
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoshifumi Saisho | -- | 1773 | 2022-06-15 07:37:11 |
Video Upload Options
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of oral hypoglycemic agents which increase urinary glucose excretion by suppressing glucose reabsorption at the proximal tubule in the kidney. SGLT2 inhibitors lower glycated hemoglobin (HbA1c) by 0.6–0.8% (6–8 mmol/mol) without increasing the risk of hypoglycemia and induce weight loss and improve various metabolic parameters including blood pressure, lipid profile and hyperuricemia.
1. Introduction
|
Generic Name |
Dosage |
SGLT1/2 Selectivity |
Half-Life (t1/2) |
Indication |
|---|---|---|---|---|
|
Ipragliflozin |
50–100 mg once daily |
254:1 |
15 h |
Type 1 and type 2 diabetes |
|
Dapagliflozin |
5–10 mg once daily |
1242:1 |
8–12 h |
Type 1 and type 2 diabetes |
|
Canagliflozin |
100 mg once daily |
155:1 |
12 h |
Type 2 diabetes |
|
Empagliflozin |
10–25 mg once daily |
2680:1 |
14–18 h |
Type 2 diabetes |
|
Luseogliflozin |
2.5–5.0 mg once daily |
1770:1 |
9 h |
Type 2 diabetes |
|
Tofogliflozin |
20 mg once daily |
2912:1 |
5 h |
Type 2 diabetes |
2. Mechanisms of Action of SGLT2 Inhibitors
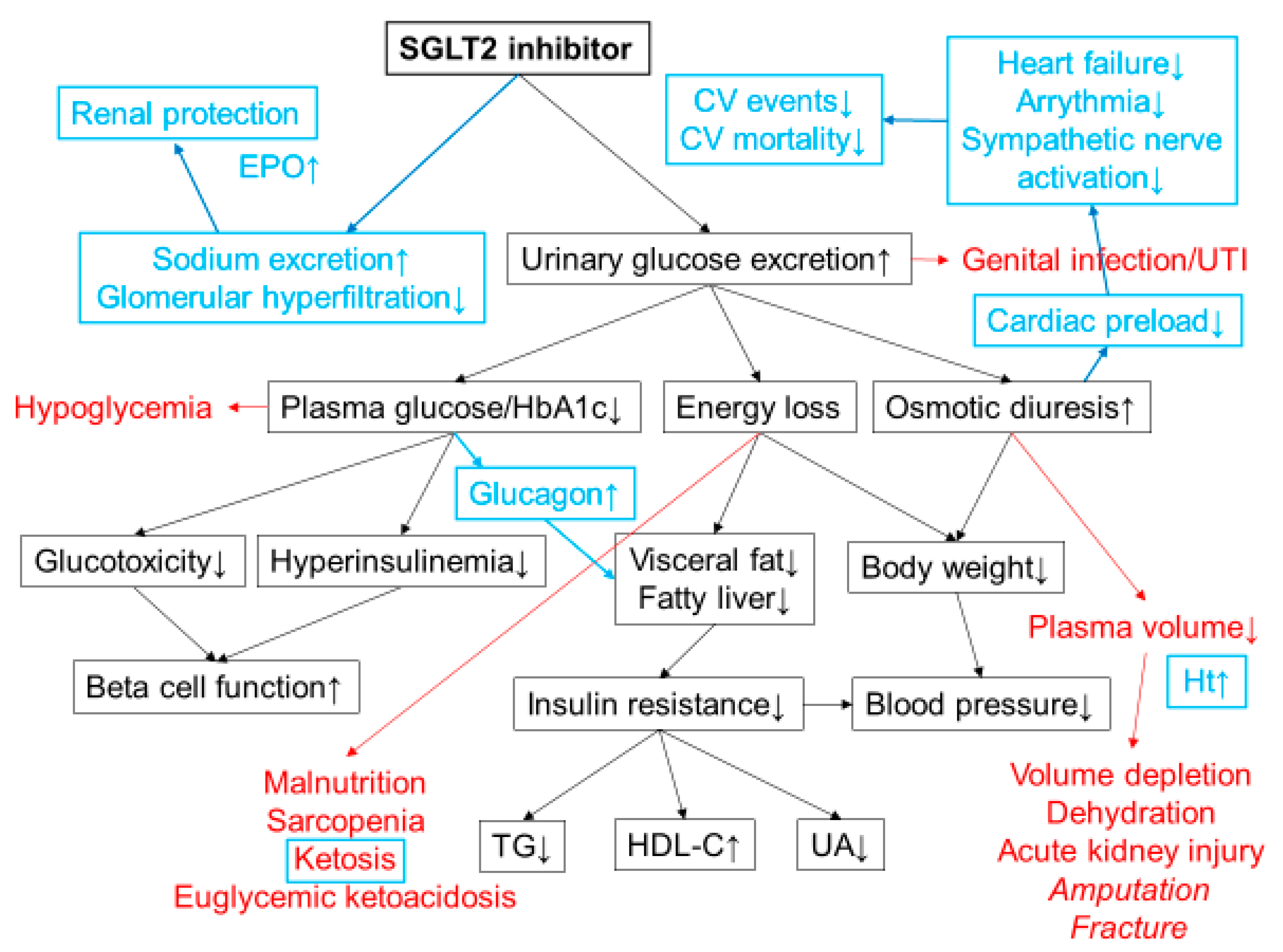
3. Clinical Efficacy of SGLT2 Inhibitors
3.1. Glucose-Lowering Effect
3.2. Body Weight, Blood Pressure, and Other Metablic Parameters
3.3. Hypoglycemia
3.4. Beta Cell Function
4. Adverse Effects
5. Positioning of SGLT2 Inhibitors in Treatment of T2DM
References
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097.
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why sglt2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328.
- Poulsen, S.B.; Fenton, R.A.; Rieg, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469.
- DeFronzo, R.A.; Hompesch, M.; Kasichayanula, S.; Liu, X.; Hong, Y.; Pfister, M.; Morrow, L.A.; Leslie, B.R.; Boulton, D.W.; Ching, A.; et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013, 36, 3169–3176.
- Norton, L.; Shannon, C.E.; Fourcaudot, M.; Hu, C.; Wang, N.; Ren, W.; Song, J.; Abdul-Ghani, M.; DeFronzo, R.A.; Ren, J.; et al. Sodium-glucose co-transporter (sglt) and glucose transporter (glut) expression in the kidney of type 2 diabetic subjects. Diabetes Obes. Metab. 2017, 19, 1322–1326.
- Nagahisa, T.; Saisho, Y. Cardiorenal protection: Potential of sglt2 inhibitors and glp-1 receptor agonists in the treatment of type 2 diabetes. Diabetes Ther. 2019, 10, 1733–1752.
- Polidori, D.; Sha, S.; Mudaliar, S.; Ciaraldi, T.P.; Ghosh, A.; Vaccaro, N.; Farrell, K.; Rothenberg, P.; Henry, R.R. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care 2013, 36, 2154–2161.
- Takebayashi, K.; Hara, K.; Terasawa, T.; Naruse, R.; Suetsugu, M.; Tsuchiya, T.; Inukai, T. Effect of canagliflozin on circulating active glp-1 levels in patients with type 2 diabetes: A randomized trial. Endocr. J. 2017, 64, 923–931.
- Neuen, B.L.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019, 7, 845–854.
- Jardine, M.J.; Zhou, Z.; Mahaffey, K.W.; Oshima, M.; Agarwal, R.; Bakris, G.; Bajaj, H.S.; Bull, S.; Cannon, C.P.; Charytan, D.M.; et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: A secondary analysis of the credence randomized trial. J. Am. Soc. Nephrol. 2020, 31, 1128–1139.
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetologia 2020, 63, 221–228.
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274.
- Nishimura, R.; Osonoi, T.; Kanada, S.; Jinnouchi, H.; Sugio, K.; Omiya, H.; Ubukata, M.; Sakai, S.; Samukawa, Y. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes. Metab. 2015, 17, 800–804.
- Henry, R.R.; Strange, P.; Zhou, R.; Pettus, J.; Shi, L.; Zhuplatov, S.B.; Mansfield, T.; Klein, D.; Katz, A. Effects of dapagliflozin on 24-hour glycemic control in patients with type 2 diabetes: A randomized controlled trial. Diabetes Technol. Ther. 2018, 20, 715–724.
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: Comparison with sitagliptin. Cardiovasc. Diabetol. 2017, 16, 8.
- McGill, J.B.; Subramanian, S. Safety of sodium-glucose co-transporter 2 inhibitors. Am. J. Cardiol. 2019, 124, S45–S52.
- Danne, T.; Garg, S.; Peters, A.L.; Buse, J.B.; Mathieu, C.; Pettus, J.H.; Alexander, C.M.; Battelino, T.; Ampudia-Blasco, F.J.; Bode, B.W.; et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium–glucose cotransporter (sglt) inhibitors. Diabetes Care 2019, 42, 1147–1154.
- Takahara, M.; Shiraiwa, T.; Matsuoka, T.A.; Katakami, N.; Shimomura, I. Ameliorated pancreatic beta cell dysfunction in type 2 diabetic patients treated with a sodium-glucose cotransporter 2 inhibitor ipragliflozin. Endocr. J. 2015, 62, 77–86.
- Polidori, D.; Mari, A.; Ferrannini, E. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia 2014, 57, 891–901.
- Saisho, Y. Changing the concept of type 2 diabetes: Beta cell workload hypothesis revisited. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 121–127.
- Saisho, Y. Beta cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 109–124.
- Saisho, Y. How can we develop more effective strategies for type 2 diabetes mellitus prevention? A paradigm shift from a glucose-centric to a beta cell-centric concept of diabetes. EMJ Diabet 2018, 6, 46–52.
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508.
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014, 124, 509–514.
- Sheu, W.H.H.; Chan, S.P.; Matawaran, B.J.; Deerochanawong, C.; Mithal, A.; Chan, J.; Suastika, K.; Khoo, C.M.; Nguyen, H.M.; Linong, J.; et al. Use of sglt-2 inhibitors in patients with type 2 diabetes mellitus and abdominal obesity: An asian perspective and expert recommendations. Diabetes Metab. J. 2020, 44, 11–32.
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thevenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the glucose transporter sglt2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015, 21, 512–517.
- Saponaro, C.; Mühlemann, M.; Acosta-Montalvo, A.; Piron, A.; Gmyr, V.; Delalleau, N.; Moerman, E.; Thévenet, J.; Pasquetti, G.; Coddeville, A.; et al. Interindividual heterogeneity of sglt2 expression and function in human pancreatic islets. Diabetes 2020, 69, 902–914.
- Suga, T.; Kikuchi, O.; Kobayashi, M.; Matsui, S.; Yokota-Hashimoto, H.; Wada, E.; Kohno, D.; Sasaki, T.; Takeuchi, K.; Kakizaki, S.; et al. Sglt1 in pancreatic alpha cells regulates glucagon secretion in mice, possibly explaining the distinct effects of sglt2 inhibitors on plasma glucagon levels. Mol. Metab. 2019, 19, 1–12.
- Kuhre, R.E.; Ghiasi, S.M.; Adriaenssens, A.E.; Wewer Albrechtsen, N.J.; Andersen, D.B.; Aivazidis, A.; Chen, L.; Mandrup-Poulsen, T.; Orskov, C.; Gribble, F.M.; et al. No direct effect of sglt2 activity on glucagon secretion. Diabetologia 2019, 62, 1011–1023.
- Bersoff-Matcha, S.J.; Chamberlain, C.; Cao, C.; Kortepeter, C.; Chong, W.H. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: A review of spontaneous postmarketing cases. Ann. Intern. Med. 2019, 170, 764–769.
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154.
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlavek, J.; Bohm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020.
- Lam, C.S.P.; Chandramouli, C.; Ahooja, V.; Verma, S. Sglt-2 inhibitors in heart failure: Current management, unmet needs, and therapeutic prospects. J. Am. Heart Assoc. 2019, 8, e013389.
- Dekkers, C.C.J.; Gansevoort, R.T. Sodium-glucose cotransporter 2 inhibitors: Extending the indication to non-diabetic kidney disease? Nephrol. Dial. Transplant. 2020, 35, i33–i42.
- Taylor, S.I.; Blau, J.E.; Rother, K.I.; Beitelshees, A.L. Sglt2 inhibitors as adjunctive therapy for type 1 diabetes: Balancing benefits and risks. Lancet Diabetes Endocrinol. 2019, 7, 949–958.
- Sims, H.; Smith, K.H.; Bramlage, P.; Minguet, J. Sotagliflozin: A dual sodium-glucose co-transporter-1 and -2 inhibitor for the management of type 1 and type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1037–1048.
- Cefalo, C.M.A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the first dual sglt inhibitor: Current outlook and perspectives. Cardiovasc. Diabetol. 2019, 18, 20.




