
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hanna Gul | -- | 3341 | 2022-06-15 13:30:30 | | | |
| 2 | Peter Tang | -19 word(s) | 3322 | 2022-06-16 03:59:16 | | |
Video Upload Options
Rheumatoid arthritis (RA) is a chronic immune-mediated systemic disease, which affects approximately 1% of the population and is characterized by a symmetrical inflammatory polyarthropathy. It has been demonstrated that drug-free remission (DFR) is possible in a proportion of RA patients achieving clinically defined remission (both on cs and b-DMARDS). Immunological, imaging and clinical associations with/predictors of DFR have all been identified, including the presence of autoantibodies, absence of Power Doppler (PD) signal on ultrasound (US), lower disease activity according to composite scores of disease activity and lower patient-reported outcome scores (PROs) at treatment cessation.
1. Introduction
2. Defining Remission in RA
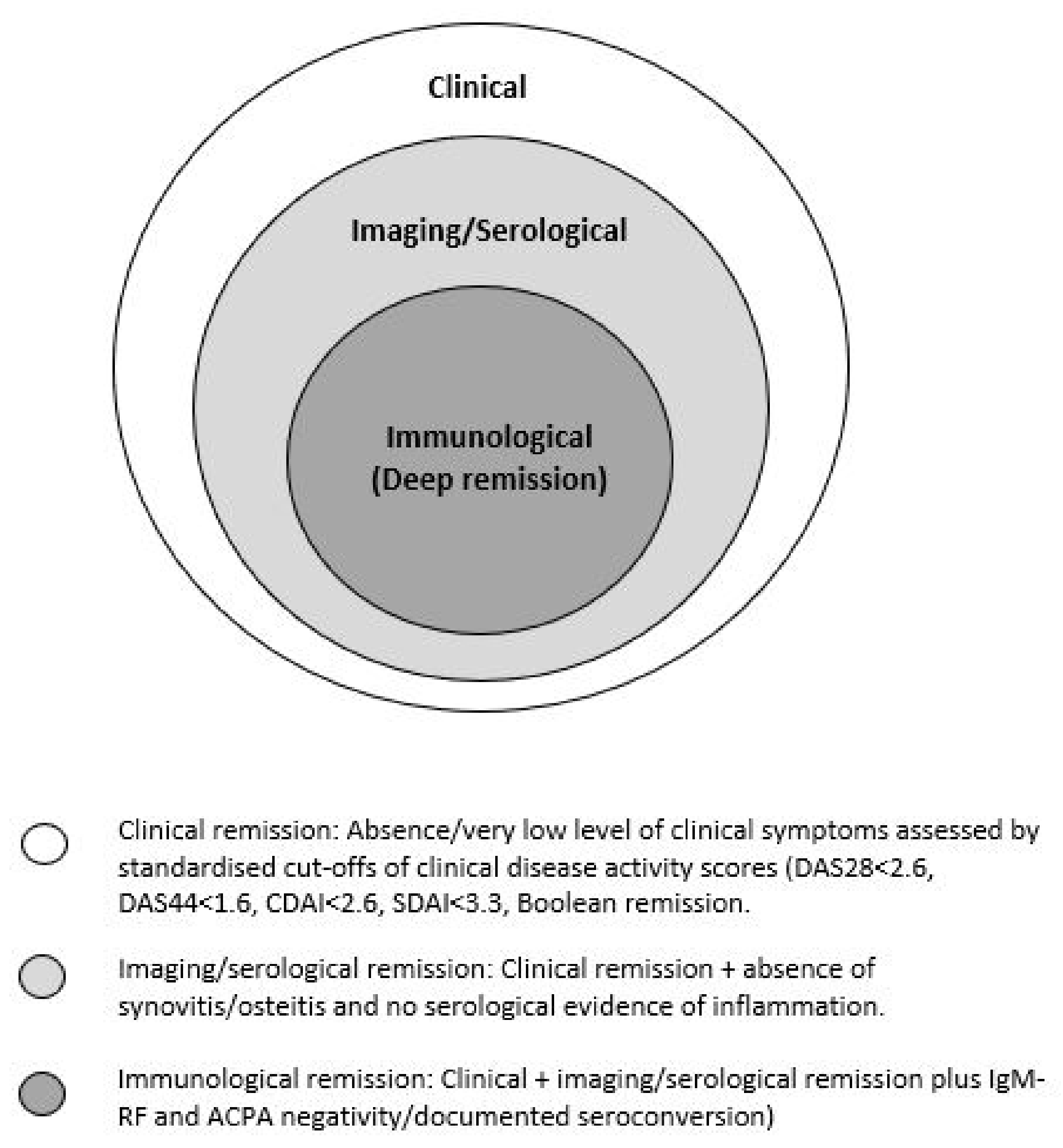
3. DFR Remission in Patients with RA Treated with cs-DMARDs
|
Study |
Design |
Authors |
n |
Treatment/Intervention |
RA Disease Duration |
Remission Criteria |
%DFR Remission |
DFR-Predicting Factors |
Follow Up Period |
|---|---|---|---|---|---|---|---|---|---|
|
Can disease-modifying anti-rheumatic drugs be discontinued in long standing rheumatoid arthritis? A 15-year follow-up |
Observational |
Tiippana et al., 2010 |
70 |
Single or combination Cs-DMARDS tapered |
Early RA |
5/6 ARA criteria fulfilled. |
16% |
N/A |
15 years |
|
Prevalence and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts |
Observational |
van der Woude et al., 2009 |
Leiden EAC cohort: 454 British EAC Cohort: 895 |
Single or combination Cs-DMARDS tapered (MTX/SSZ/HcQ) |
Early RA |
Had to fulfil 3 criteria: (1) No current use of DMARDs/corticosteroids, (2) No swollen joints, and (3) Classification as DMARD-free remission by the patient’s rheumatologist. |
Leiden EAC cohort: 15% British EAC Cohort: 9.4% |
Absence of autoantibodies ((ACPA and IgM-RF) and short symptom duration at presentation |
Minimum of 1 year after discontinuation of DMARD therapy |
|
KIMERA |
Observational |
Jung et al., 2020 |
234 |
Single or combination therapy with cs DMARDs; methotrexate (MTX)/sulfasalazine combined with high-dose glucocorticoid; MTX combined with TNF-inhibitors tapered |
Early RA |
(1) Non-use of cs or bDMARDs and glucocorticoids, (2) DAS28 <2.6, and (3) no swollen joints. |
46.1% |
Early RA and lower disease activity (DAS28 <2.26) at csDMARD withdrawal |
48 months |
|
Randomized placebo-controlled study of stopping second-line drugs in RA |
RCT |
Ten Wolde et al., 1996 |
285 |
Placebo or withdrawal of at least one 2nd line cs-DMARD (chloroquine, HCQ, gold, d-penicillamine, SSZ, AZA or MTX) |
Established RA. Median duration 8–9 years. |
5/6 ARA criteria fulfilled |
62% |
Lower maintenance dose of second line drug and absence of RF |
52 weeks |
|
D-penicillamine withdrawal in rheumatoid arthritis |
Double blind RCT |
Ahern et al., 1984 |
38 |
Tapering of d-penicillamine |
Established RA (6–11 years) |
5/6 ARA criteria fulfilled |
21% |
None |
12 months |
|
BeST |
Multi center randomized single blind trial |
Markusse et al., 2015 |
508 |
MTX/combination cs DMARD/ combination cs-DMARD +prednisolone/combination cs DMARD with MTX and Infliximab |
Early disease (symptom duration < 2 years) |
DAS44 <1.6 |
14% |
Absence of ACPA and using MTX rather than SSZ as the last csDMARD before withdrawal |
10 years |
|
tREACH |
RCT |
Kuijper et al., 2016 |
281 |
Triple cs-DMARD (MTX, SSZ and HCQ) with glucocorticoid bridging or MTX monotherapy with glucocorticoid bridging TNFi and MTX if the DAS28 was >2.4. |
Early RA |
DAS28 <1.6 |
2.4% |
N/A |
2 year |
|
IMPROVED |
RCT |
Heimans et al., 2016 |
610 |
MTX and prednisolone, then tapered |
Early RA or Undifferentiated arthritis |
DAS44 <1.6 |
21% |
Absence of ACPA |
2 year |
|
BioRRA |
Interventional cohort study |
Baker et al., 2019 |
44 |
Cessation of cs-DMARDs |
Established RA |
DAS28-CRP < 2.4 |
48% |
Absence of RF, shorter time from diagnosis to starting first DMARD, shorter symptom duration at time of diagnosis, longer disease duration fulfilment of ACR/EULAR Boolean remission criteria and longer time since last DMARD change Absence of genes within peripheral CD4+ T cells; FAM102B and ENSG00000227070 Presence of gene within peripheral CD4+ T cells: ENSG00000228010 |
6 months |
4. Predicting DFR for Patients Receiving Treatment with cs-DMARDs
5. DFR Remission in Patients with RA Treated with Biological Therapies (b-DMARDs)
|
Study |
Design |
Authors |
n |
Treatment/Intervention Drug Withdrawn in Italics |
RA Disease Duration |
Remission Criteria |
%DFR Remission in Biologic Treatment Arm |
DFR Predicting Factors |
Follow Up Period |
|---|---|---|---|---|---|---|---|---|---|
|
IVEA |
Double blind RCT |
Quinn MQ et al., 2006 |
20 |
1. Infliximab + MTX 2. MTX |
6 months |
DAS28 |
70 |
- |
12 months |
|
BeSt |
RCT |
van den Broek M et al., 2011 |
128 |
4th study arm: Combination with infliximab |
23 months |
DAS44 |
56 |
Lower baseline HAQ ACPA negative Lower baseline disease activity Younger age Non-smoker |
24 months |
|
IDEA |
Double blind RCT |
Nam JL et al., 2014 |
112 |
1. Infliximab +MTX 2. MTX + single dose IV methylprednisolone |
78 weeks |
DAS44 |
76% |
- |
78 weeks |
|
HONOR |
Open label non randomized |
Yamaguchi A et al., 2020 |
52 |
Adalimumab |
7 years |
DAS28 |
21 |
A baseline DAS28 of <2.22 or <1.98 Shorter disease duration |
60 months |
|
RRR * |
Observational |
Tanaka Y et al., 2010 |
114 |
Infliximab |
6 years |
LDA |
55 |
A baseline DAS28 of <2.22 or <1.98 |
12 months |
|
OPTIMA |
RCT |
Smolen J et al., 2013 |
1032 |
Adalimumab + MTX |
≤12 months |
DAS28 |
66% |
Good baseline functional status |
52 weeks |
|
PRIZE |
Double blind RCT |
Emery P et al., 2014 |
306 |
1. ½ dose Etanercept + MTX 2. Placebo + MTX 3. Placebo alone |
≤12 months |
DAS2 |
23–40% |
- |
39 weeks |
|
CERTAIN |
Double blind RCT |
Smolen J et al., 2015 |
194 |
1. Certolizumab + MTX 2. Placebo |
6 months–10 years |
CDAI |
18.8% |
- |
52 weeks |
|
Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? |
Observational |
Saleem et al., 2011 |
47 |
TNFi (Various) + MTX 1. Initial therapy 2. Delayed therapy |
12 months |
DAS28 |
59%15% |
Male gender First line TNFi Shorter disease duration Higher and naïve T-cells and fewer IRCs at baseline |
24 months |
|
EMPIRE |
Double blind RCT |
Nam et al., 2013 |
110 |
1. Etanercept + MTX 2. MTX + placebo |
≤3 months |
DAS28 |
28.1% |
Starting TNFi earlier in disease course |
52 weeks |
|
TARA |
Single blind RCT |
Van Mulligen et al., 2020 |
189 94 DMARD 95 TNFi |
TNFi or csDMARD (Various) 1. csDMARD taper first 2. TNFi taper first |
Not stated |
DAS44 |
15% |
- |
24 months |
|
AVERT |
Double blind RCT |
Emery P et al., 2015 |
351 |
Abatacept + MTX |
<1 year |
DAS28 |
15% |
Lower baseline PRO scores |
18 months |
|
DREAM |
Observational |
Nishimoto N et al., 2014 |
187 |
Tocilizumab |
7.8 years |
LDA |
9% |
Lower multi-biomarker assay scores (serological) RF negative |
12 months |
|
ACT RAY |
RCT |
Huizinga TW et al., 2015 |
556 |
Tocilizumab |
8 years |
DAS28 |
6% |
Shorter disease duration, few/absent erosions |
12 months |
|
RETRO |
RCT |
Haschka J et al., 2016 |
101 |
Various |
NK |
DAS28 |
48.1% |
ACPA negative Lower baseline disease activity Male gender Lower multi-biomarker assay scores (serological) RF negative |
12 months |
|
PredictRA |
Double blind RCT |
Emery et al., 2020 |
122 |
Adalimumab taper vs. withdrawal |
Mean 12.9 years |
DAS28 |
55% (withdrawal arm) |
- |
36 weeks |
|
ANSWER |
Cohort |
Hashimoto et al., 2018 |
181 |
Various |
NK |
DAS28 |
21.5% |
Boolean remission at baseline Sustained remission period No glucocorticoid use at time of discontinuation TNFi discontinuation (vs. other b-DMARD) |
12 months |
* NK = not known.
6. Predictors of DFR for Patients Receiving Treatment with b-DMARDs
6.1. Clinical and Demographic Variables
6.2. Patient Reported Outcomes (PRO) Measures
6.3. Imaging Variables
6.4. Immunological Variables
6.5. Serum Biomarkers and Multi-Biomarker Assays
6.6. Deep/Multi-Level Remission
References
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911.
- Quinn, M.A.; Emery, P. Window of opportunity in early rheumatoid arthritis: Possibility of altering the disease process with early intervention. Clin. Exp. Rheumatol. 2003, 21, S154–S157.
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; de Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637.
- Hughes, L.D.; Done, J.; Young, A. A 5 item version of the Compliance Questionnaire for Rheumatology (CQR5) successfully identifies low adherence to DMARDs. BMC Musculoskelet. Disord. 2013, 14, 286.
- Grijalva, C.G.; Chung, C.P.; Arbogast, P.G.; Stein, C.M.; Mitchel, E.F., Jr.; Griffin, M.R. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med. Care 2007, 45, S66–S76.
- Betegnie, A.L.; Gauchet, A.; Lehmann, A.; Grange, L.; Roustit, M.; Baudrant, M.; Bedouch, P.; Allenet, B. Why Do Patients with Chronic Inflammatory Rheumatic Diseases Discontinue Their Biologics? An Assessment of Patients’ Adherence Using a Self-report Questionnaire. J. Rheumatol. 2016, 43, 724–730.
- Cruyssen, B.; Looy, S.; Wyns, B.; Westhovens, R.; Durez, P.; Van den Bosch, F.; Veys, E.; Mielants, H.; Clerck, L.; Peretz, A.; et al. DAS28 best reflects the physician’s clinical judgment of response to infliximab therapy in rheumatoid arthritis patients: Validation of the DAS28 score in patients under infliximab treatment. Arthritis Res. Ther. 2005, 7, R1063–R1071.
- Van der Heijde, D.M.; van’t Hof, M.A.; van Riel, P.L.; Theunisse, L.A.; Lubberts, E.W.; van Leeuwen, M.A.; van Rijswijk, M.H.; van de Putte, L.B. Judging disease activity in clinical practice in rheumatoid arthritis: First step in the development of a disease activity score. Ann. Rheum. Dis. 1990, 49, 916–920.
- Van der Maas, A.; Lie, E.; Christensen, R.; Choy, E.; de Man, Y.A.; van Riel, P.; Woodworth, T.; den Broeder, A.A. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: An OMERACT cohort validation study. Ann. Rheum. Dis. 2013, 72, 1800–1805.
- Saleem, B.; Brown, A.K.; Keen, H.; Nizam, S.; Freeston, J.; Wakefield, R.; Karim, Z.; Quinn, M.; Hensor, E.; Conaghan, P.G.; et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann. Rheum. Dis. 2011, 70, 792–798.
- Saleem, B.; Brown, A.K.; Keen, H.; Nizam, S.; Freeston, J.; Karim, Z.; Quinn, M.; Wakefield, R.; Hensor, E.; Conaghan, P.G.; et al. Disease remission state in patients treated with the combination of tumor necrosis factor blockade and methotrexate or with disease-modifying antirheumatic drugs: A clinical and imaging comparative study. Arthritis Rheum. 2009, 60, 1915–1922.
- Saleem, B.; Nizam, S.; Emery, P. Can remission be maintained with or without further drug therapy in rheumatoid arthritis? Clin. Exp. Rheumatol. 2006, 24, S33–S36.
- Brown, A.K.; Conaghan, P.G.; Karim, Z.; Quinn, M.A.; Ikeda, K.; Peterfy, C.G.; Hensor, E.; Wakefield, R.J.; O’Connor, P.J.; Emery, P. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 2958–2967.
- Brown, A.K.; Quinn, M.A.; Karim, Z.; Conaghan, P.G.; Peterfy, C.G.; Hensor, E.; Wakefield, R.J.; O’Connor, P.J.; Emery, P. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: Evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006, 54, 3761–3773.
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011, 63, 573–586.
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26.
- Aletaha, D.; Nell, V.P.; Stamm, T.; Uffmann, M.; Pflugbeil, S.; Machold, K.; Smolen, J.S. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: Validation of a clinical activity score. Arthritis Res. Ther. 2005, 7, R796–R806.
- Bykerk, V.P.; Massarotti, E.M. The new ACR/EULAR remission criteria: Rationale for developing new criteria for remission. Rheumatol. 2012, 51 (Suppl. 6), 16–20.
- El Miedany, Y.; El Gaafary, M.; Youssef, S.; Ahmed, I.; Bahlas, S.; Hegazi, M.; Nasr, A. Optimizing therapy in inflammatory arthritis: Prediction of relapse after tapering or stopping treatment for rheumatoid arthritis patients achieving clinical and radiological remission. Clin. Rheumatol. 2016, 35, 2915–2923.
- Ben Abdelghani, K.; Miladi, S.; Souabni, L.; Kassab, S.; Chekili, S.; Laatar, A.; Zakraoui, L. Role of ultrasound in assessing remission in rheumatoid arthritis. Diagn. Interv. Imaging 2015, 96, 3–10.
- Nguyen, H.; Ruyssen-Witrand, A.; Gandjbakhch, F.; Constantin, A.; Foltz, V.; Cantagrel, A. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: A systematic review and meta-analysis. Rheumatology 2014, 53, 2110–2118.
- Raffeiner, B.; Grisan, E.; Botsios, C.; Stramare, R.; Rizzo, G.; Bernardi, L.; Punzi, L.; Ometto, F.; Doria, A. Grade and location of power Doppler are predictive of damage progression in rheumatoid arthritis patients in clinical remission by anti-tumour necrosis factor alpha. Rheumatology 2017, 56, 1320–1325.
- Peluso, G.; Michelutti, A.; Bosello, S.; Gremese, E.; Tolusso, B.; Ferraccioli, G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 172–175.
- Saleem, B.; Brown, A.K.; Quinn, M. Prediction offlare and long-term outcome in DMARD treated RA patients in remission: The value of imaging and new remission criteria. Ann. Rheum. Dis. 2011, 70, 88.
- Ponchel, F.; Burska, A.N.; Hunt, L.; Gul, H.; Rabin, T.; Parmar, R.; Buch, M.H.; Conaghan, P.G.; Emery, P. T-cell subset abnormalities predict progression along the Inflammatory Arthritis disease continuum: Implications for management. Sci. Rep. 2020, 10, 3669.
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D.S.; Naredo, E.; Fautrel, B.; van Vollenhoven, R. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: Current evidence and future directions. Ann. Rheum. Dis. 2016, 75, 1428–1437.
- Benjamin, O.; Bansal, P.; Goyal, A.; Lappin, S.L. Disease Modifying Anti-Rheumatic Drugs (DMARD); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021.
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell. Physiol. 2019, 234, 10018–10031.
- Ahern, M.J.; Hall, N.D.; Case, K.; Maddison, P.J. D-penicillamine withdrawal in rheumatoid arthritis. Ann. Rheum. Dis. 1984, 43, 213–217.
- Van der Leeden, H.; Dijkmans, B.A.; Hermans, J.; Cats, A. A double-blind study on the effect of discontinuation of gold therapy in patients with rheumatoid arthritis. Clin. Rheumatol. 1986, 5, 56–61.
- ten Wolde, S.; Breedveld, F.C.; Hermans, J.; Vandenbroucke, J.P.; van de Laar, M.A.; Markusse, H.M.; Janssen, M.; van den Brink, H.R.; Dijkmans, B.A. Randomised placebo-controlled study of stopping second-line drugs in rheumatoid arthritis. Lancet 1996, 347, 347–352.
- Kremer, J.M.; Rynes, R.I.; Bartholomew, L.E. Severe flare of rheumatoid arthritis after discontinuation of long-term methotrexate therapy. Double-blind study. Am. J. Med. 1987, 82, 781–786.
- Gøtzsche, P.C.; Hansen, M.; Stoltenberg, M.; Svendsen, A.; Beier, J.; Faarvang, K.L.; Wangel, M.; Rydgren, L.; Halberg, P.; Juncker, P.; et al. Randomized, placebo controlled trial of withdrawal of slow-acting antirheumatic drugs and of observer bias in rheumatoid arthritis. Scand. J. Rheumatol. 1996, 25, 194–199.
- De Silva, M.; Hazleman, B.L. Long-term azathioprine in rheumatoid arthritis: A double-blind study. Ann. Rheum. Dis. 1981, 40, 560–563.
- Landewé, R.B.; Boers, M.; Verhoeven, A.C.; Westhovens, R.; van de Laar, M.A.; Markusse, H.M.; van Denderen, J.C.; Westedt, M.L.; Peeters, A.J.; Dijkmans, B.A.; et al. COBRA combination therapy in patients with early rheumatoid arthritis: Long-term structural benefits of a brief intervention. Arthritis Rheum. 2002, 46, 347–356.
- Mottonen, T.; Hannonen, P.; Leirisalo-Repo, M.; Nissila, M.; Kautiainen, H.; Korpela, M.; Laasonen, L.; Julkunen, H.; Luukkainen, R.; Vuori, K.; et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: A randomised trial. FIN-RACo trial group. Lancet 1999, 353, 1568–1573.
- Marchesoni, A.; Battafarano, N.; Arreghini, M.; Panni, B.; Gallazzi, M.; Tosi, S. Radiographic progression in early rheumatoid arthritis: A 12-month randomized controlled study comparing the combination of cyclosporin and methotrexate with methotrexate alone. Rheumatology 2003, 42, 1545–1549.
- Klarenbeek, N.B.; Koevoets, R.; van der Heijde, D.M.; Gerards, A.H.; Ten Wolde, S.; Kerstens, P.J.; Huizinga, T.W.; Dijkmans, B.A.; Allaart, C.F. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1815–1821.
- Van der Woude, D.; Young, A.; Jayakumar, K.; Mertens, B.J.; Toes, R.E.; van der Heijde, D.; Huizinga, T.W.; van der Helm-van Mil, A.H. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: Results from two large early arthritis cohorts. Arthritis Rheum. 2009, 60, 2262–2271.
- Klarenbeek, N.B.; van der Kooij, S.M.; Guler-Yuksel, M.; van Groenendael, J.H.; Han, K.H.; Kerstens, P.J.; Huizinga, T.W.; Dijkmans, B.A.; Allaart, C.F. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: Exploratory analyses from the BeSt study. Ann. Rheum. Dis. 2011, 70, 315–319.
- Baker, K.F.; Skelton, A.J.; Lendrem, D.W.; Scadeng, A.; Thompson, B.; Pratt, A.G.; Isaacs, J.D. Predicting drug-free remission in rheumatoid arthritis: A prospective interventional cohort study. J. Autoimmun. 2019, 105, 102298.
- Kuijper, T.M.; Luime, J.J.; de Jong, P.H.; Gerards, A.H.; van Zeben, D.; Tchetverikov, I.; de Sonnaville, P.B.; van Krugten, M.V.; Grillet, B.A.; Hazes, J.M.; et al. Tapering conventional synthetic DMARDs in patients with early arthritis in sustained remission: 2-year follow-up of the tREACH trial. Ann. Rheum. Dis. 2016, 75, 2119–2123.
- Van Mulligen, E.; Weel, A.E.A.M.; Kuijper, T.M.; Hazes, J.M.W.; van der Helm- van Mil, A.H.M.; de Jong, P.H.P. The impact of a disease flare during tapering of DMARDs on the lives of rheumatoid arthritis patients. Semin. Arthritis Rheum. 2020, 50, 423–431.
- Flurey, C.A.; Morris, M.; Richards, P.; Hughes, R.; Hewlett, S. It’s like a juggling act: Rheumatoid arthritis patient perspectives on daily life and flare while on current treatment regimes. Rheumatology 2014, 53, 696–703.
- Markusse, I.M.; Dirven, L.; Gerards, A.H.; van Groenendael, J.H.; Ronday, H.K.; Kerstens, P.J.; Lems, W.F.; Huizinga, T.W.; Allaart, C.F. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res. Ther. 2015, 17, 232.
- Van den Broek, M.; Huizinga, T.W.; Dijkmans, B.A.; Allaart, C.F. Drug-free remission: Is it already possible? Curr. Opin. Rheumatol. 2011, 23, 266–272.
- Yamaguchi, A.; Hirata, S.; Kubo, S.; Fukuyo, S.; Hanami, K.; Nakano, K.; Nakayamada, S.; Saito, K.; Tanaka, Y. 5-year remission rate after the discontinuation of adalimumab in patients with rheumatoid arthritis: Long-term follow-up results of the HONOR study. Mod. Rheumatol. 2020, 30, 799–806.
- Saleem, B.; Keen, H.; Goeb, V.; Parmar, R.; Nizam, S.; Hensor, E.M.; Churchman, S.M.; Quinn, M.; Wakefield, R.; Conaghan, P.G.; et al. Patients with RA in remission on TNF blockers: When and in whom can TNF blocker therapy be stopped? Ann. Rheum. Dis. 2010, 69, 1636–1642.
- Tanaka, Y.; Hirata, S.; Kubo, S.; Fukuyo, S.; Hanami, K.; Sawamukai, N.; Nakano, K.; Nakayamada, S.; Yamaoka, K.; Sawamura, F.; et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann. Rheum. Dis. 2015, 74, 389–395.
- Ghiti Moghadam, M.; Vonkeman, H.E.; Ten Klooster, P.M.; Tekstra, J.; van Schaardenburg, D.; Starmans-Kool, M.; Brouwer, E.; Bos, R.; Lems, W.F.; Colin, E.M.; et al. Stopping Tumor Necrosis Factor Inhibitor Treatment in Patients with Established Rheumatoid Arthritis in Remission or With Stable Low Disease Activity: A Pragmatic Multicenter, Open-Label Randomized Controlled Trial. Arthritis Rheumatol. 2016, 68, 1810–1817.
- Westhovens, R.; Robles, M.; Ximenes, A.C.; Wollenhaupt, J.; Durez, P.; Gomez-Reino, J.; Grassi, W.; Haraoui, B.; Shergy, W.; Park, S.H.; et al. Maintenance of remission following 2 years of standard treatment then dose reduction with abatacept in patients with early rheumatoid arthritis and poor prognosis. Ann. Rheum. Dis. 2015, 74, 564–568.
- Huizinga, T.W.; Conaghan, P.G.; Martin-Mola, E.; Schett, G.; Amital, H.; Xavier, R.M.; Troum, O.; Aassi, M.; Bernasconi, C.; Dougados, M. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann. Rheum. Dis. 2015, 74, 35–43.
- Van den Broek, M.; Klarenbeek, N.B.; Dirven, L.; van Schaardenburg, D.; Hulsmans, H.M.; Kerstens, P.J.; Huizinga, T.W.; Dijkmans, B.A.; Allaart, C.F. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: Subanalysis of the BeSt study. Ann. Rheum. Dis. 2011, 70, 1389–1394.
- Tanaka, Y.; Takeuchi, T.; Mimori, T.; Saito, K.; Nawata, M.; Kameda, H.; Nojima, T.; Miyasaka, N.; Koike, T. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann. Rheum. Dis. 2010, 69, 1286–1291.
- Haschka, J.; Englbrecht, M.; Hueber, A.J.; Manger, B.; Kleyer, A.; Reiser, M.; Finzel, S.; Tony, H.P.; Kleinert, S.; Feuchtenberger, M.; et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: Interim results from the prospective randomised controlled RETRO study. Ann. Rheum. Dis. 2016, 75, 45–51.
- Kavanaugh, A.; Smolen, J.S. The when and how of biologic agent withdrawal in rheumatoid arthritis: Learning from large randomised controlled trials. Clin. Exp. Rheumatol. 2013, 31, S19–S21.
- Kavanaugh, A.; Lee, S.J.; Curtis, J.R.; Greenberg, J.D.; Kremer, J.M.; Soto, L.; Etzel, C.J.; Cox, V.; Yoshida, K.; Reed, G.W.; et al. Discontinuation of tumour necrosis factor inhibitors in patients with rheumatoid arthritis in low-disease activity: Persistent benefits. Data from the Corrona registry. Ann. Rheum. Dis. 2015, 74, 1150–1155.
- Boers, M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1771–1774.
- Aletaha, D.; Funovits, J.; Keystone, E.C.; Smolen, J.S. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum. 2007, 56, 3226–3235.
- Smolen, J.S.; Emery, P.; Fleischmann, R.; van Vollenhoven, R.F.; Pavelka, K.; Durez, P.; Guérette, B.; Kupper, H.; Redden, L.; Arora, V.; et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: The randomised controlled OPTIMA trial. Lancet 2014, 383, 321–332.
- Emery, P.; Burmester, G.R.; Bykerk, V.P.; Combe, B.G.; Furst, D.E.; Barré, E.; Karyekar, C.S.; Wong, D.A.; Huizinga, T.W. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: Results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann. Rheum. Dis. 2015, 74, 19–26.
- Saleem, B.; Brown, A.K.; Quinn, M.; Karim, Z.; Hensor, E.M.; Conaghan, P.; Peterfy, C.; Wakefield, R.J.; Emery, P. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann. Rheum. Dis. 2012, 71, 1316–1321.
- Scire, C.A.; Montecucco, C.; Codullo, V.; Epis, O.; Todoerti, M.; Caporali, R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: Power Doppler signal predicts short-term relapse. Rheumatology 2009, 48, 1092–1097.
- Naredo, E.; Valor, L.; De la Torre, I.; Montoro, M.; Bello, N.; Martinez-Barrio, J.; Martinez-Estupinan, L.; Nieto, J.C.; Ovalles-Bonilla, J.G.; Hernandez-Florez, D.; et al. Predictive value of Doppler ultrasound-detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid arthritis. Rheumatology 2015, 54, 1408–1414.
- Iwamoto, T.; Ikeda, K.; Hosokawa, J.; Yamagata, M.; Tanaka, S.; Norimoto, A.; Sanayama, Y.; Nakagomi, D.; Takahashi, K.; Hirose, K.; et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: High predictive values of total gray-scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res. 2014, 66, 1576–1581.
- Alivernini, S.; Peluso, G.; Fedele, A.L.; Tolusso, B.; Gremese, E.; Ferraccioli, G. Tapering and discontinuation of TNF-α blockers without disease relapse using ultrasonography as a tool to identify patients with rheumatoid arthritis in clinical and histological remission. Arthritis Res. Ther. 2016, 18, 39.
- McQueen, F.M.; Benton, N.; Perry, D.; Crabbe, J.; Robinson, E.; Yeoman, S.; McLean, L.; Stewart, N. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1814–1827.
- Benton, N.; Stewart, N.; Crabbe, J.; Robinson, E.; Yeoman, S.; McQueen, F.M. MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann. Rheum. Dis. 2004, 63, 555–561.
- Foltz, V.; Gandjbakhch, F.; Etchepare, F.; Rosenberg, C.; Tanguy, M.L.; Rozenberg, S.; Bourgeois, P.; Fautrel, B. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012, 64, 67–76.
- Detert, J.; Bastian, H.; Listing, J.; Weiß, A.; Wassenberg, S.; Liebhaber, A.; Rockwitz, K.; Alten, R.; Krüger, K.; Rau, R.; et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann. Rheum. Dis. 2013, 72, 844–850.
- Lamers-Karnebeek, F.B.; Luime, J.J.; Ten Cate, D.F.; Teerenstra, S.; Swen, N.; Gerards, A.H.; Hendrikx, J.; van Rooyen, E.M.; Voorneman, R.; Haagsma, C.; et al. Limited value for ultrasonography in predicting flare in rheumatoid arthritis patients with low disease activity stopping TNF inhibitors. Rheumatology 2017, 56, 1560–1565.
- Fautrel, B.; Pham, T.; Alfaiate, T.; Gandjbakhch, F.; Foltz, V.; Morel, J.; Dernis, E.; Gaudin, P.; Brocq, O.; Solau-Gervais, E.; et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: Results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study). Ann. Rheum. Dis. 2016, 75, 59–67.
- Nishimoto, N.; Amano, K.; Hirabayashi, Y.; Horiuchi, T.; Ishii, T.; Iwahashi, M.; Iwamoto, M.; Kohsaka, H.; Kondo, M.; Matsubara, T.; et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod. Rheumatol. 2014, 24, 17–25.
- Tanaka, Y.; Hirata, S. Intensive intervention can lead to a treatment holiday from biological DMARDs in patients with rheumatoid arthritis. Drugs 2014, 74, 2129–2139.
- Cope, A.P. T cells in rheumatoid arthritis. Arthritis Res. Ther. 2008, 10 (Suppl. 1), S1.
- Ponchel, F.; Morgan, A.W.; Bingham, S.J.; Quinn, M.; Buch, M.; Verburg, R.J.; Henwood, J.; Douglas, S.H.; Masurel, A.; Conaghan, P.; et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 2002, 100, 4550–4556.
- Rech, J.; Hueber, A.J.; Finzel, S.; Englbrecht, M.; Haschka, J.; Manger, B.; Kleyer, A.; Reiser, M.; Cobra, J.F.; Figueiredo, C.; et al. Prediction of disease relapses by multibiomarker disease activity and autoantibody status in patients with rheumatoid arthritis on tapering DMARD treatment. Ann. Rheum. Dis. 2016, 75, 1637–1644.
- Van der Helm-van Mil, A.H.; Knevel, R.; Cavet, G.; Huizinga, T.W.; Haney, D.J. An evaluation of molecular and clinical remission in rheumatoid arthritis by assessing radiographic progression. Rheumatology 2013, 52, 839–846.
- Hambardzumyan, K.; Bolce, R.; Saevarsdottir, S.; Cruickshank, S.E.; Sasso, E.H.; Chernoff, D.; Forslind, K.; Petersson, I.F.; Geborek, P.; van Vollenhoven, R.F. Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: Results from the SWEFOT trial. Ann. Rheum. Dis. 2015, 74, 1102–1109.
- Li, W.; Sasso, E.H.; Emerling, D.; Cavet, G.; Ford, K. Impact of a multi-biomarker disease activity test on rheumatoid arthritis treatment decisions and therapy use. Curr. Med. Res. Opin. 2013, 29, 85–92.
- Curtis, J.R.; van der Helm-van Mil, A.H.; Knevel, R.; Huizinga, T.W.; Haney, D.J.; Shen, Y.; Ramanujan, S.; Cavet, G.; Centola, M.; Hesterberg, L.K.; et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res. 2012, 64, 1794–1803.
- Gul, H.L.; Eugenio, G.; Rabin, T.; Burska, A.; Parmar, R.; Wu, J.; Ponchel, F.; Emery, P. Defining remission in rheumatoid arthritis: Does it matter to the patient? A comparison of multi-dimensional remission criteria and patient reported outcomes. Rheumatology 2020, 59, 613–621.
- Gul, H.; Ponchel, F.; Emery, P. OP0182 IN RA PATIENTS IN REMISSION, WHICH BIOMARKERS PREDICT SUCCESSFUL TAPERING OF CSDMARDS? Ann. Rheum. Dis. 2021, 80, 110.




