Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasile Valeriu Lupu | -- | 1220 | 2023-06-13 08:09:12 | | | |
| 2 | Conner Chen | Meta information modification | 1220 | 2023-06-15 05:12:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Morariu, I.; Avasilcai, L.; Vieriu, M.; Lupu, V.V.; Morariu, B.; Lupu, A.; Morariu, P.; Pop, O.; Starcea, I.M.; Trandafir, L. Irritable Bowel Syndrome. Encyclopedia. Available online: https://encyclopedia.pub/entry/45477 (accessed on 07 February 2026).
Morariu I, Avasilcai L, Vieriu M, Lupu VV, Morariu B, Lupu A, et al. Irritable Bowel Syndrome. Encyclopedia. Available at: https://encyclopedia.pub/entry/45477. Accessed February 07, 2026.
Morariu, Ionela-Daniela, Liliana Avasilcai, Madalina Vieriu, Vasile Valeriu Lupu, Branco-Adrian Morariu, Ancuța Lupu, Paula-Cristina Morariu, Oana-Lelia Pop, Iuliana Magalena Starcea, Laura Trandafir. "Irritable Bowel Syndrome" Encyclopedia, https://encyclopedia.pub/entry/45477 (accessed February 07, 2026).
Morariu, I., Avasilcai, L., Vieriu, M., Lupu, V.V., Morariu, B., Lupu, A., Morariu, P., Pop, O., Starcea, I.M., & Trandafir, L. (2023, June 13). Irritable Bowel Syndrome. In Encyclopedia. https://encyclopedia.pub/entry/45477
Morariu, Ionela-Daniela, et al. "Irritable Bowel Syndrome." Encyclopedia. Web. 13 June, 2023.
Copy Citation
IBS is a widespread functional GI disorder that determines symptoms such as chronic abdominal pain, flatulence, bloating, and altered bowel habits. Depending on diagnostic standards and the regional area, this pathology has a prevalence between 5% and 20% in adults. IBS can occur among patients of any age, even among children, more precisely 13.5% worldwide, and adolescents, rarely manifesting in older patients. IBS has a slightly higher prevalence among women than males and between 18 and 39 years of age.
irritable bowel syndrome
low-FODMAP diet
oligosaccharides
1. Overview
IBS is a widespread functional GI disorder that determines symptoms such as chronic abdominal pain, flatulence, bloating, and altered bowel habits [1][2]. Depending on diagnostic standards and the regional area, this pathology has a prevalence between 5% and 20% in adults [3][4][5]. IBS can occur among patients of any age, even among children, more precisely 13.5% worldwide [6], and adolescents, rarely manifesting in older patients. IBS has a slightly higher prevalence among women than males and between 18 and 39 years of age [3][4].
Although there is currently no specific biomarker for IBS, the diagnosis was established based on clinical history. Until 2006, diagnosing it seemed difficult for doctors because symptoms could change over time, but with the formulation of diagnostic criteria, the work of physicians became easier. Based on Rome IV diagnostic criteria and their most recent revision in 2016, IBS represents recurrent abdominal pain, which occurred weekly three months prior, coupled with a minimum of two of the subsequent criteria: influenced by bowel movements, associated with changes in the frequency and/or appearance. Following that classification, patients are grouped into three categories according to the pattern of the most frequent bowel movements: IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D), IBS with mixed bowel habits (IBS-M), or IBS unclassified (IBS-U) [7].
2. Pathophysiology
The pathophysiology of IBS is very complex and still incompletely understood, as it involves altered enteric neurotransmitters, intestinal microbiota imbalances, neuroendocrine disorders, visceral hypersensitivity, changes in intestinal barrier function, and changes in motility and the response to maladaptive stress response [8][9]. It has been found to be an alteration of bidirectional communication through the brain–intestinal axis caused by an intricate association of biological, psychological, and social variables that underlie the condition. Communication between the brain and the gut is mediated by the autonomic nervous system. A decrease in parasympathetic activity and an increase in sympathetic nervous system activity are frequently observed in patients with IBS. The decrease in vagal tone, which influences peripheral inflammation and permeability, as well as gastrointestinal motility and sensitivity, can be caused by stress [10]. On the contrary, the vagus nerve can indirectly detect the gut microenvironment and transmit this information to the brain [11][12].
Bacterial overgrowth in the small intestine in the majority of patients provides evidence that gut microbiota is at the forefront of the pathophysiology of IBS [8]. Bloating, constipation, diarrhoea, and flatulence are the main symptoms of intestinal bacterial overgrowth. In approximately 25% of patients, the onset of IBS precedes an enteric infection [13]. As a result, post-infectious IBS is a subtype of enteric pathology dominated by diarrhoea, with a high risk of acquisition in women with severe enteritis or after prolonged antibiotic treatment. The intestinal tract presents an increased number of T cells in the lamina propria, intraepithelial lymphocytes, mast cells at the mucosa level and enteroendocrine cells containing serotonin, thus sustaining the development of a pro-inflammatory environment. In chronic inflammation, juxtaposing mast cell mediators with enteric nerves contributes to the visceral hypersensitivity seen in post-infectious IBS [14].
Food is an additional element that contributes to the pathophysiology of IBS [15][16]. Short-chain carbohydrate fermentation reveals the process through which enteric bacteria and the presence of food allergies, nonimmune food sensitivities, changes in gut hormones, and changes in the gut microbiome produce symptoms of IBS. The use of non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics, infections, and stress are known triggers for IBS symptoms. However, [17] ingesting foods high in FODMAPs and foods high in biogenic amines, which produce histamine [15][18], has been associated with the onset of gastrointestinal symptoms in IBS [14][17][19].
However, it has been found that early life experiences (such as dysfunctional family factors and trauma from psychological and physical abuse) are linked to IBS susceptibility. Anxiety and depression influence pain sensitivity, gut motility, immune function, and QoL [12][14][20][21].
3. Diagnosis
The diagnosis of IBS requires the presence of characteristic symptoms within the last 3 months and the appearance 6 months ago. The Bristol stool form scale can help with the problematic subtyping of IBS because it is based on stool form [22][23][24].
The diagnosis of IBS is made after complete anamnesis based on the characteristic symptoms and results of various preliminary laboratory analyses, including complete blood count (CBC), determination of C-reactive protein (CRP), rapid erythrocyte sedimentation rate (ESR), and serological tests for coeliac disease [11][25][26][27].
Faecal lactoferrin (FL) and faecal calprotectin (fCal) are two biomarkers of intestinal inflammation that are useful for diagnosis. Their analysis is superior to serological tests (e.g., ESR and CRP) for differentiating inflammatory bowel disease (IBD) from IBS. Studies showed that measuring fCal in IBS led to a 67% reduction in the number of adults that require a colonoscopy. The determination of fCal in patients less than 45 years old is necessary to rule out IBD.
Although not widely available, rapid testing is available for both FL and fCal. The combination of CRP and fCal tests provides an even greater discrimination of IBS from IBD [28][29].
However, fCal is not a definitive marker for the diagnosis of IBD and may be elevated in obesity, infection, malignancy, or due to certain drugs (e.g., proton pump inhibitors or non-steroidal anti-inflammatory drugs) [25].
The lysozyme, polymorphonuclear neutrophil elastase, neutrophil lipocalin, and myeloperoxidase are other faecal proteins that have been investigated as biomarkers in IBS. Because these were limited studies, their relevance to the diagnosis of IBS is still uncertain [28].
To rule out other symptoms, a digital abdominal and rectal examination is required. This could confirm stool consistency, including rectal impaction, and it can detect dyssynergic defecation (paradoxical contraction on rectal examination during exertion) or low rectal masses [25].
Endoscopy is the ‘golden investigation’ for diagnosis of diseases of the gastrointestinal tract. It allows direct visualisation and offers the possibility of performing biopsies and establishing a histological diagnosis. However, despite those benefits, it is unpleasant to patients and may cause complications. Therefore, simple, non-invasive, and cheap tests to distinguish between intestinal diseases are beneficial [28].
For IBS, it is important to perform colon cancer screening with the help of colonoscopy. Colonoscopy is a frequent test performed to determine whether a disease, such as IBD, microscopic colitis, or colon cancer, is not the cause of a patient’s digestive symptoms. Polyps, haemorrhoids, and diverticula are just some of the lesions identified in patients with IBS during colonoscopy [11][27].
4. Therapeutic and Nutritional Management
Management of IBS includes three directions (Figure 1): pharmacological therapy (antidepressants, antispasmodics, and laxatives), interventions on hygienic-dietary revitalisation [30][31][32], and psychotherapy (cognitive behavioural psychotherapy, dynamic psychotherapy, hypnotherapy, and biofeedback-assisted stress management intervention) [11][18][33].
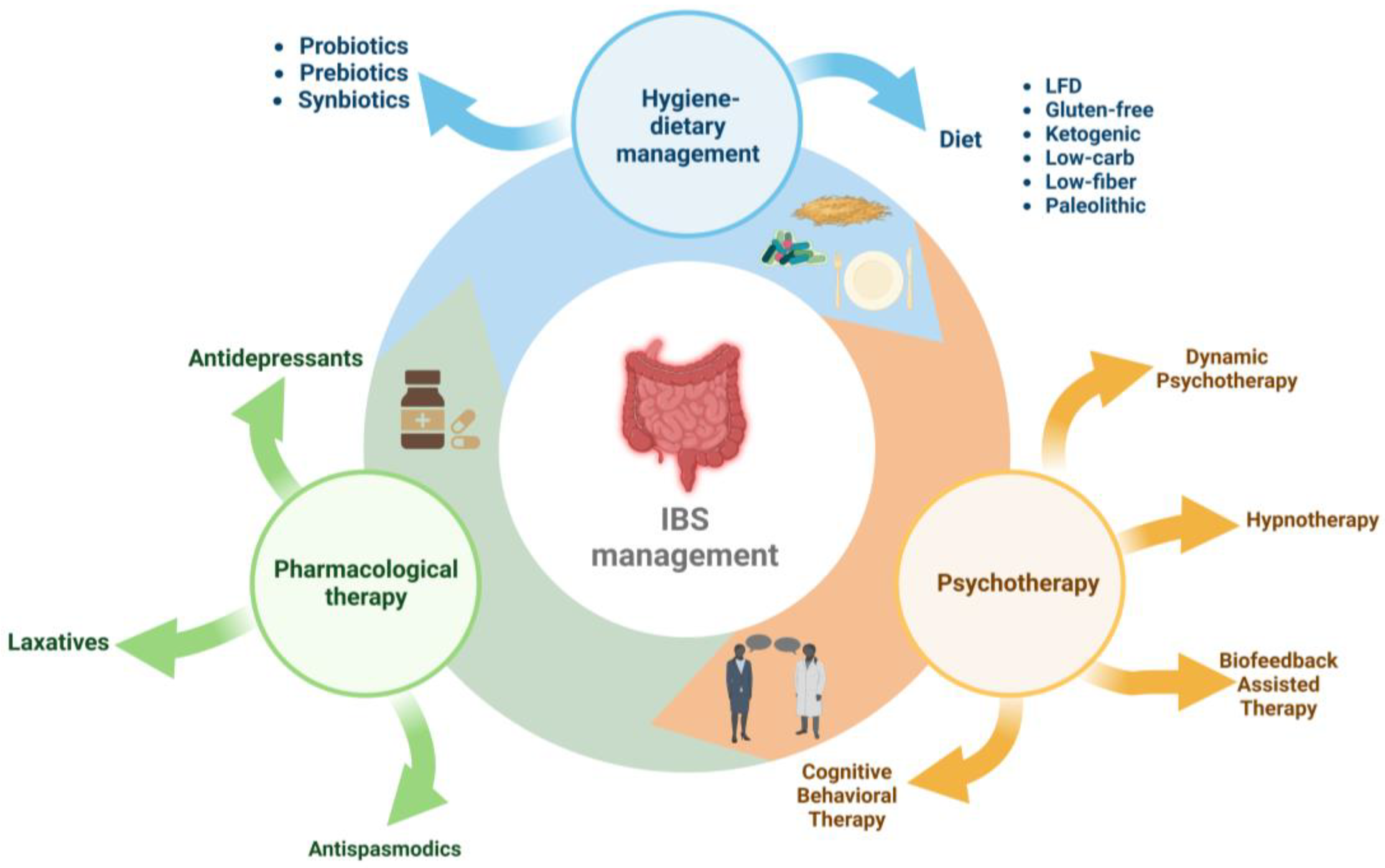
Figure 1. Therapeutic and nutritional management of irritable bowel syndrome.
References
- Agarwal, N.; Spiegel, B.M.R. The Effect of Irritable Bowel Syndrome on Health-Related Quality of Life and Health Care Expenditures. Gastroenterol. Clin. N. Am. 2011, 40, 11–19.
- Reznikov, E.A.; Suskind, D.L. Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies. Nutrients 2023, 15, 668.
- Andrews, E.B.; Eaton, S.C.; Hollis, K.A.; Hopkins, J.S.; Ameen, V.; Hamm, L.R.; Cook, S.F.; Tennis, P.; Mangel, A.W. Prevalence and Demographics of Irritable Bowel Syndrome: Results from a Large Web-Based Survey. Aliment. Pharmacol. Ther. 2005, 22, 935–942.
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4.
- Testa, A.; Imperatore, N.; Rispo, A.; Rea, M.; Tortora, R.; Nardone, O.M.; Lucci, L.; Accarino, G.; Caporaso, N.; Castiglione, F. Beyond Irritable Bowel Syndrome: The Efficacy of the Low Fodmap Diet for Improving Symptoms in Inflammatory Bowel Diseases and Celiac Disease. Dig. Dis. 2018, 36, 271–280.
- Rhys-Jones, D.; Varney, J.E.; Muir, J.G.; Gibson, P.R.; Halmos, E.P. Application of The FODMAP Diet in a Paediatric Setting. Nutrients 2022, 14, 4369.
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Gut 2021, 70, 1214–1240.
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136.
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35, 5–13.
- Manabe, N.; Tanaka, T.; Hata, J.; Kusunoki, H.; Haruma, K. Pathophysiology Underlying Irritable Bowel Syndrome -From the Viewpoint of Dysfunction of Autonomic Nervous System Activity-. J. Smooth Muscle Res. 2009, 45, 15–23.
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44.
- Ancona, A.; Petito, C.; Iavarone, I.; Petito, V.; Galasso, L.; Leonetti, A.; Turchini, L.; Belella, D.; Ferrarrese, D.; Addolorato, G.; et al. The Gut–Brain Axis in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Dig. Liver Dis. 2021, 53, 298–305.
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, Gut Microbiota, and Irritable Bowel Syndrome: What Are the Relations? World J. Gastroenterol. 2022, 28, 1204–1219.
- Singh, R.; Salem, A.; Nanavati, J.; Mullin, G.E. The Role of Diet in the Treatment of Irritable Bowel Syndrome. Gastroenterol. Clin. N. Am. 2018, 47, 107–137.
- Böhn, L.; Störsrud, S.; Simrén, M. Nutrient Intake in Patients with Irritable Bowel Syndrome Compared with the General Population. Neurogastroenterol. Motil. 2013, 25, 23–30.e1.
- Chirila, I.; Drug, V.L.; Morariu, I.D. Food Related to Functional Digestive Disorders in Working Age Adults. Neurogastroenterol. Motil. 2017, 29, 133–134.
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary Poorly Absorbed, Short-Chain Carbohydrates Increase Delivery of Water and Fermentable Substrates to the Proximal Colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882.
- Camilleri, M.; Boeckxstaens, G. Dietary and Pharmacological Treatment of Abdominal Pain in IBS. Gut 2017, 66, 966–974.
- Gibson, P.R.; Varney, J.; Malakar, S.; Muir, J.G. Food Components and Irritable Bowel Syndrome. Gastroenterology 2015, 148, 1158–1174.e4.
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome. J. Affect. Disord. 2020, 266, 429–446.
- Shiha, M.G.; Aziz, I. Review Article: Physical and Psychological Comorbidities Associated with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2021, 54, S12–S23.
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional Bowel Disorders. Gastroenterology 2006, 130, 1480–1491.
- Patel, N.; Shackelford, K. Irritable Bowel Syndrome; StatPearls Publishing: Tampa, FL, USA, 2022.
- Black, C.J. Review Article: Diagnosis and Investigation of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2021, 54, S33–S43.
- Dalrymple, J.; Bullock, I. Diagnosis and Management of Irritable Bowel Syndrome in Adults in Primary Care: Summary of NICE Guidance. BMJ 2008, 336, 556–558.
- Nakov, R.; Snegarova, V.; Dimitrova-Yurukova, D.; Velikova, T. Biomarkers in Irritable Bowel Syndrome: Biological Rationale and Diagnostic Value. Dig. Dis. 2022, 40, 23–32.
- Linedale, E.C.; Andrews, J.M. Diagnosis and Management of Irritable Bowel Syndrome: A Guide for the Generalist. Med. J. Aust. 2017, 207, 309–315.
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal Calprotectin for Screening of Patients with Suspected Inflammatory Bowel Disease: Diagnostic Meta-Analysis. BMJ 2010, 341, c3369.
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent Advances in the Treatment of Irritable Bowel Syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715.
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Mihai, C.; Cijevschi-Prelipcean, C.; Drug, V.L. The Role of Diet in the Gastro-Esophageal Reflux and Dyspepsia Overlap. Eur. J. Clin. Investig. 2015, 45, 33.
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Drug, V.L. The Role of Diet in the Overlap between Gastroesophageal Reflux Disease and Functional Dyspepsia. Turk. J. Gastroenterol. 2016, 27, 73–80.
- Camilleri, M.; Boeckxstaens, G. Irritable Bowel Syndrome: Treatment Based on Pathophysiology and Biomarkers. Gut 2023, 72, 590–599.
- Exarchopoulou, K.; Papageorgiou, A.; Bacopoulou, F.; Koumantarou Malisiova, E.; Vlachakis, D.; Chrousos, G.P.; Darviri, C. A Biofeedback-Assisted Stress Management Program for Patients with Irritable Bowel Syndrome: A Randomised Controlled Trial. EMBnet J. 2021, 26, e980.
- Galica, A.N.; Galica, R.; Dumitrașcu, D.L. Diet, Fibers, and Probiotics for Irritable Bowel Syndrome. J. Med. Life 2022, 15, 174–179.
- Moayyedi, P.; Simrén, M.; Bercik, P. Evidence-Based and Mechanistic Insights into Exclusion Diets for IBS. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 406–413.
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a Diet Low in FODMAPs Reduce Symptoms Associated with Functional Gastrointestinal Disorders? A Comprehensive Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 897–906.
- Altobelli, E.; Del Negro, V.; Angeletti, P.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940.
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a Low FODMAP Diet in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gut 2022, 71, 1117–1126.
- Zhan, Y.; Zhan, Y.; Dai, S. Is a Low FODMAP Diet Beneficial for Patients with Inflammatory Bowel Disease? A Meta-Analysis and Systematic Review. Clin. Nutr. 2018, 37, 123–129.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
976
Revisions:
2 times
(View History)
Update Date:
15 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




