaining values near 100% of CO
2 uptake with 30% of steam, after 15 min.
Water is believed to work as a co-sorbent that boosts CO
2 chemical reactivity, by creating a new carbonation pathway consisting of two mechanisms
[25][175]. The primary mechanism forms an alternative transient compound, Mg(OH)
2, with a larger molar volume than that of MgO (
the following Equation
(24)). The secondary mechanism acts at the pore structural level, by expanding the inner pore volume and, thus, diminishing the resistance through diffusion in its inside (
the following Equation
) (25)) [21][25][27,175]. The overall result is the higher reactivity of the Mg-based sorbent toward CO
2, that is, higher CO
2 uptake capacity.
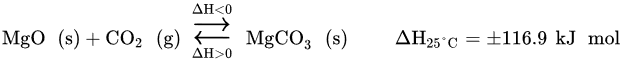 In practice, most of Mg-based sorbents do not exhibit the expected theoretical capture capacity. In fact, these materials are characterized with low CO2 capture capacity due to having slow kinetic reactivity [21][25][27,175]. As an example, commercial MgO powder presents a CO2 capture capacity of only 20 mg CO2/g MgO at 200 °C [26][176]. There are two main factors that are believed to explain both the poor capture capacity and the slow kinetics. Regarding the first one, this is related to the fact that MgO has a low surface area and, hence, does not expose its basic sites sufficiently well for CO2 sorption. Taking the same example of the commercially available MgO as above, its surface area is between 8 and 35 m2/g [21][27]. Moreover, MgO has a volume expansion of 2.49 times [21][27] caused by the formation of MgCO3. This product layer ends up covering with dense layers the adjacent basic active sites of the MgO sorbent, inhibiting the CO2 sorption to proceed. This evidence supports the fact that the poor sorption capacity is a surface phenomenon [25][175]. The other reason lies in MgO’s intrinsically high lattice enthalpy. Low porosity is also often related to low kinetics, since MgO’s strait pores obstruct the CO2 diffusion through them and, thus, delay the adsorption equilibrium [25][175]. In addition, MgO has a poor thermal and mechanical stability.
The circulation of the Mg-based sorbent between both the carbonation and the regeneration reactors is illustrated in Figure 17.
In practice, most of Mg-based sorbents do not exhibit the expected theoretical capture capacity. In fact, these materials are characterized with low CO2 capture capacity due to having slow kinetic reactivity [21][25][27,175]. As an example, commercial MgO powder presents a CO2 capture capacity of only 20 mg CO2/g MgO at 200 °C [26][176]. There are two main factors that are believed to explain both the poor capture capacity and the slow kinetics. Regarding the first one, this is related to the fact that MgO has a low surface area and, hence, does not expose its basic sites sufficiently well for CO2 sorption. Taking the same example of the commercially available MgO as above, its surface area is between 8 and 35 m2/g [21][27]. Moreover, MgO has a volume expansion of 2.49 times [21][27] caused by the formation of MgCO3. This product layer ends up covering with dense layers the adjacent basic active sites of the MgO sorbent, inhibiting the CO2 sorption to proceed. This evidence supports the fact that the poor sorption capacity is a surface phenomenon [25][175]. The other reason lies in MgO’s intrinsically high lattice enthalpy. Low porosity is also often related to low kinetics, since MgO’s strait pores obstruct the CO2 diffusion through them and, thus, delay the adsorption equilibrium [25][175]. In addition, MgO has a poor thermal and mechanical stability.
The circulation of the Mg-based sorbent between both the carbonation and the regeneration reactors is illustrated in Figure 17.