
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alvaro Ruiz-Fernandez | -- | 1754 | 2022-06-15 16:27:12 | | | |
| 2 | Beatrix Zheng | -157 word(s) | 1597 | 2022-06-16 04:22:28 | | |
Video Upload Options
Nanosecond Pulsed Electric Field (nsPEF) is an electrostimulation technique first developed in 1995; nsPEF requires the delivery of a series of pulses of high electric fields in the order of nanoseconds into biological tissues or cells. They primary effects in cells is the formation of membrane nanopores and the activation of ionic channels, leading to an incremental increase in cytoplasmic Ca2+ concentration, which triggers a signaling cascade producing a variety of effects: from apoptosis up to cell differentiation and proliferation. Further, nsPEF may affect organelles, making nsPEF a unique tool to manipulate and study cells. This technique is exploited in a broad spectrum of applications, such as: sterilization in the food industry, seed germination, anti-parasitic effects, wound healing, increased immune response, activation of neurons and myocites, cell proliferation, cellular phenotype manipulation, modulation of gene expression, and as a novel cancer treatment.
1. A Brief History on the Development of Electric Pulses Technology
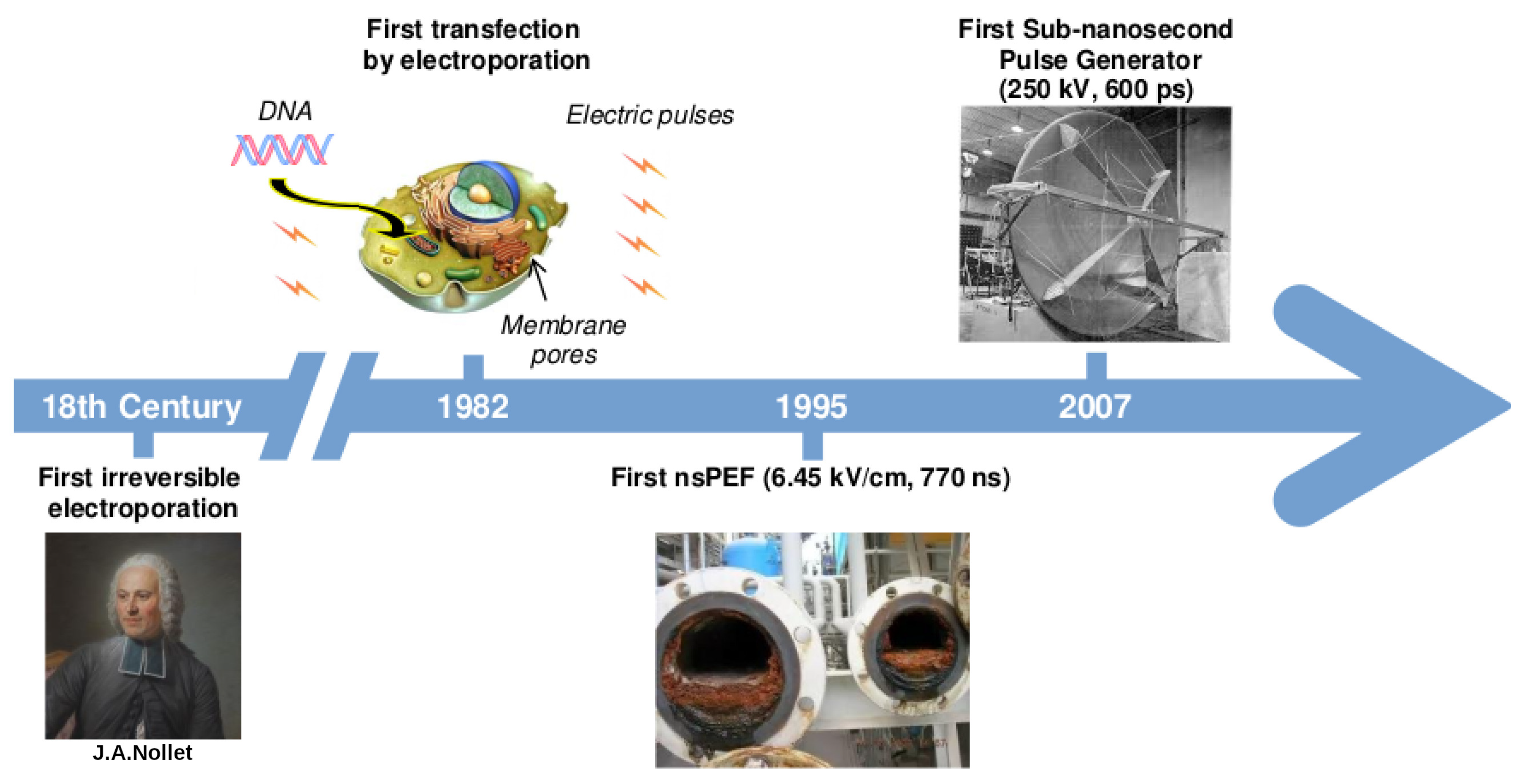
2. nsPEF Applications
2.1. In Human Health
-
Activation of excitable cells:Cardiac cells: nsPEF (10–80 kV/cm, 4 ns, 1–20 pulses with 200/400/600 ms intervals) can indirectly lead to cardiac cell excitation. Of note, these results challenge the concept of chronaxie: minimum time required for an electric current to double the strength of the rheobase in order to stimulate a muscle or a neuron. The use of nsPEF technology to excite cardiac cells and mobilize intracellular Ca2+ may prove valuable for cardiac pacing and defibrillation [15]. For other related studies see [16][17][18].Neurons: nsPEF (27.8 kV/cm, 10 ns, single pulse) was sufficient to initiate action potentials. The observed effect was repeatable and stable. These results highlight the potential use of ultrashort pulsed electric fields for stimulation of subcortical structures and suggest they may be used as a wireless alternative for deep brain stimulation [19]. For other related studies see [20][21][22][23].
-
Phenotype manipulation:Differentiation: nsPEF (1.5–25 kV/cm, 300 ns, 5 pulses) can induce proliferation and myotubule maturation or nodule formation in myoblasts and osteoblasts, respectively. Myoblasts were isolated from hind-limb skeletal muscle of four-week-old mice PtenMKO, and primary human osteoblasts were obtained from a vendor (Sciencell®) [24].
-
ntiparasitic: Cystic echinococcosis is a widely endemic helminthic disease caused by infection with metacestodes (larval stage) of the Echinococcus granulosus tapeworm. Application of nsPEF (21 KV/cm, 300 ns, 100 pulses) caused a significant increase in the death rate of protoscolices (future heads of the adult worms) [31]. For related studies see [32][33].
-
Immune response: Using in vivo experiments, nsPEF (15 kV, 100 ns, 400 pulses) induced translocation of calreticulin in rat tumor cell-surfaces, a molecular pattern associated with damage that is indicative of immunogenic cell death (ICD). The nsPEF also triggered CD8-dependent inhibition of secondary tumor growth, concluded by comparing the tumor size using rats depleted of CD8+cytotoxic T-cells under the same nsPEF treatment. The first group showed an average size of only 3% of the primary tumor size compared with the 54% shown by the CD8+-depleted rats. Additionally, with immunohistochemistry it was observed that CD8+ T-cells were highly enriched in the first group. Furthermore, it was shown that vaccinating rats with isogenic tumor cells (MCA205 fibrosarcoma cell line) treated with nsPEF (50 kV, 100 ns, 500 pulses) stimulates an immune response that inhibits the growth of secondary tumors in a CD8+-dependent manner [38]. This work opens the door to the fabrication of cell-based vaccines using nsPEF stimulation to promote an improved immune response. For other related studies reporting tumor ablation through an antitumor immune response using nsPEF see [39][40][41][42][43].
-
Cancer: This is by far the most-studied nsPEF application, with 46 in vitro studies up to 2016 [44] and over 100 so far. Recently, preclinical animal studies have demonstrated that nsPEF can induce local and systemic CD8+T-cell mediated adaptive immune response against tumors [40][43]. In clinical trials, nsPEF proved to be a safe and effective therapy against basal cell carcinoma [45][46]. There are other novel techniques to combat cancer that also use electric fields, known as electrochemotherapy [47][48], irreversible electroporation [7], and electro-gene therapy [7]. Electrochemotherapy and electro-gene therapy use electroporation to achieve the anti-tumoral effect of other agents. In irreversible electroporation, cytoplasmic membranes of tumor cells cannot recover from permeabilization, causing cell death mainly by necrosis. Unlike the just mentioned electro-technique, nsPEF is cell-dependent. A possible explanation for this may be related to apoptosis (programmed cell death type 1 [49]), which is a tightly controlled cell process and different in each cell type [50]. Thus, if nsPEF induces apoptosis, as seems to be the case, it is expected to exhibit cell-dependent responses. This makes nsPEF an extraordinary tool, with specific responses based on tuning the intensity, duration, and number of pulses. There are several examples of cell dependence and nsPEF. Stacey et al. in 2002 demonstrated that exposing cancer cells to nsPEF with 60 kV/cm could induce DNA damage [51]. Beebe et al. in 2002 studied the antitumor effects of nsPEF on Jurkat cells, with pulses at 60, 150, and 300 kV/cm [52]. Xinh ua Chen et al. in 2012 applied nsPEF with 900 pulses at 68 kV/cm to ablate hepatocellular carcinoma [53]. Nuccitelli et al. in 2013 inhibited human pancreatic carcinoma using 100 pulses of 100 ns duration and 30 kV/cm [54]. More importantly for nsPEF as cancer treatment, tumor cells are more sensitive to nsPEF than normal cells [55].
2.2. Industrial
-
Cell proliferation: nsPEF (10 kV/cm, 100 ns) can increase Arthrospira platensis SAG 21.99 (a cyanobacteria) cell growth after repeated pulses in the exponential growth phase. The effect was most pronounced five days after treatment. Treatments with nsPEF might improve sustainable and economical microalgae-based biorefineries [57]. For other studies see [24][58][59].
-
Fermentation industry: nsPEF (15 kV/cm, 100 ns, 20 pulse) increased avermectin (anthelmintic and insecticidal agent) production in Streptomyces avermitilis by 42% and reduced the time needed for reaching a plateau in the fermentation process from 5 to 7 days [60]. For other related studies see [61].
-
Food industry: Microalgae are a novel food ingredient of increasing interest as they can be grown on non-arable lands and fixates CO2 when grown photoautotrophically. Treatment with nsPEF (5–100 kV/cm, 2–100 ns) reduced total bacterial contamination >log10 in Chlorella vulgaris cultures without compromising the microalgae. For related studies see [62][63].
References
- Meijerink, M.R.; Scheffer, H.J.; Narayanan, G. Irreversible Electroporation in Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2018.
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845.
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186.
- Tsong, T.Y. On electroporation of cell membranes and some related phenomena. Bioelectrochem. Bioenerg. 1990, 24, 271–295.
- Orlowski, S.; Belehradek, J., Jr.; Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733.
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29.
- Davalos, R.V.; Mir, L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223.
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320.
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488.
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in food processing and biorefinery. J. Membr. Biol. 2014, 247, 1279–1304.
- Saldaña, G.; Álvarez, I.; Condón, S.; Raso, J. Microbiological aspects related to the feasibility of PEF technology for food pasteurization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1415–1426.
- Schoenbach, K.H.; Alden, R.W.; Fox, T.J. Biofouling prevention with pulsed electric fields. In Proceedings of the 1996 International Power Modulator Symposium, Boca Raton, FL, USA, 25–27 June 1996; pp. 75–78.
- Baum, C.E. Producing large transient electromagnetic fields in a small region: An electromagnetic implosion. In Ultra-Wideband Short-Pulse Electromagnetics 8; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–104.
- Heeren, T.; Camp, J.T.; Kolb, J.F.; Schoenbach, K.H.; Katsuki, S.; Akiyama, H. 250 kV sub-nanosecond pulse generator with adjustable pulse-width. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 884–888.
- Wang, S.; Chen, J.; Chen, M.T.; Vernier, P.T.; Gundersen, M.A.; Valderrábano, M. Cardiac myocyte excitation by ultrashort high-field pulses. Biophys. J. 2009, 96, 1640–1648.
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401.
- Pakhomov, A.G.; Xiao, S.; Novickij, V.; Casciola, M.; Semenov, I.; Mangalanathan, U.; Kim, V.; Zemlin, C.; Sozer, E.; Muratori, C.; et al. Excitation and electroporation by MHz bursts of nanosecond stimuli. Biochem. Biophys. Res. Commun. 2019, 518, 759–764.
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and injury of adult ventricular cardiomyocytes by nano-to millisecond electric shocks. Sci. Rep. 2018, 8, 8233.
- Romanenko, S.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Ultrashort pulsed electric fields induce action potentials in neurons when applied at axon bundles. In Proceedings of the 2016 9th International Kharkiv Symposium on Physics and Engineering of Microwaves, Millimeter and Submillimeter Waves (MSMW), Kharkiv, Ukraine, 20–24 June 2016; pp. 1–5.
- Pakhomov, A.G.; Semenov, I.; Casciola, M.; Xiao, S. Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1273–1281.
- Casciola, M.; Xiao, S.; Pakhomov, A.G. Damage-free peripheral nerve stimulation by 12-ns pulsed electric field. Sci. Rep. 2017, 7, 10453.
- Lamberti, P.; Tucci, V.; Zeni, O.; Romeo, S. Analysis of ionic channel currents under nsPEFs-stimulation by a circuital model of an excitable cell. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference (MELECON), Palermo, Italy, 16–18 June 2020; pp. 411–414.
- Roth, C.C.; Tolstykh, G.P.; Payne, J.A.; Kuipers, M.A.; Thompson, G.L.; DeSilva, M.N.; Ibey, B.L. Nanosecond pulsed electric field thresholds for nanopore formation in neural cells. J. Biomed. Opt. 2013, 18, 035005.
- Vadlamani, R.A.; Nie, Y.; Detwiler, D.A.; Dhanabal, A.; Kraft, A.M.; Kuang, S.; Gavin, T.P.; Garner, A.L. Nanosecond pulsed electric field induced proliferation and differentiation of osteoblasts and myoblasts. J. R. Soc. Interface 2019, 16, 20190079.
- Zhang, K.; Guo, J.; Ge, Z.; Zhang, J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci. Rep. 2014, 4, 5836.
- Morotomi-Yano, K.; Uemura, Y.; Katsuki, S.; Akiyama, H.; Yano, K. Activation of the JNK pathway by nanosecond pulsed electric fields. Biochem. Biophys. Res. Commun. 2011, 408, 471–476.
- Muratori, C.; Pakhomov, A.G.; Gianulis, E.; Meads, J.; Casciola, M.; Mollica, P.A.; Pakhomova, O.N. Activation of the phospholipid scramblase TMEM16F by nanosecond pulsed electric fields (nsPEF) facilitates its diverse cytophysiological effects. J. Biol. Chem. 2017, 292, 19381–19391.
- Beebe, S.J.; Blackmore, P.F.; White, J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004, 25, 1077.
- Guo, S.; Jackson, D.L.; Burcus, N.I.; Chen, Y.J.; Xiao, S.; Heller, R. Gene electrotransfer enhanced by nanosecond pulsed electric fields. Mol. Ther. Methods Clin. Dev. 2014, 1, 14043.
- Estlack, L.E.; Roth, C.C.; Thompson, G.L.; Lambert, W.A.; Ibey, B.L. Nanosecond pulsed electric fields modulate the expression of Fas/CD95 death receptor pathway regulators in U937 and Jurkat Cells. Apoptosis 2014, 19, 1755–1768.
- Zhang, R.; Aji, T.; Shao, Y.; Jiang, T.; Yang, L.; Lv, W.; Chen, Y.; Chen, X.; Wen, H. Nanosecond pulsed electric field (nsPEF) disrupts the structure and metabolism of human Echinococcus granulosus protoscolex in vitro with a dose effect. Parasitol. Res. 2017, 116, 1345–1351.
- Chen, X.; Zhang, R.; Aji, T.; Shao, Y.; Chen, Y.; Wen, H. Novel interventional management of hepatic hydatid cyst with nanosecond pulses on experimental mouse model. Sci. Rep. 2017, 7, 4491.
- Chen, X.; Zhang, R.; Wen, H. Experimental nanopulse ablation of multiple membrane parasite on ex vivo hydatid cyst. BioMed Res. Int. 2018, 2018, 8497283.
- Zhang, J.; Blackmore, P.F.; Hargrave, B.Y.; Xiao, S.; Beebe, S.J.; Schoenbach, K.H. Nanosecond pulse electric field (nanopulse): A novel non-ligand agonist for platelet activation. Arch. Biochem. Biophys. 2008, 471, 240–248.
- Hargrave, B.; Li, F. Nanosecond pulse electric field activation of platelet-rich plasma reduces myocardial infarct size and improves left ventricular mechanical function in the rabbit heart. J. Extra-Corpor. Technol. 2012, 44, 198.
- Xiao, S.; Kiyan, T.; Blackmore, P.; Schoenbach, K. Pulsed Power for Wound Healing. In Proceedings of the 2008 IEEE International Power Modulators and High-Voltage Conference, Las Vegas, NV, USA, 27–31 May 2008; pp. 69–72.
- Hargrave, B.; Li, F. Nanosecond Pulse Electric Field Activated-Platelet Rich Plasma Enhances the Return of Blood Flow to Large and Ischemic Wounds in a Rabbit Model. Physiol. Rep. 2015, 3, e12461.
- Nuccitelli, R.; Berridge, J.C.; Mallon, Z.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS ONE 2015, 10, e0134364.
- Chen, R.; Sain, N.M.; Harlow, K.T.; Chen, Y.J.; Shires, P.K.; Heller, R.; Beebe, S.J. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur. J. Cancer 2014, 50, 2705–2713.
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640.
- Skeate, J.G.; Da Silva, D.M.; Chavez-Juan, E.; Anand, S.; Nuccitelli, R.; Kast, W.M. Nano-Pulse Stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE 2018, 13, e0191311.
- Nuccitelli, R.; Tran, K.; Lui, K.; Huynh, J.; Athos, B.; Kreis, M.; Nuccitelli, P.; De Fabo, E.C. Non-thermal nanoelectroablation of UV-induced murine melanomas stimulates an immune response. Pigment Cell Melanoma Res. 2012, 25, 618–629.
- Nuccitelli, R.; McDaniel, A.; Anand, S.; Cha, J.; Mallon, Z.; Berridge, J.C.; Uecker, D. Nano-Pulse Stimulation is a physical modality that can trigger immunogenic tumor cell death. J. Immunother. Cancer 2017, 5, 32.
- Napotnik, T.B.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavčič, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12.
- Nuccitelli, R.; Wood, R.; Kreis, M.; Athos, B.; Huynh, J.; Lui, K.; Nuccitelli, P.; Epstein, E.H., Jr. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: Proof of method. Exp. Dermatol. 2014, 23, 135–137.
- Garon, E.B.; Sawcer, D.; Vernier, P.T.; Tang, T.; Sun, Y.; Marcu, L.; Gundersen, M.A.; Koeffler, H.P. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int. J. Cancer 2007, 121, 675–682.
- Mir, L.M.; Orlowski, S.; Belehradek, J., Jr.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72.
- Serša, G.; Čemažar, M.; Miklavčič, D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum (II) in mice. Cancer Res. 1995, 55, 3450–3455.
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498.
- Salido, G.M.; Rosado, J.A. Apoptosis: Involvement of Oxidative Stress and Intracellular Ca2+ Homeostasis; Universty of Extremadure: Badajoz, Spain, 2009; pp. 229–235.
- Stacey, M.; Stickley, J.; Fox, P.; O’Donnell, C.; Schoenbach, K.; Beebe, S.; Buescher, S. Increased cell killing and DNA damage in cells exposed to ultra-short pulsed electric fields. In Proceedings of the Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 20–24 October 2002; pp. 79–82.
- Beebe, S.J.; Fox, P.; Rec, L.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292.
- Chen, X.; Zhuang, J.; Kolb, J.F.; Schoenbach, K.H.; Beebe, S.J. Long term survival of mice with hepatocellular carcinoma after pulse power ablation with nanosecond pulsed electric fields. Technol. Cancer Res. Treat. 2012, 11, 83–93.
- Nuccitelli, R.; Huynh, J.; Lui, K.; Wood, R.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of human pancreatic carcinoma in a murine xenograft model without recurrence. Int. J. Cancer 2013, 132, 1933–1939.
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074.
- Stacey, M.; Stickley, J.; Fox, P.; Statler, V.; Schoenbach, K.; Beebe, S.; Buescher, S. Differential effects in cells exposed to ultra-short, high intensity electric fields: Cell survival, DNA damage, and cell cycle analysis. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 542, 65–75.
- Buchmann, L.; Frey, W.; Gusbeth, C.; Ravaynia, P.S.; Mathys, A. Effect of nanosecond pulsed electric field treatment on cell proliferation of microalgae. Bioresour. Technol. 2019, 271, 402–408.
- Zhang, Y.; Dong, F.; Liu, Z.; Guo, J.; Zhang, J.; Fang, J. Nanosecond pulsed electric fields promoting the proliferation of porcine iliac endothelial cells: An in vitro study. PLoS ONE 2018, 13, e0196688.
- Dong, F.; Liu, Z.; Zhang, J.; Fang, J.; Guo, J.; Zhang, Y. Nspefs Promoting the Proliferation of Piec Cells: An in Vitro Study. In Proceedings of the 2017 IEEE International Conference on Plasma Science (ICOPS), Atlantic City, NJ, USA, 21–25 May 2017; p. 1.
- Guo, J.; Ma, R.; Su, B.; Li, Y.; Zhang, J.; Fang, J. Raising the avermectins production in Streptomyces avermitilis by utilizing nanosecond pulsed electric fields (nsPEFs). Sci. Rep. 2016, 6, 25949.
- Rajabi, F.; Gusbeth, C.; Frey, W.; Maisch, J.; Nick, P. Nanosecond pulsed electrical fields enhance product recovery in plant cell fermentation. Protoplasma 2020, 257, 1585–1594.
- Prorot, A.; Arnaud-Cormos, D.; Lévêque, P.; Leprat, P. Bacterial stress induced by nanosecond pulsed electric fields (nsPEF): Potential applications for food industry and environment. In Proceedings of the IV International Conference on Environmental, Industrial and Applied Microbiolog, Malaga, Spain, 14–16 September 2011.
- Haberkorn, I.; Buchmann, L.; Häusermann, I.; Mathys, A. Nanosecond pulsed electric field processing of microalgae based biorefineries governs growth promotion or selective inactivation based on underlying microbial ecosystems. Bioresour. Technol. 2020, 319, 124173.
- Su, B.; Guo, J.; Nian, W.; Feng, H.; Wang, K.; Zhang, J.; Fang, J. Early growth effects of nanosecond pulsed electric field (nsPEFs) exposure on Haloxylon ammodendron. Plasma Process. Polym. 2015, 12, 372–379.
- Eing, C.J.; Bonnet, S.; Pacher, M.; Puchta, H.; Frey, W. Effects of nanosecond pulsed electric field exposure on Arabidopsis thaliana. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1322–1328.
- Songnuan, W.; Kirawanich, P. Early growth effects on Arabidopsis thaliana by seed exposure of nanosecond pulsed electric field. J. Electrost. 2012, 70, 445–450.




