
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Itzae Adonai Gutiérrez Hurtado | -- | 1826 | 2022-06-16 22:23:48 | | | |
| 2 | Rita Xu | Meta information modification | 1826 | 2022-06-17 04:20:49 | | |
Video Upload Options
The importance of nutrition in human health is becoming increasingly clear. Despite the growing number of publications in this field, the quality of evidence supporting most nutritional recommendations is classified as "low". To improve the quality of evidence to support nutritional recommendations, the quality of research in this field must be improved. Randomized clinical trials (RCT) are a design that can help to provide high-quality evidence; however, conducting a RCT based on a nutritional intervention can be difficult due to the heterogeneous nature of the intervention and the number of variables that must be considered. Following a review of methodological and ethical standards, as well as four extensions of the CONSORT (Consolidated Standards of Reporting Trials) guidelines applicable to nutritional interventions, a series of definitions, examples, diagrams, and algorithms of key aspects that should be considered when conducting a RCT based on a nutritional intervention were identified.
1. Introduction
To improve the quality of evidence for nutritional recommendations, the quality of nutritional interventions must be improved, as many nutritional interventions currently have serious methodological limitations which results in questionable results [1][3].
A RCT, regardless of intervention type, must meet two requirements: manipulation of one variable (the independent variable) and random allocation of the intervention between study groups [4]. A nutritional intervention is a set of actions intended to change a nutritional aspect in an individual or population. It can range from nutrient administration to the implementation of a nutrition education program and can be conducted through a RCT [5].
The World Health Organization (WHO) classifies nutritional interventions into four types:
- Behavioral interventions, which aim to modify eating habits by changing them.
- Fortification, which is the addition of nutrients to basic foods.
- Supplementation, which entails administering a specific nutrient to a specific population.
- Regulatory interventions, the goal of which is to regulate certain activities in order to modify nutrition and improve health [6].
Improperly conducting a RCT can significantly reduce the value of the results and the usefulness of an investigation, wasting time and money while also exposing study participants to unnecessary risks. As a result, CONSORT (Consolidated Standards of Reporting Trials) has developed a set of guidelines for reporting RCTs, with the goal of ensuring research quality and providing enough evidence to make well-informed health decisions[7][8][9].
The CONSORT guidelines were first published in 1996, but initially only considered clinical trials with pharmacological treatments. However, an analysis of RCTs published up to 2000 revealed that non-pharmacological therapies accounted for one in four publications. This highlighted the need for updates to improve the quality of methodology and final reports of non-pharmacological RCTs [7][8].
In general, four CONSORT extensions can be extremely beneficial in the development of nutritional interventions. “Non-Pharmacologic Treatment Interventions” is a useful addition to most nutritional interventions. If the intervention involves the consumption of an herbal compound or a nutritional supplement containing herbs, the extension “Controlled Trials of Herbal Interventions” should be considered. Furthermore, depending on the chosen design and the research objective, the “Non-Inferiority and Equivalence Trials” extension may be useful; for example, in the case of a parallel design where the efficacy of one nutritional intervention is compared to another or a pharmacological therapy is compared to a nutritional intervention. Finally, the “Cluster Trials” extension will be useful when the intervention is aimed at groups[7][10][11][12].
Despite updates and extensions of the CONSORT guidelines, there is no specific guidance that takes into account the needs of a RCT based on the different types of nutritional interventions. Therefore, it is necessary to select the most appropriate CONSORT extension according to the characteristics and type of nutritional intervention, which raises the possibility of bias in the methodology or reporting, as well as the certainty of the results when evaluated using tools such as GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) or equivalent [13].
2. Considerations for the Nutrition Intervention
2.1. Study Population
A fundamental aspect of any RCT is to establish appropriate selection criteria to obtain a homogeneous sample. From an ethical and methodological point of view, it is advantageous that the study population has a high probability of benefiting directly from the intervention. It is essential to take into account in the selection of the study population, variables that may influence the results, such as age, gender, socio-economic and socio-cultural characteristics, and health status (e.g. primary disease, stage, type, co-morbidities, pharmacological therapies, related to the intervention), to reduce the risk of bias [14][15].
In many cases the outcomes of nutritional interventions are affected by not directly assessing the effect of the intervention on disease outcomes. For example, one of the most important challenges in establishing evidence of the therapeutic effect of the low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet in irritable bowel syndrome (IBS) is that most studies do not assess differences in dietary response between different IBS subgroups; furthermore, in many cases a validated assessment scale, such as the IBS-SSS (IBS Severity Scoring System), is not used [16].
2.2. Participants, Care Providers and Care Settings
Unlike RCTs based on pharmacological therapies, in some types of nutritional interventions it is necessary to consider and describe the characteristics of the care providers (those delivering the intervention), and even, in some cases, the characteristics of the setting where the intervention is delivered [8].
While the randomization process may reduce the risk of bias, it has been shown that in non-pharmacological interventions, the experience of the care providers influences the outcomes, i.e. the more experienced the provider, the more likely they are to have better outcomes. For this reason, the CONSORT extension "Non-Pharmacologic Treatment Interventions" invites, among other things, to report, as appropriate, on the qualification of the care provider, years of practice, number of interventions performed and specific training prior to the start of the trial. For care settings, it is important to report when the intervention is performed in a hospital setting, as there is a positive association between hospital volume and outcomes [8].
2.3. Intervention
In pharmacological therapies, the drug is clearly the independent variable; however, in most nutritional interventions, it is difficult to identify the elements that function as "active ingredients", which should be considered as the independent variable [7]. For example, the therapeutic effect of the low FODMAP diet does not result from the administration of a compound, but from the restriction of some oligosaccharides, disaccharides, monosaccharides and polyols found in various foods.
Considering the difficulty in identifying the active ingredient(s) of a nutritional intervention, the first step in conducting this kind of studies is to identify the dietary components that can modify the dependent variables. Subsequently, the researcher must adapt the intervention to the characteristics of the target population, and finally standardize the intervention. In the standardization process, in case the intervention involves nutritional counselling, the administration of a menu, or any educational intervention, it is advisable to plan the number of sessions so that the effects of the intervention can be seen, the form of application (individual or group), the strategies for monitoring the sessions, the means of identifying the exchange of information between the participants of the different study groups, the validity of the instruments used to provide and collect information, the resources to assess adherence to the intervention and the total duration of the study. In addition to standardizing the intervention, it is recommended to have a strategy for assessing providers' adherence to the protocol to ensure that the intervention is uniform [7][8].
In some cases, the nutritional intervention will consist of the administration of a supplement, and when this is the case, it is required to include the specific name of the substance, the health registration, the dose in standardized measurements, the time of administration (duration and schedule) and the route of administration; if the supplement is an herbal compound, e.g. green tea, it is necessary to follow CONSORT's "Recommendations for reporting randomized controlled trials of herbal interventions", which, among other requirements, suggests including the scientific name of the plant, the characteristics of the product (e.g. the part of the plant used, whether the herb is fresh or dried), the concentration of the active ingredient, the form of standardization and the supplier, among others [11].
Finally, when assessing the effect of a specific nutrient, the following considerations are recommended:
- To assess the effect of a nutrient, it is best to know its levels prior to the intervention, as these may influence the study subjects' responses. For measurement, it is necessary to use a valid and reliable biomarker, e.g. serum 25-hydroxyvitamin D concentration, to determine the vitamin D level. In some cases, when the hypothesis suggests that a nutrient deficiency is linked to the disease, the levels can be used as an inclusion criterion. When effect thresholds are unknown, however, including participants with a wide range of levels of the nutrient under study allows determination of whether the intervention is more or less effective as a function of nutritional status [17][18][19][9].
- Administer doses within a plausible range to identify the threshold response to the nutrient. In addition, it is recommended to perform multiple measurements of the nutrient under study and assess whether changes in nutritional status are reflected in changes in the study variables, i.e. whether increasing nutrient levels produces the desired effect on the condition under study [17].
- Consider other nutrients involved in the response. Because nutrients interact with each other, an organism's ability to respond to one nutrient may depend on the status of another; for example, bone gain following calcium and vitamin D supplementation depends on protein intake. It is also important to consider whether co-administration with foods, nutrients or bioactive compounds in the diet may affect the bioavailability or efficacy of the nutrient under study [9][18][17].
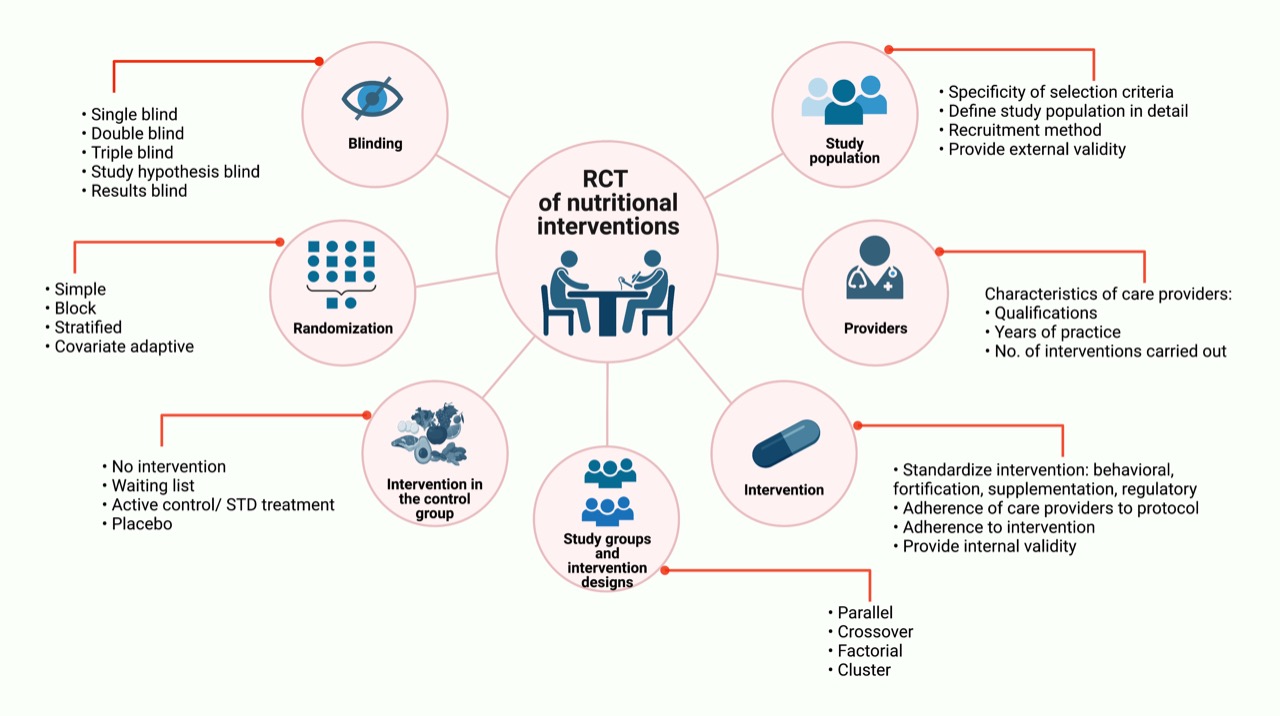
Figure 1. Randomized Clinical Trials based on nutritional interventions. Major aspects to be consider in RCTs based on nutritional interventions are: study population, care providers, intervention, study groups and intervention designs, intervention in the control group, randomization and blinding. Some of these methodological aspects apply not only to those based on nutritional interventions. RCT: Randomized Clinical Trials; STD treatment: Standard treatment; No: Number.
References
- Paolo Magni; Dennis M Bier; Sergio Pecorelli; Carlo Agostoni; Arne Astrup; Furio Brighenti; Robert Cook; Emanuela Folco; Luigi Fontana; Robert A Gibson; et al.Ranieri GuerraGordon H GuyattJohn Pa IoannidisAnn S JacksonDavid KlurfeldMaria MakridesBasil MathioudakisAlessandro MonacoChirag J PatelGiorgio RacagniHolger J SchünemannRaanan ShamirNiv ZmoraAndrea Peracino Perspective: Improving Nutritional Guidelines for Sustainable Health Policies: Current Status and Perspectives.. Advances in Nutrition 2017, 8, 532-545, 10.3945/an.116.014738.
- D. del Olmo; V. Alcázar; T. López del Val; Nutrición basada en la evidencia: presente, limitaciones y futuro. Endocrinología y Nutrición 2005, 52, 2-7, 10.1016/s1575-0922(05)74648-9.
- Heidi M. Staudacher; Peter M. Irving; Miranda C.E. Lomer; Kevin Whelans; The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proceedings of the Nutrition Society 2017, 76, 628-628, 10.1017/s0029665117002816.
- Christopher J. Miller; Shawna N. Smith; Marianne Pugatch; Experimental and quasi-experimental designs in implementation research. Psychiatry Research 2019, 283, 112452, 10.1016/j.psychres.2019.06.027.
- Academy of Nutrition and Dietetics. Nutrition Terminology Reference Manual: Dietetics Language for Nutrition Care 2019; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2019.
- WHO Nutrition Interventions. e-Library of Evidence for Nutrition Actions (eLENA). Available online: https://www.who.int/elena/about/background/en/ (accessed on 26 October 2021).
- Isabelle Boutron; Douglas G. Altman; David Moher; Kenneth F. Schulz; Philippe Ravaud; for the CONSORT NPT Group; CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Annals of Internal Medicine 2017, 167, 40-47, 10.7326/m17-0046.
- Isabelle Boutron; David Moher; Douglas G. Altman; Kenneth F. Schulz; Philippe Ravaud; for the CONSORT Group; Extending the CONSORT Statement to Randomized Trials of Nonpharmacologic Treatment: Explanation and Elaboration. Annals of Internal Medicine 2008, 148, 295-309, 10.7326/0003-4819-148-4-200802190-00008.
- Alice H Lichtenstein; Kristina Petersen; Kathryn Barger; Karen E Hansen; Cheryl A M Anderson; David J Baer; Johanna W Lampe; Helen Rasmussen; Nirupa R Matthan; Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Advances in Nutrition 2020, 12, 4-20, 10.1093/advances/nmaa109.
- Marion K Campbell; Gilda Piaggio; Diana R Elbourne; Douglas G Altman; for the CONSORT Group; Consort 2010 statement: extension to cluster randomised trials. BMJ 2012, 345, e5661-e5661, 10.1136/bmj.e5661.
- Joel J. Gagnier; Heather Boon; Paula Rochon; David Moher; Joanne Barnes; Claire Bombardier; Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. Journal of Clinical Epidemiology 2006, 59, 1134-1149, 10.1016/j.jclinepi.2005.12.020.
- Piaggio, G.; Elbourne, D.R.; Pocock, S.J.; Evans, S.J.W.; Altman, D.G.; CONSORT Group. Reporting of Noninferiority and Equivalence Randomized Trials: Extension of the CONSORT 2010 Statement. JAMA 2012, 308, 2594–2604.
- Connie M Weaver; Alice H Lichtenstein; Penny M Kris-Etherton; Perspective: Guidelines Needed for the Conduct of Human Nutrition Randomized Controlled Trials. Advances in Nutrition 2020, 12, 1-3, 10.1093/advances/nmaa083.
- Jeovany Martínez-Mesa; David Gonzalez-Chica; Rodrigo Pereira Duquia; Renan Rangel Bonamigo; Joao Bastos; Sampling: how to select participants in my research study?. Anais Brasileiros de Dermatologia 2016, 91, 326-330, 10.1590/abd1806-4841.20165254.
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869.
- Jinsheng Wang; Pengcheng Yang; Lei Zhang; Xiaohua Hou; A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Frontiers in Nutrition 2021, 8, 560, 10.3389/fnut.2021.683191.
- Robert P Heaney; Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutrition Reviews 2013, 72, 48-54, 10.1111/nure.12090.
- Robert P Heaney; Design and analysis of clinical trials of nutrients: author reply.. Nutrition Reviews 2014, 72, 354-354, 10.1111/nure.12118.
- Adrian R. Martineau; Design and analysis of clinical trials of nutrients: commentary.. Nutrition Reviews 2014, 72, 353-353, 10.1111/nure.12119.




