Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoshimi Seida | -- | 4046 | 2022-06-15 03:03:21 | | | |
| 2 | Catherine Yang | Meta information modification | 4046 | 2022-06-15 03:32:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Seida, Y.; Tokuyama, H. Hydrogel Adsorbents. Encyclopedia. Available online: https://encyclopedia.pub/entry/24038 (accessed on 05 March 2026).
Seida Y, Tokuyama H. Hydrogel Adsorbents. Encyclopedia. Available at: https://encyclopedia.pub/entry/24038. Accessed March 05, 2026.
Seida, Yoshimi, Hideaki Tokuyama. "Hydrogel Adsorbents" Encyclopedia, https://encyclopedia.pub/entry/24038 (accessed March 05, 2026).
Seida, Y., & Tokuyama, H. (2022, June 15). Hydrogel Adsorbents. In Encyclopedia. https://encyclopedia.pub/entry/24038
Seida, Yoshimi and Hideaki Tokuyama. "Hydrogel Adsorbents." Encyclopedia. Web. 15 June, 2022.
Copy Citation
The performance of hydrogel adsorbents depends on the constituents of the gels and the functions produced by the polymer networks of the gels. Research on hydrogels utilizing the characteristic functions of polymer networks has increased over the last decade. The functions of polymer networks are key to the development of advanced adsorbents for the removal of various pollutants.
polymer hydrogel
adsorbent
adsorption
1. Basic Requirements for Adsorbents
The design and use of adsorbents in the adsorption separation process are essential for adsorption separation technologies. Adsorbents that meet requirements like material cost, minimal environmental impact, stability, uptake rate, reusability, and adsorption capacity for target adsorbates will be selected in practice. In the modern design of adsorbents, the physicochemical structure of the adsorbent can be tailored to target pollutants. In the design of the adsorption separation process, the parameters of both the adsorption equilibrium and the mass transfer in adsorbents are crucial [1][2]. The adsorption isotherms, the curves of semi-empirical adsorption equilibrium showing the adsorption capacity as a function of adsorbate concentration, represent the performance of the adsorbent. Adsorbent users can estimate the performance of adsorbents under the condition of use based on the adsorption isotherms and relevant conditions. Therefore, adsorption isotherms are indispensable for the practical use of adsorbents, although data acquisition of adsorption isotherms is time-consuming. The adsorption isotherm or adsorption capacity of adsorbents depends on a range of factors such as temperature, ionic strength, solvent composition, particle size, and adsorber operation [1][2]. In the design of adsorption processes and evaluation of adsorbents using literature data of adsorption isotherms or capacities, it is necessary to confirm the conditions under which the adsorption data in the literature were collected, to evaluate the adsorption process properly. It is also essential to consider desorption and regeneration methods involving simple operations and minimal waste. Because extra solvent wastes are produced in the recovery and regeneration processes, the desorption process should also be optimized.
2. Gel Adsorbents
Some adsorbents include a local gel phase. In most cases, only an adsorbent in which the entire adsorbent consists of a gel phase is called a gel adsorbent. Polymer hydrogels have several advantages in their application as adsorbents. The polymer networks create percolation pores with the desired solvent content and pore size. The specific surface area of polymer hydrogels is not typically discussed because of their network structure with a large amount of solvent. Open pores created by the polymer networks in the gels enable adsorbates to percolate deep inside the gels quickly, thus facilitating the adsorption capacity of the gels. Gels with high solvent content exhibit excellent diffusional permeability toward a range of substances. The diffusivity of solutes in gels has been extensively studied, both experimentally and theoretically. Various mathematical models used to determine the diffusion coefficient in gels have been developed and summarized in studies published in the 1990s [3][4]. Nevertheless, the study of diffusivity remains an interesting scientific topic of discussion [5][6][7][8][9][10][11]. The development of porous structures in polymer gels can potentially increase the number of effective adsorption sites and reduce mass transfer resistance. Many methods for synthesizing porous hydrogels, such as phase separation [12][13][14], emulsion [15][16][17][18], ice crystal [19], and foam [20][21], have been reported. A solution environment that is different from the outer solution can be created inside the gels depending on the nature of the polymers, such as the hydrophilic–hydrophobic balance of polymers and the existence of fixed charges in the polymers. A gel liquid-phase system with an arbitrary size, interface, and less solvent disturbance is unique and valuable. One of the significant advantages of particulate gels is their ease of liquid separation for solvent extraction. The solvent extraction phase can be immobilized on the gels; rendering the handling of solvent easy. When stimuli-responsive polymers are used, the structure and physical properties of the gel can be reversibly controlled by the stimuli to which the polymers respond. The stimuli-responsive property of the gel produces a dynamic shift in the adsorption isotherm [22], which is effective in adsorptive separation systems. The network structure of gels can create integrated reaction fields, characteristic of polymer hydrogels formed by a three-dimensional polymer network and immobilized solvent, as mentioned above. The characteristics and functions of the polymer network include open pores with a large pore volume, a scaffold for functional group immobilization, compatibility with solvents, stimuli-responsiveness, strength imparting, and a specific environment different from the outer solution in terms of pH, ionic strength, and redox potential.
3. Classification of Hydrogels Based on the Roles or Functions of the Polymer Networks
Polymer gels as adsorbents were classified based on the roles and functions of the polymer networks in their applications as follows.
3.1. Matrix for Immobilization
The simple use of hydrogels is for the immobilization of supports and carriers. Even in this simple application of hydrogels, they have various advantages. The incorporation of diverse materials, simple solid–liquid separation, stabilization of the immobilized substances maintaining high dispersion, accumulation and enrichment of target substances inside the gels, and the size control of stable solvent droplets with the size of gels are distinct advantages of the gels. The difference between hydrogels and emulsions or liquid immobilizing capsules is that water is immobilized in the polymer network with percolating open pores, and mass transfer across the surface of the gels is not restricted. The size of gels and pores are controllable, and mass transfer in the gels is not reduced, owing to the large amount of water and the percolating open pores in the hydrogels, which are also effective for the immobilized microorganisms and macromolecules, such as antibodies and enzymes, to maintain their activity over a long time. Furthermore, gels can produce a hierarchical reaction field because zoning of embedded substances can be achieved owing to the immobilization property of the polymer network.
The immobilization of fine particles in gels enables easy handling of fine particles in the liquid-phase adsorption process. This advantage of gels is significant in adsorption separation operations in batch processes, especially in the use of nano- and microparticles as adsorbents. The polymer network of the gel enabled the highly dispersed immobilization of nanoparticles (Figure 1), which contributed to the increase in the effective surface area of the nanoparticles. Nanoparticles can be embedded by the polymerization of gel monomers in the suspension of nanoparticles or by their synthesis inside the gel matrix. Various composite gels immobilizing nanoparticles have been reported in the last decade, including amidoximated poly(methacrylic-co-acrylonitrile) composite gel immobilizing magnetic CoFe nanoparticles for the removal of Cr3+, Cd2+, and dyes [23], Fe2O3 nanoparticles immobilizing polyacrylamide/chitosan composite hydrogel for the removal of methylene blue [24], hydrous ferric oxide immobilizing poly(trans-Aconitic acid/2-hydroxyethyl acrylate) hydrogel for the removal of Pb2+, Cu2+, Cd2+, and Ni2+ [25], zirconia nanoparticles immobilizing poly(N, N-dimethyl acrylamide) hydrogel for the removal of As2O3 and AsO43− [26], and iron hydroxide (lepidocrocite) immobilizing poly(N, N′-dimethylaminopropyl acrylamide, methyl chloride quaternary) cationic hydrogel for the removal of AsO43− from groundwater [27]. These gels increased the adsorption capacity by combining them with nanoparticles. Some examples of the adsorption mechanisms of nanoparticles are illustrated in Figure 2 [27][28][29]. The adsorption mechanism is classified into inner-sphere surface coordination complexes (strong bonding), outer-sphere ion-pair complexes, and electrostatic interactions. As2O3 and AsO43− were adsorbed on the zirconia and iron hydroxide nanoparticles via the inner-sphere complex. The large surface area and surface charge of the nanoparticles would also contribute to the adsorption capacity. From this perspective, composites with metal nanoparticles produced positive adsorption results. Bimetallic nanoparticles, such as CoFe, NiFe, and CuNi, have been studied in water treatment for the removal of heavy metals and decomposition of dyes from aqueous solutions owing to their specific adsorption behaviors for pollutants, although the mechanism of adsorption on the nanoparticles has not been extensively studied [30][31].
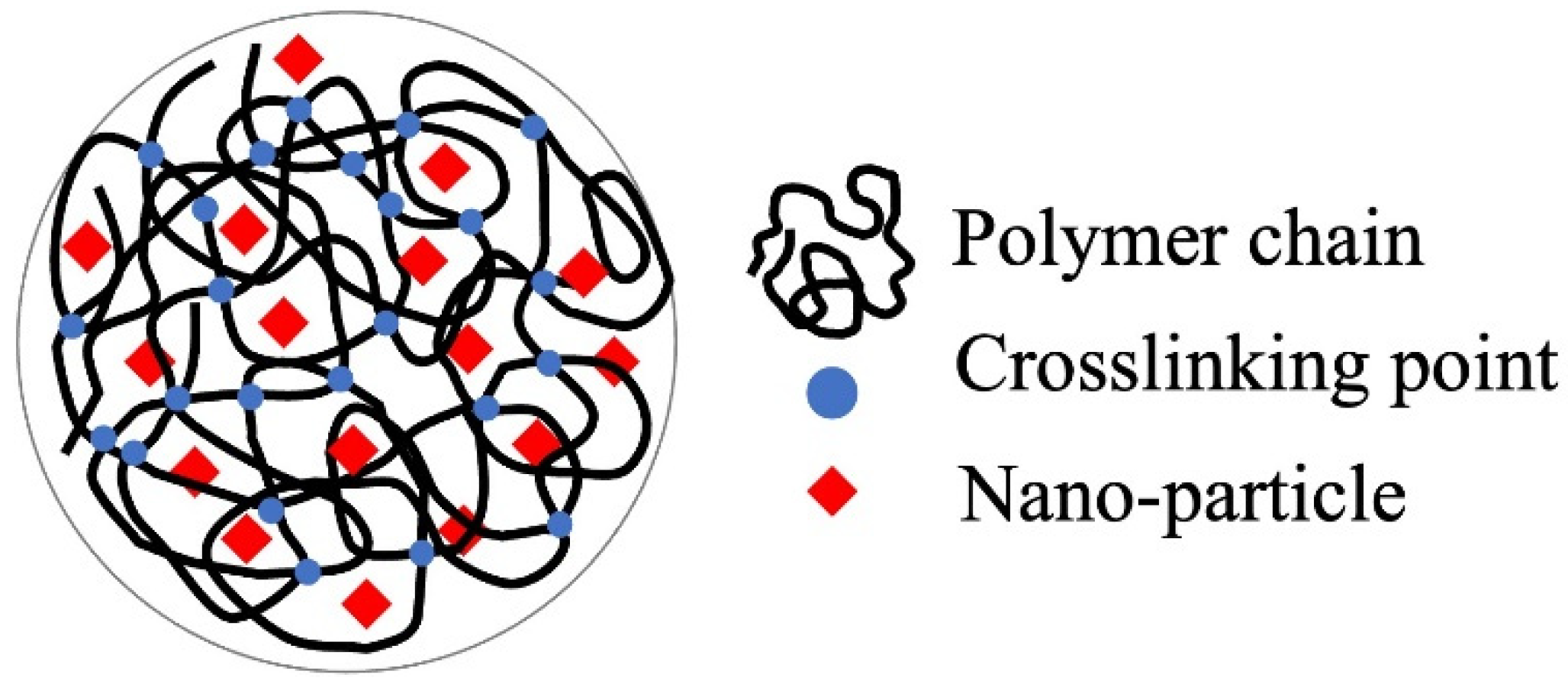
Figure 1. Hydrogel for the immobilization support and carrier.
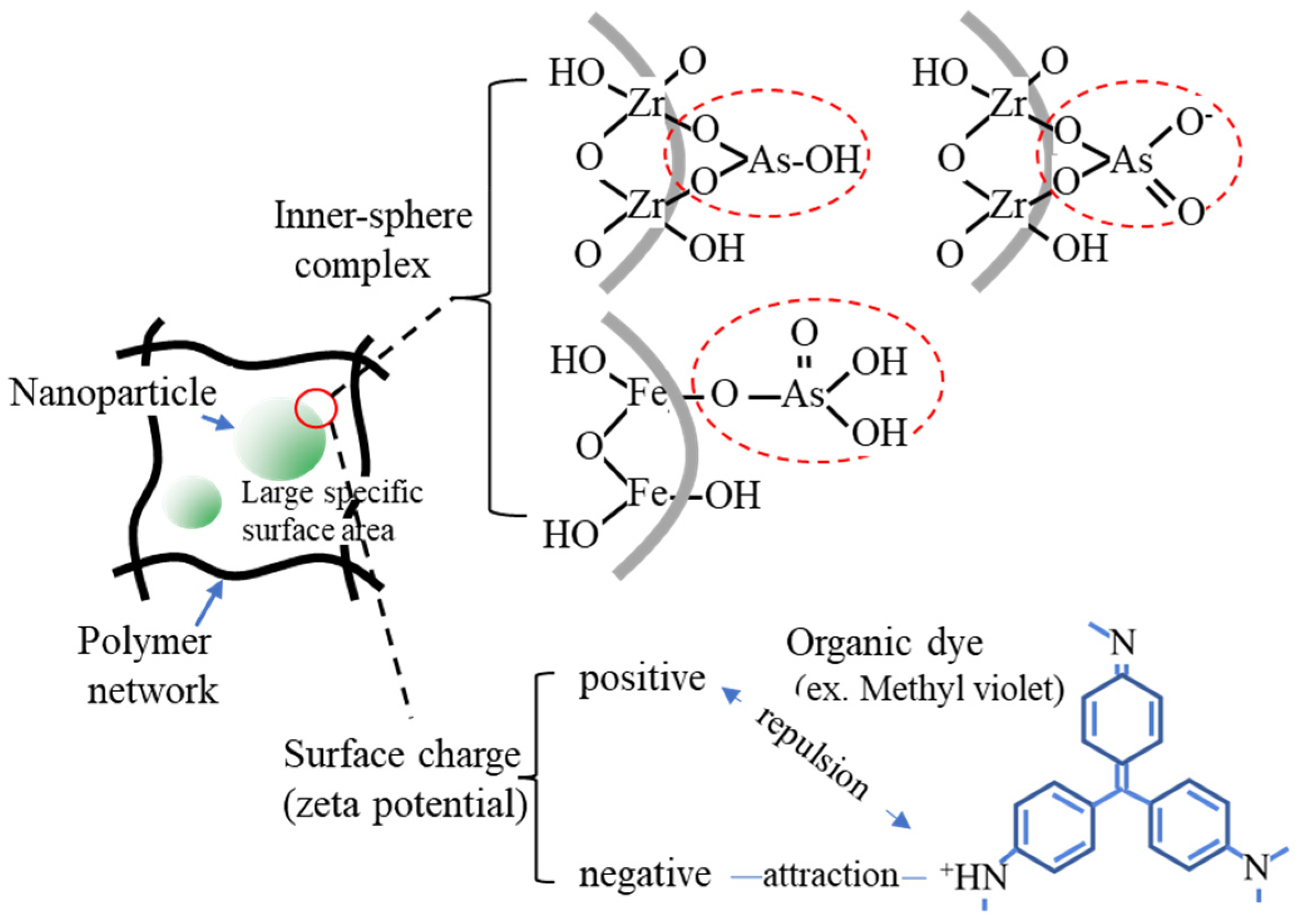
Figure 2. Examples of the adsorption mechanism on the nanoparticles.
3.2. Scaffold Fixing Functional Groups
A polymer network is the backbone of hydrogel adsorbents. Functional groups that function as active sites for the adsorption of pollutants are immobilized on the polymer network. The functional groups can be freely introduced into the polymer network, and the solvent content can be adjusted intentionally in hydrogel adsorbents. Thus, the basis of adsorbent design is to use the functional groups that interact with the target adsorbates in preference. Polymeric gel adsorbents can be prepared by polymerizing monomers with desired functional groups or by post-chemical modification of the polymer network to obtain the desired functional groups. Increasing the adsorbent’s surface area and grafting the branched polymer onto the backbone polymer chains are generally used to increase the number of effective adsorption sites in the adsorbents, as mentioned above. Most studies focus on the adsorption performance, but not the adsorption mechanism, despite the importance of the adsorption mechanism in practical process control. The interactions facilitating adsorption are determined by the type of adsorbate, functional groups involved in the adsorption with their spatial layout, and solution conditions such as pH and salinity. In the case of rigid polymers such as chitosan, for instance, the conformation of the chitosan polymer and pH-dependent charge of the amine groups in chitosan affect the coordination bonding between heavy-metal ions and the functional groups of the chitosan (amine groups and hydroxyl groups) [32].
Many hydrogels, including poly(acrylic acid) [33], poly(N-isopropyl acrylamide) [34][35][36][37][38][39], poly(N,N-dimethylacetamide-co-2-hydroxyethyl methacrylate) [40], amidoximated poly(methacrylic-co-acrylonitrile) [23], poly(2-hydroxyethyl methacrylate)-grafted copolymer [41], poly(glycidyl methacrylate-glycine) [42], poly(1-vinyl-2-pyrrolidone-co-sodium acrylate) [43], and poly(maleic acid) with cyclic groups of poly (1, 4-dioxa-7, 12-diazacyclotetradecane-8, 11-dione) and poly (1,4-diazocane-5,8-dione) [44][45] have been studied in the last decade to investigate their adsorption properties over various targets. As typical adsorption characteristics of hydrogels, Sammaddar et al. (2019) [46] and Qi et al. (2021) [47] reviewed the adsorption capacity of the hydrogel adsorbents such as acrylic acid, amine, alginic acid, cellulose and its derivatives, pectin, and chitosan gels for the removal of pollutants from the aqueous system. In addition to these hydrogel adsorbents, hybrid and composite hydrogels involving natural polymer blend composites and clay/natural polymer composites have been synthesized, and their adsorption properties for hazardous pollutants have been reviewed [48][49][50]. Shalla et al. (2018) reviewed hydrogel composites for the removal of recalcitrant organic dyes, including synthetic polymer-natural polymer, clay polymer, graphene polymer, and metal oxide–polymer composites [51]. As ionic dyes adsorb via electrostatic interactions, it is essential to increase both the number of adsorption sites and their dispersibility using materials with a high affinity for dyes in the composites.
3.3. Stimuli-Responsive Network
Thermoresponsive hydrogels are representative adsorbents of this type. These gels reveal a change in the hydrophilic–hydrophobic balance of the polymer network depending on the temperature. This property is often observed in amphiphilic polymers. Representative amphiphilic polymers include poly(N-isopropyl acrylamide), poly(N-substituted acrylamide), and their copolymers. Polymers with a lower critical solution temperature (LCST) in an aqueous system exhibit hydrophilicity with hydration below the LCST. At temperatures above the LCST, the polymers exhibit hydrophobicity with dehydration. This property enables gels to adsorb and desorb organic molecules with hydrophobic moieties through temperature control across the LCST [22].
Figure 3a shows the basic concept of temperature-controlled adsorption and the separation of organic contaminants in water using thermoresponsive hydrogels. The critical temperature of the adsorption–desorption control corresponded to the LCST of the polymers used in the gels. LCST can be controlled by the copolymerization of a small number of co-monomers that differ from PNIPA in terms of hydrophobicity. Various thermoresponsive copolymer hydrogels, such as N-isopropyl acrylamide (NIPA)-co-acrylamide, NIPA-co-polyacrylic acid (PAA), NIPA-co-dimethyl acrylamide (DMA), and NIPA-co-acryloyl piperidine, have been synthesized, and their thermoresponsive adsorption properties for organic molecules have been reported in the 1990s. The adsorption amount of adsorbates in this system depends on the difference in the hydrophobicity of the adsorbates. This principle of adsorption control was applied to the chromatographic separation of steroids in 1996 by Kanazawa et al. (1996) [52]. This chromatographic separation process in the aqueous phase demonstrated organic-eluent-free separation. Changes in the volume of the gel occur along with the control of the hydrophilic–hydrophobic balance in this system. Therefore, when applying the gel to column separation, it is necessary to devise ways to use it, such as immobilizing the gel in a porous support. The stimuli to which the temperature-responsive gels respond can be diversified by copolymerizing a small amount of a second stimulus-responsive monomer into the gel [53].
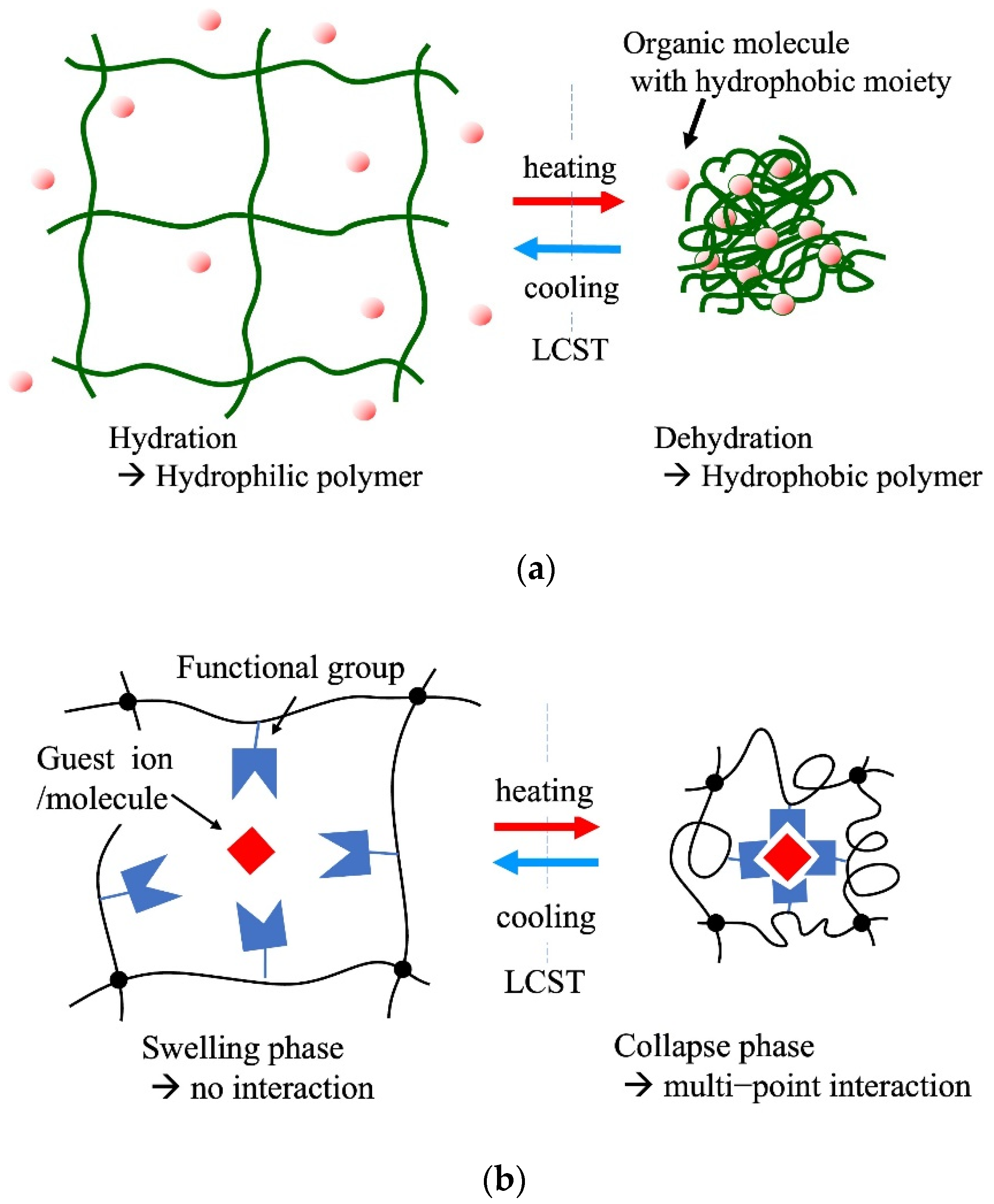
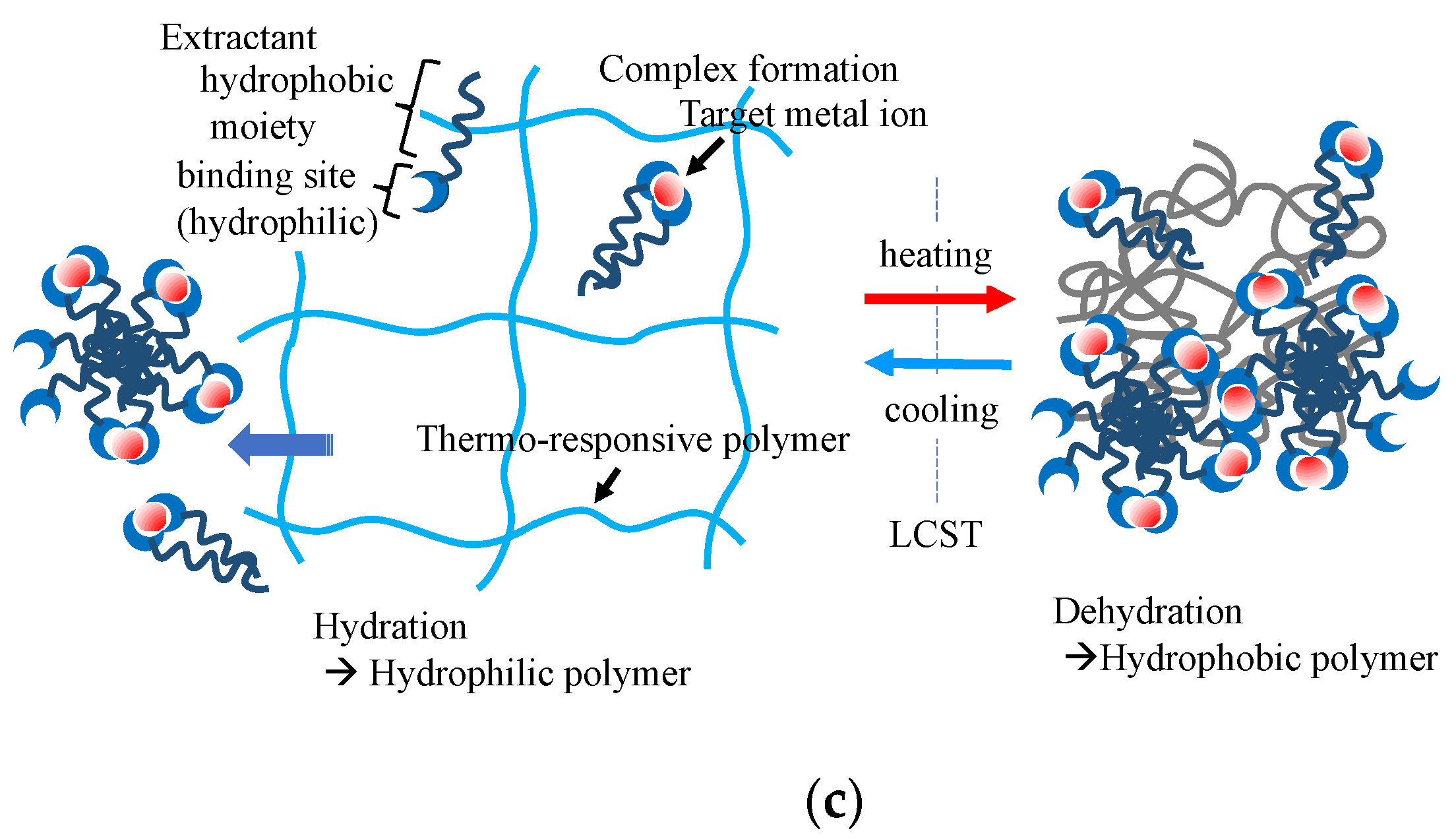 Figure 3. Thermoresponsive adsorption control using amphiphilic polymer hydrogel in aqueous systems, (a) adsorption through hydrophobic interaction between the thermoresponsive hydrogel and the molecules with hydrophobic moieties, (b) selective multipoint interaction, (c) solid-phase extraction of metal ions.
Figure 3. Thermoresponsive adsorption control using amphiphilic polymer hydrogel in aqueous systems, (a) adsorption through hydrophobic interaction between the thermoresponsive hydrogel and the molecules with hydrophobic moieties, (b) selective multipoint interaction, (c) solid-phase extraction of metal ions.3.4. Contaminant Uptake with Self-Assembling and Gelation
Pollutants in an aqueous system can be removed by self-assembly of gelator molecules and gelation triggered by target substances using modern gelator molecules. This is similar to the adsorption and concentration of contaminants in the coagulation process using coagulants. In the case of Al-based coagulants, as many of the organic pollutants dissolved in water are negatively charged, the coagulants electrostatically couple with the pollutants, followed by coagulation to form gel-like flocks through hydrogen bonding among the aluminum hydroxides produced from the aluminum-based coagulants. The adsorption and removal of heavy-metal ions by polymers with gelation are similar to the method by which heavy-metal ions directly chelate with polymers to precipitate or aggregate. Chelating polymer coagulants adsorb metal ions via chelate bonding between the metal ions and ligands in the polymers. The suitable ligands for target metal ions depend on the type of metal ion (d10 type or transition metal type). Small molecular gelators, so-called molecular gels and low-molecular-weight gelators (LMWG), produce self-assembled supramolecular polymer gels reversibly with non-covalent interactions, such as van der Waals forces, π-π stacking or hydrophobic effects, dipole–dipole, charge-transfer or coordination interactions, and hydrogen bonding (Figure 4) [54].
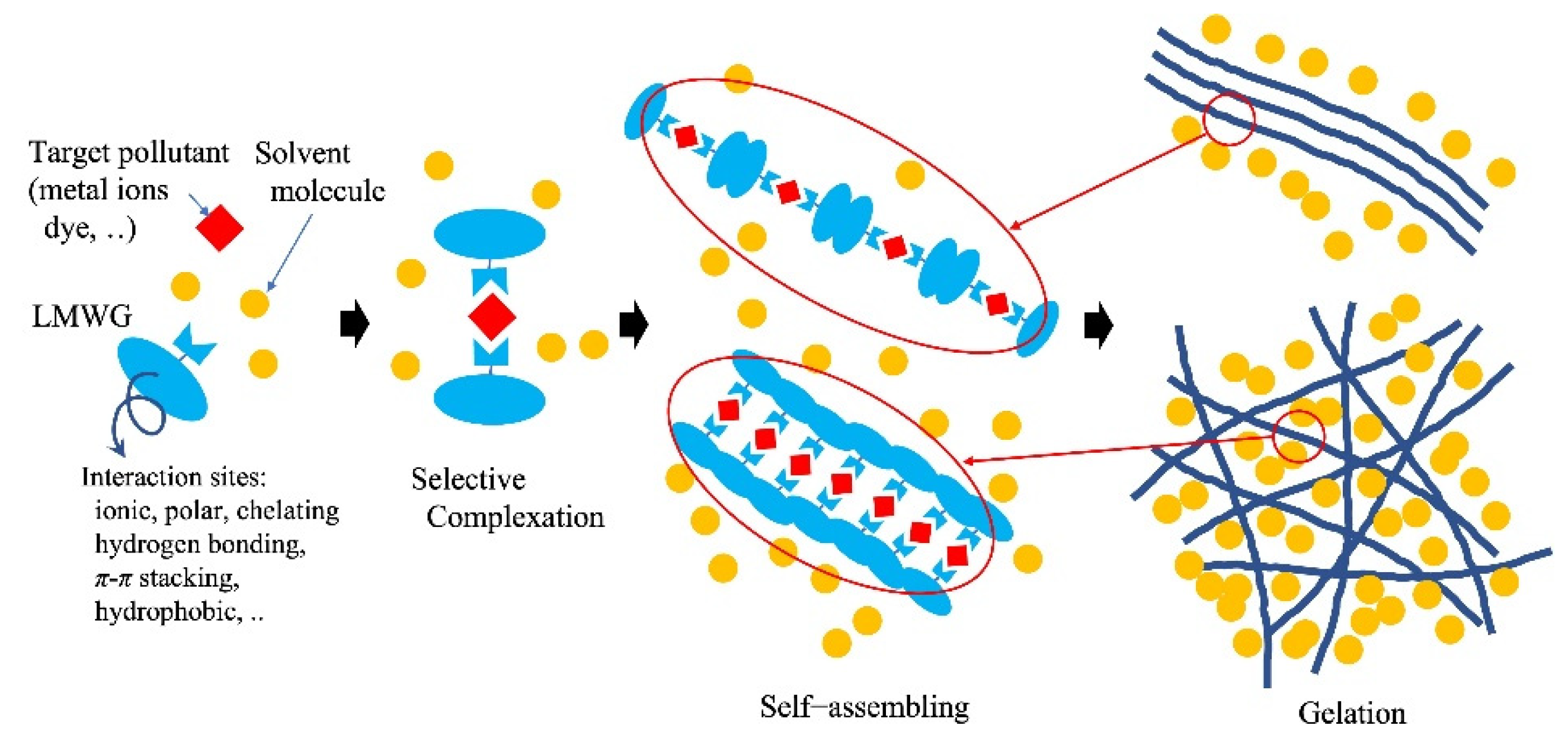
Figure 4. Example of metal ion removal by low-molecular-weight gelator (molecular gels).
3.5. Molecular and Ion Imprint Networks
A complex between a molecule that uses a template and functional monomers is first formed during molecular imprinting. The monomers are then copolymerized with a crosslinking agent. The template molecule is then removed to obtain a molecular imprint polymer (MIP) with a binding space complementary to the template molecule (Figure 5a) [55][56]. Various methods have been proposed to improve the selective adsorption performance of imprinted gels. The fragment imprint method attains selective molecular recognition ability in the gel by using a part of the structure of the target molecule or a substance that is similar to the structure of a pseudo-template molecule (Figure 5a) [57][58]. The internal immobilization method uses a template molecule with an ionic functional group close to the target molecule.
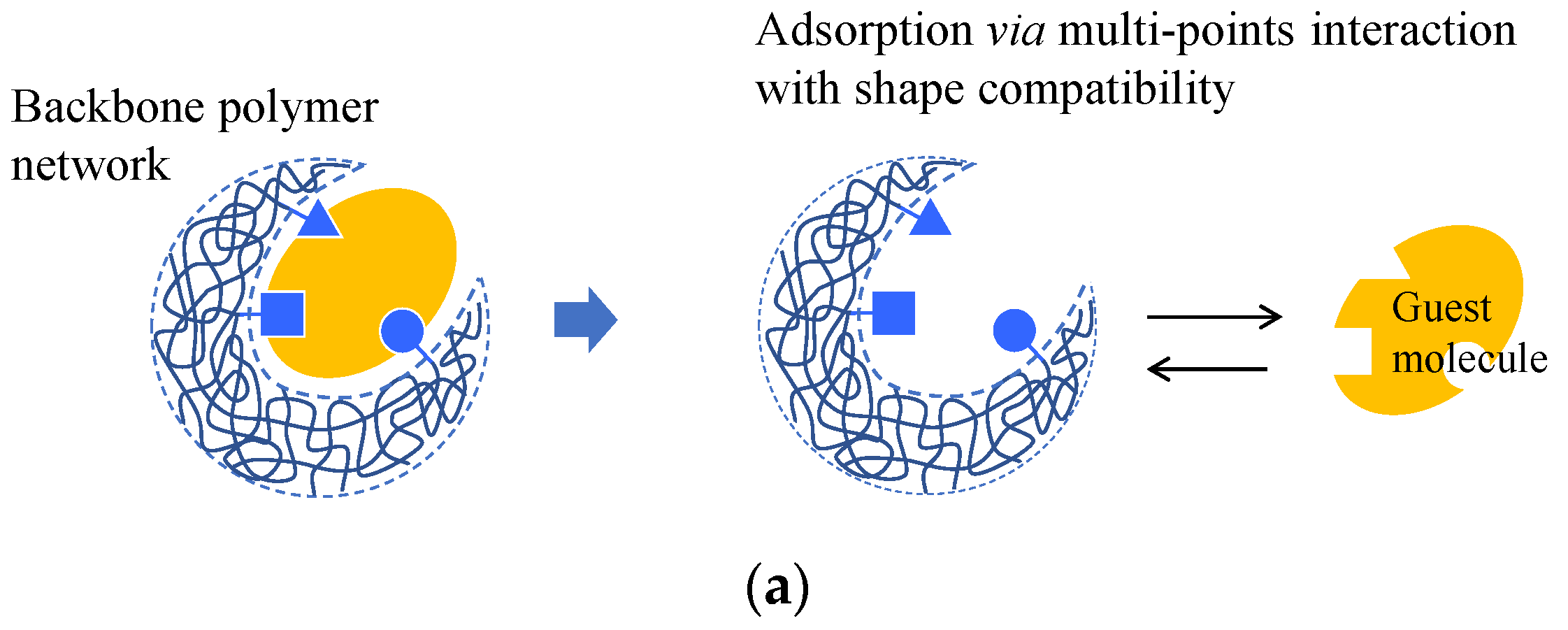
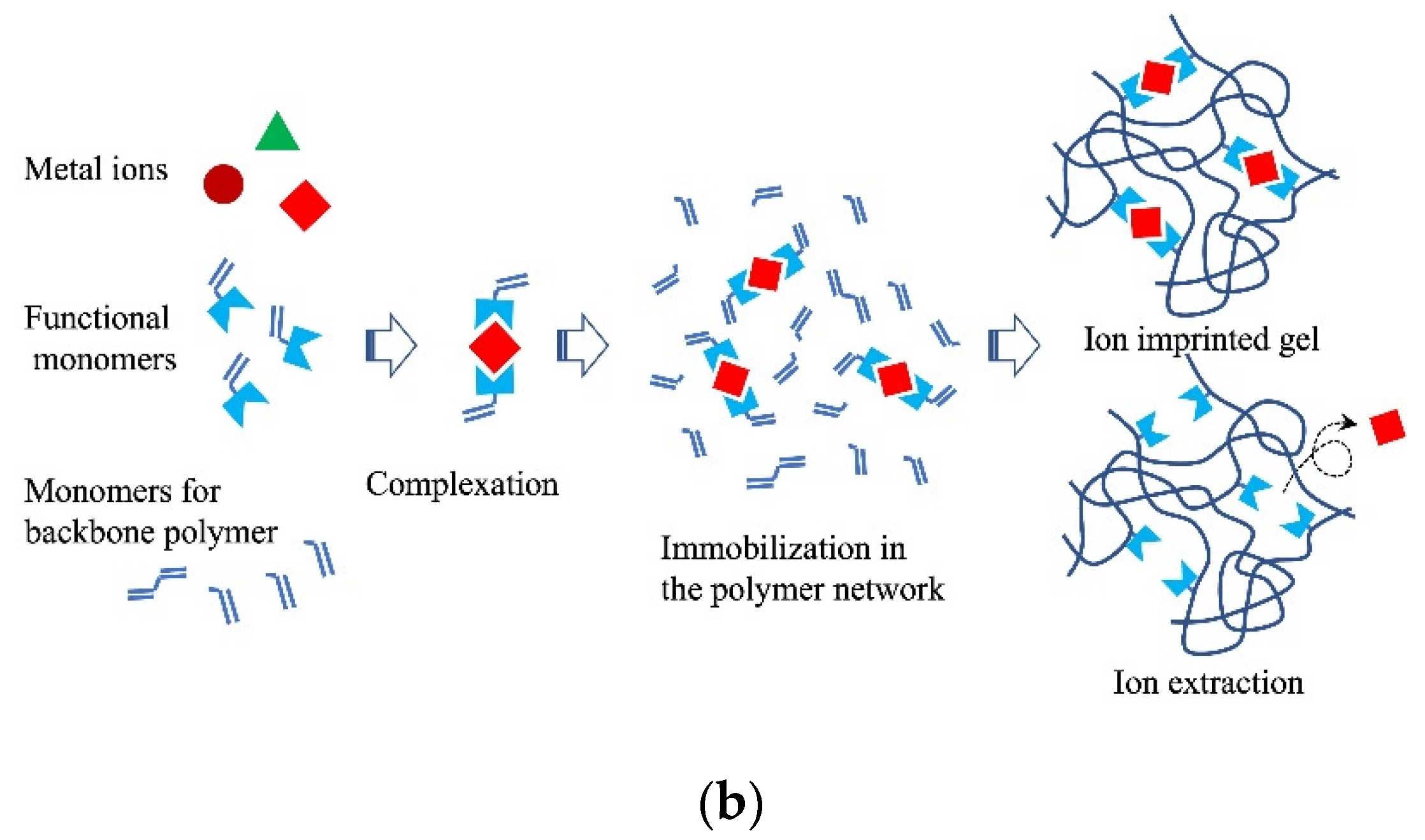
Figure 5. Schematic diagram of (a) molecular imprint and (b) ion imprint gels.
Ion imprinting differs from molecular imprinting. Metal ions can be selectively separated using an ion-imprinted polymer gel. The metal salt of the target metal ion is first conjugated to the monomers of the gel. The conjugate is then polymerized with a crosslinking agent to obtain an ion-imprinted polymer gel with high selectivity (Figure 5b). Highly selective monitoring of metals (Hg(II), Pb(II), Cd(II), and As(V)) using ion-imprinted polymers was demonstrated by Pankaj et al. (2015) [59].
For molecular and ion-imprinted polymer gel adsorbents, it is essential to create pathways with easy access to the adsorption sites in the adsorbents. Li et al. (2014) reported a porous organic polymer (POP) with a high surface area and size-adjustable open pores as adsorbents for heavy metals [60]. This adsorbent, named nano-trap, exhibited high selectivity for Hg in its adsorption capacity (1 g-Hg of 1 g-Hg/1 g POP). Imprint gels using temperature-responsive polymer networks have also been developed to improve adsorption and desorption rates by controlling the swelling volume of the gel. Selectivity and adsorption capacity are trade-off factors. The adsorption capacity of imprint gels is small and its increase without decreasing the selectivity is an essential subject for imprint gels. In the case of macromolecules, mass transfer (diffusion) in the imprint gels is slow. Thus, improvement of the gel structure with access pathways to the imprinted sites is also crucial for rapid adsorption.
3.6. Reaction Field
This type of gel utilizes chemical reactions in the gel to adsorb target substances effectively or effectively degrade the targets accumulated in the gel by adsorption. The gel serves as a field for the adsorption, accumulation, and reaction of the target substances. Over the past few decades, such gel applications have been developed as immobilization carriers for microorganisms and enzymes. In recent years, many studies have reported novel hydrogels with chemically reactive polymer networks or reaction assisting polymer networks, controllable inner environments, and highly dispersed catalytic materials.
Nakano et al. (2001) reported the adsorption and separation of hexavalent chromium using crosslinked condensed tannin gel [61]. Cr oxidizes tannin via a redox reaction to form a carboxyl group on the tannin gel. The Cr was reduced from Cr(VI) to Cr(III) by the tannin and adsorbed on the carboxyl and hydroxyl groups of the tannin gel via the ion-exchange method (Figure 6a). This reaction can be increased by adjusting the pH of the aqueous phase, enabling a large-scale removal of Cr (287 mg/g). The idea of incorporating a redox reaction mechanism into a gel to convert difficult-to-separate metal ions into easily adsorbable metal ions paves the way for developing a simple separation process that inculcates multiple separation processes within the gel particles. In this system, Cr(VI) is adsorbed via electrostatic interactions with positively charged amine groups of the gel. Gallic acid facilitates the reduction of Cr(VI) to less toxic Cr(III). Along with the reduction of Cr(VI), oxidation of phenolic hydroxy groups in the gel to carbonyl groups and low pH conditions due to gallic acid promote the adsorption of Cr(III) by chelating mechanism, resulting in a large adsorption capacity of 476.2 mg/g.
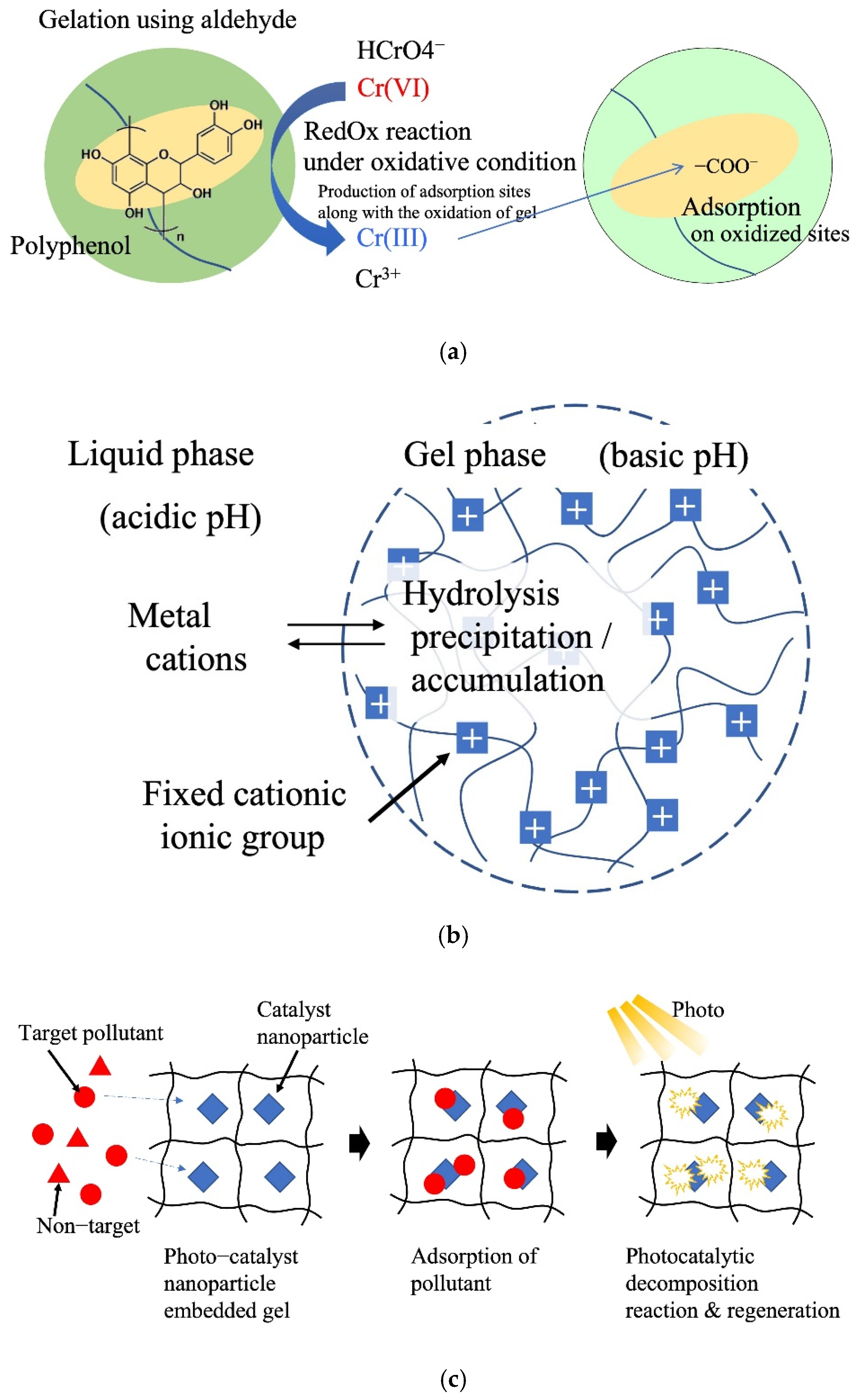
Figure 6. Reactive adsorption system. (a) Tannin gel redox adsorption system. (b) Hydrolysis of metal cations. (c) Adsorption and catalysis reaction.
In the ionic gels of aqueous systems, the pH inside the gel becomes basic or acidic because of the Donnan equilibrium between the gel and outer liquid phase [62]. Utilizing this property of ionic gels, the precipitation of heavy-metal ions as hydroxides in the gel is possible. Gotoh et al. (2017) reported the recovery of metal ions through hydrolysis of metal ions in a cationic hydrogel using poly(N-[3-dimethylaminopropyl]acrylamide) gel (Figure 6b) [63]. The pH of the gel is controlled by varying the type and concentration of ionic groups. This system enables the immobilization of a large number of metal ions in gels in the form of poorly soluble hydroxides. Application for the remediation of soils contaminated with heavy-metal ions using this in situ immobilization has been proposed.
In recent years, many hydrogel adsorbents with highly dispersed and fixed photocatalytic nanoparticles have been reported. The adsorption and photocatalytic functions of the nanoparticles were applied to water and wastewater treatment. Yang et al. (2017) developed a composite hydrogel catalyst composed of poly(hydroxyethyl acrylate/N-methyl maleic acid) and CuS nanoparticles [64]. The gel adsorbed and concentrated sulfamethoxazole (SMX) antibiotics in the composite hydrogel. Subsequently, 90% of SMX was degraded upon visible-light irradiation by the photocatalytic reaction with embedded CuS nanoparticles (Figure 6c). Zhang et al. (2018) reported Fe3O4 nanoparticle-embedded porous hydrogel beads as catalysts for the Fenton reaction of methyl orange [18]. Zhu et al. (2021) reported poly(triethylene glycol methyl ether methacrylate) hydrogels as carriers of phosphotungstic acid for acid catalytic reactions in water [65]. Tokuyama et al. (2022) developed TiO2 nanoparticle-loaded poly(NIPA) fibers for the adsorption and photocatalytic degradation of organic pollutants in aquatic media [66].
3.7. Dissociation Control of Functional Groups in the Polymer Network
Changes in the dissociation of ionic groups on the polymer networks occur depending on the volumetric change in the crosslinked polymer hydrogels. Weak acid-type or weak base-type ionic groups on the polymer chains undergo a shift in their dissociation equilibrium pKa, with changes in pH inside the gel when the density of the ionic groups in the gels changes with the swelling or collapse of the gel (Figure 7). This phenomenon is considered to occur because of various phenomena, such as a change in the dielectric constant, counter ion condensation, and dehydration of the polymer. By utilizing this property, the adsorption control of target ions (the counter ions of the ionic groups in the gels) is achieved through volumetric swelling control of the gels. Thermoresponsive polymer gels are one of the candidates for this application because of their simple and easy volume control. Gotoh et al. (2010) reported the temperature-swing adsorption of phosphoric acid by poly(N-isopropyl acrylamide-co-N-[3-dimethylaminopropyl] acrylamide) copolymer hydrogels [67]. Honda et al. (2021) synthesized N-[3-(dimethylamino)propyl]methacrylamide (DMAPM)-co-N-tert-butyl acrylamide (TBAM) nanogels and applied them for the separation of carbon dioxide [68]. Nagasawa et al. (2019) reported the influence of the hydrophobicity of the backbone polymer on the thermoresponsive adsorption and recovery of carbon dioxide in a poly(N-isopropylacrylamide-N-tert-buthyacrylamide) copolymer gel slurry system [69]. A molecular design that produces a larger pKa shift is expected to yield a high-performance gel. To use this property, the mechanical strength of the gels to withstand repeated swelling operations is required. In addition, the gels must be micronized for a rapid response and adsorption. This process is energy-consuming when thermoresponsive gels are used because of the temperature-swing operation of the entire system. Therefore, it is crucial to devise methods to reduce energy consumption in practical applications. Effective utilization of the heat generated in chemical factories, steel mills, incinerators, etc. would increase the feasibility of these processes. Thin membranes fabricated on porous supports with nanosized particles of TBAM gels successfully achieved the energy-saving separation of carbon dioxide [68].
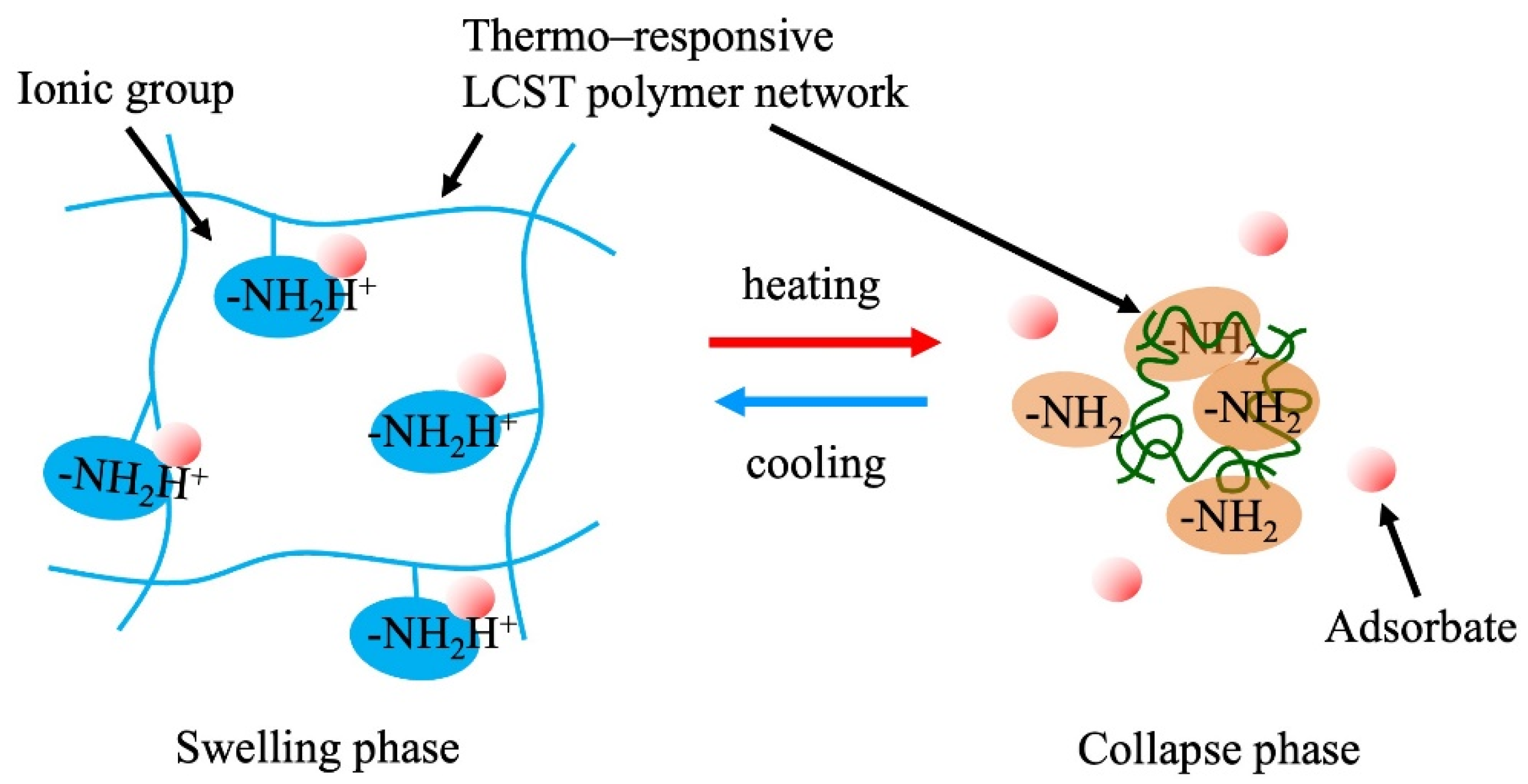
Figure 7. Adsorption control via pKa shift in the stimuli-responsive ionic hydrogels.
3.8. Diversification of the Application
Composite or conjugated forms of hydrogel adsorbents can increase the performance of the gel and the availability of the gels as adsorbents, considering the advantages of their use, cost, and material sharing from an ecological point of view. Adsorbents that exhibit advanced functions have been developed based on composite design. An example is the hydrogel embedding of magnetic nanoparticles [23]. Solid–liquid separation of fine adsorbent particles is easily performed using magnetic force in a liquid-phase separation system. Rehman et al. (2016) reported Fe3O4 nanoparticle-immobilized poly(3-acrylamidopropyl trimethylammonium chloride) microgels for the removal of AsO43− [70]. The magnetic field-responsive microgels were easily recovered from aqueous media for reuse. Because the adsorbents are lost over repeated use, the design of gels with a highly stable magnetic recovery rate is the subject of this study. In the application of spilled oil recovery, Xu et al. (2018) reported that poly(vinyl alcohol)/cellulose nanofiber (PVA/CNF) oil adsorbent gels with Fe3O4 nanoparticles effectively absorb oil spreading on the water surface, and the gels can be easily collected from the surface by the application of a magnetic field [71].
Xiao et al. (2018) developed light-to-thermal conversion phase-change composite hydrogels consisting of sodium acetate trihydrate (NaAc3H2O), acrylamide–acrylic acid sodium copolymer, and CuS for the storage of solar heat energy by light-to-thermal conversion (Figure 8a). The composite hydrogel exhibited good energy conversion in a stable form during the phase change of NaAc3H2O. It restricted molecular water movement via hydrogen bonding with the functional groups on the polymer network of the gel, when NaAc3H2O was melting, without water leakage [72].
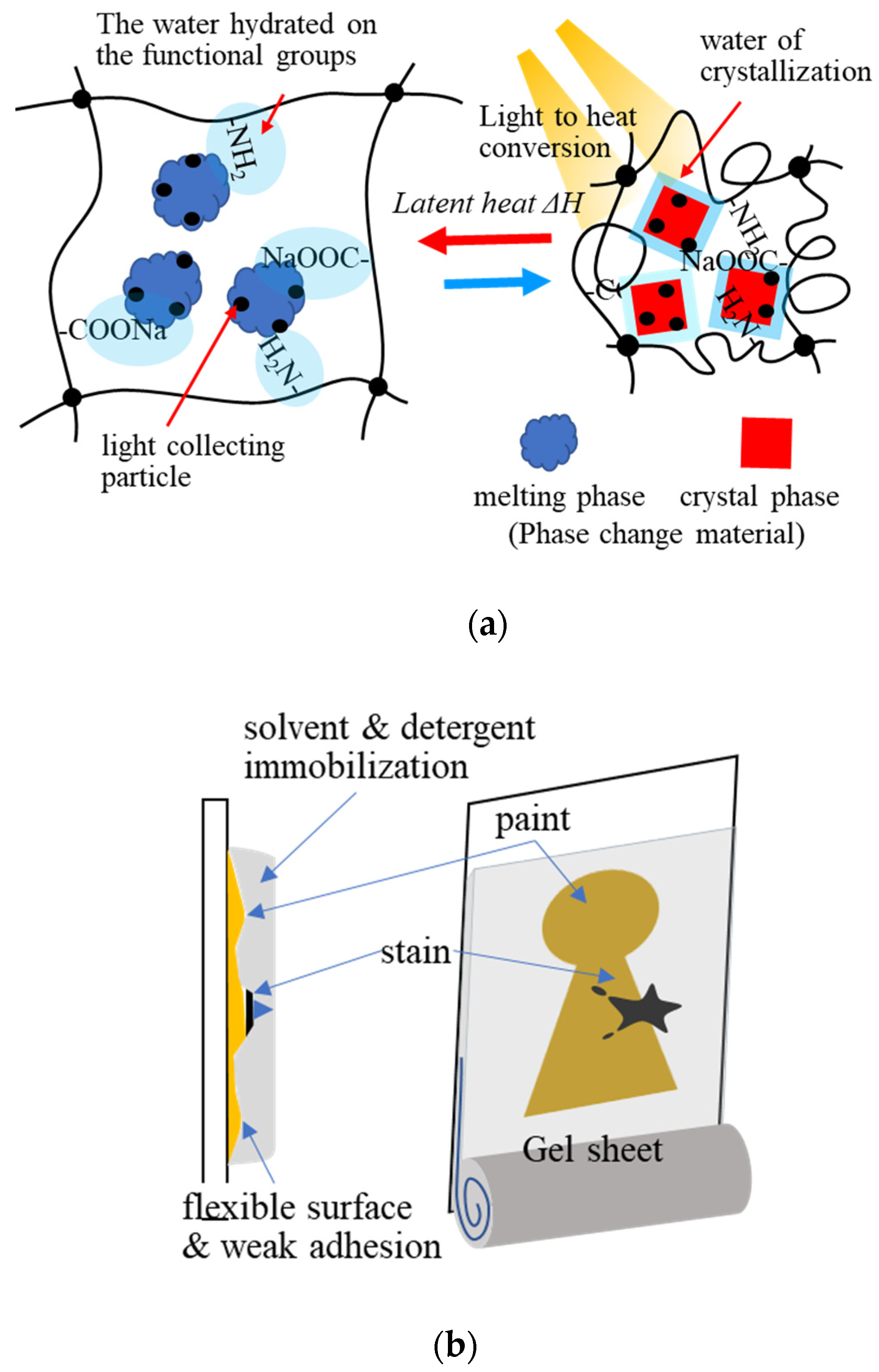
Figure 8. Application of the hydrogel adsorbents: (a) heat accumulation with light-to-thermal conversion, (b) conservation science: schematic image of the gel cleaning for painting art restoration.
Applications of gel adsorbents have diversified in recent years. The adsorption and absorption properties of hydrogels have been applied in various fields [74][75][76][77]. One of the applications of hydrogels is dressing materials [73]. Hydrogels can maintain a humid environment, absorb exuded tissue fluid, and exchange gas; making them a potential wound dressing material. Clean moist healing that adheres to the skin, absorbs exudate, disinfects the site, releases drugs through wound dressing, through fine particles of hydrogels are the scope of this application. In conservation science, in the 1980s, Richard Wolbers developed a “gel cleaning method” using gels such as gellan gum, agarose, and Pemulen TR-2 to restore and clean cultural properties In the gel cleaning method, stains on cultural properties are transferred to the gel. It is remarkably effective for cleaning the vertical surfaces of artworks such as paintings (Figure 8b). Gel cleaning with less volatilization of organic solvents (acetone, hexane, etc.) is also helpful for preserving the working environment. Hydrogel functions, such as solvent immobilization, surface flexibility, adhesion, adsorption, absorption, and extraction, are used in this technique. The multifunctional properties of hydrogel adsorbents can be further applied in different fields.
References
- Ruthven, D.M. Principles of Adsorption and Adsorption Process; John Wiley & Sons, Inc.: NewYork, NY, USA, 1984.
- Suzuki, M. Adsorption Engineering; Chemical Engineering Monographs; Elsevier Science: Tokyo, Japan, 1990; 306p.
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395.
- Masaro, L.; Zhu, X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775.
- Hagel, V.; Haraszti, T.; Boehm, H. Diffusion and interaction in PEG-DA hydrogels. Biointerphases 2013, 8, 36.
- Hadjiev, N.A.; Amsden, B.G. An assessment of the ability of the obstruction-scaling model to estimate solute diffusion coefficients in hydrogels. J. Control. Release 2015, 199, 10–16.
- Puguan, J.M.C.; Yu, X.; Kim, H. Diffusion characteristics of different molecular weight solutes in Ca–alginate gel beads. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 158–165.
- Ban, T.; Kaji, M.; Nagatsu, Y.; Tokuyama, H. Propagating Precipitation Waves in Disordered Media. ACS Omega 2017, 2, 8027–8032.
- Gosecki, M.; Kazmierski, S.; Gosecka, M. Diffusion-Controllable Biomineralization Conducted In Situ in Hydrogels Based on Reversibly Cross-Linked Hyperbranched Polyglycidol. Biomacromolecules 2017, 18, 3418–3431.
- Zhou, Y.; Li, J.; Zhang, Y.; Dong, D.; Zhang, E.; Ji, F.; Qin, Z.; Yang, J.; Yao, F. Establishment of a Physical Model for Solute Diffusion in Hydrogel: Understanding the Diffusion of Proteins in Poly(sulfobetaine methacrylate) Hydrogel. J. Phys. Chem. B 2017, 121, 800–814.
- Tokuyama, H.; Nakahata, Y.; Ban, T. Diffusion coefficient of solute in heterogeneous and macroporous hydrogels and its correlation with the effective crosslinking density. J. Membr. Sci. 2020, 595, 117533.
- Uyama, H. Polymeric Monolith—New Fabrication Methods and Applications. Kobunshi Ronbunshu 2010, 67, 489–496.
- Nischang, I. Porous polymer monoliths: Morphology, porous properties, polymer nanoscale gel structure and their impact on chromatographic performance. J. Chromatogr. A 2013, 1287, 39–58.
- Hosoda, N.; Tsujimoto, T.; Uyama, H. Plant oil-based green composite using porous poly(3-hydroxybutyrate). Polym. J. 2014, 46, 301–306.
- Tokuyama, H.; Kanehara, A. Novel Synthesis of Macroporous Poly(N-isopropylacrylamide) Hydrogels Using Oil-in-Water Emulsions. Langmuir 2007, 23, 11246–11251.
- Tokuyama, H.; Sumida, H.; Kanehara, A.; Nii, S. Effect of surfactants on the porous structure of poly(N-isopropylacrylamide) hydrogels prepared by an emulsion templating method. Colloid Polym. Sci. 2009, 287, 115–121.
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320.
- Zhang, S.; Fan, X.; Zhang, F.; Zhu, Y.; Chen, J. Synthesis of Emulsion-Templated Magnetic Porous Hydrogel Beads and Their Application for Catalyst of Fenton Reaction. Langmuir 2018, 34, 3669–3677.
- Sedlačík, T.; Nonoyama, T.; Guo, H.; Kiyama, R.; Nakajima, T.; Takeda, Y.; Kurokawa, T.; Gong, J.P. Preparation of Tough Double- and Triple-Network Supermacroporous Hydrogels through Repeated Cryogelation. Chem. Mater. 2020, 32, 8576–8586.
- Teodosiu, C.; Wenkert, R.; Tofan, L.; Paduraru, C. Advances in preconcentration/removal of environmentally relevant heavy metal ions from water and wastewater by sorbents based on polyurethane foam. Rev. Chem. Eng. 2014, 30, 403–420.
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745.
- Seida, Y.; Nakano, Y.; Ichida, H. Surface Properties of Temperature-Sensitive N-isopropylacrylamide-Copolymer Gels. Kagaku Kogaku Ronbunshu 1992, 18, 346–352.
- Ajmal, M.; Siddiq, M.; Aktas, N.; Sahiner, N. Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 5, 43873–43884.
- Zhang, C.; Dai, Y.; Wu, Y.; Lu, G.; Cao, Z.; Cheng, Z.; Wang, K.; Yang, H.; Xia, Y.; Wen, X.; et al. Facile preparation of polyacrylamide/chitosan/Fe3O4 composite hydrogels for effective removal of methylene blue from aqueous solution. Carbohydr. Polym. 2020, 234, 115882.
- Zhang, Y.; Li, Z. Heavy metals removal using hydrogel-supported nanosized hydrous ferric oxide: Synthesis, characterization, and mechanism. Sci. Total Environ. 2017, 580, 776–786.
- Tokuyama, H.; Kitamura, E.; Seida, Y. Development of zirconia nanoparticle-loaded hydrogel for arsenic adsorption and sensing. React. Funct. Polym. 2020, 146, 104427.
- Safi, S.R.; Gotoh, T.; Iizawa, T.; Nakai, S. Development and regeneration of composite of cationic gel and iron hydroxide for adsorbing arsenic from ground water. Chemosphere 2019, 217, 808–815.
- Zheng, Y.-M.; Zou, S.-W.; Nanayakkara, K.N.; Matsuura, T.; Chen, J.P. Adsorptive removal of arsenic from aqueous solution by a PVDF/zirconia blend flat sheet membrane. J. Membr. Sci. 2011, 374, 1–11.
- Zheng, Y.-M.; Yu, L.; Chen, J.P. Removal of methylated arsenic using a nanostructured zirconia-based sorbent: Process performance and adsorption chemistry. J. Colloid Interface Sci. 2012, 367, 362–369.
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of na-noparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269.
- Harikumar, P.S.; Hridya, T.K. Application of CuNi bimetallic nanoparticle as an adsorbent for the removal of heavy metals from aqueous solution. Int. J. Environ. Anal. Chem. 2021, 101, 869–883.
- Elwakeel, K.Z. Environmental Application of Chitosan Resins for the Treatment of Water and Wastewater: A Review. J. Dispers. Sci. Technol. 2010, 31, 273–288.
- Seida, Y.; Imai, T.; Murata, K.; Nakano, Y. Thermo-responsivity of the polyacrylate-adsorbed metal cation and its hydration structure. Adsorpt. Sci. Technol. 2008, 26, 423–432.
- Tokuyama, H.; Yanagawa, K.; Sakohara, S. Temperature swing adsorption of heavy metals on novel phosphate-type adsorbents using thermosensitive gels and/or polymers. Sep. Purif. Technol. 2006, 50, 8–14.
- Tokuyama, H.; Iwama, T. Temperature-Swing Solid-Phase Extraction of Heavy Metals on a Poly(N-isopropylacrylamide) Hydrogel. Langmuir 2007, 23, 13104–13108.
- Tokuyama, H.; Iwama, T. Solid-phase extraction of indium(III) ions onto thermosensitive poly(N-isopropylacrylamide). Sep. Purif. Technol. 2009, 68, 417–421.
- Tokuyama, H.; Hisaeda, J.; Nii, S.; Sakohara, S. Removal of heavy metal ions and humic acid from aqueous solutions by co-adsorption onto thermosensitive polymers. Sep. Purif. Technol. 2010, 71, 83–88.
- Cui, G.; Bai, Y.; Li, W.; Gao, Z.; Chen, S.; Qiu, N.; Satoh, T.; Kakuchi, T.; Duanb, Q. Synthesis and characterization of Eu(III) com-plexes of modified D-glucosamine and poly(N-isopropylacrylamide). Mat. Sci. Eng. C 2017, 78, 603–608.
- Tokuyama, H.; Kanehara, A. Temperature swing adsorption of gold(III) ions on poly(N-isopropylacrylamide) gel. React. Funct. Polym. 2007, 67, 136–143.
- Ramos-Jacques, A.L.; Lujan-Montelongo, J.A.; Silva-Cuevas, C.; Cortez-Valadez, M.; Estevez, M.; Hernandez-Martínez, A.R. Lead (II) removal by poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Eur. Polym. J. 2018, 101, 262–272.
- Soleyman, R.; Pourjavadi, A.; Monfared, A.; Khorasani, Z. Novel salep-based chelating hydrogel for heavy metal removal from aqueous solutions. Polym. Adv. Technol. 2016, 27, 999–1005.
- Chen, C.Y.; Chiang, C.L.; Chen, C.R. Removal of heavy metal ions by a chelating resin containing glycine as chelating groups. Sep. Purif. Technol. 2007, 54, 396–403.
- Ibrahim, A.G.; Saleh, A.S.; Elsharma, E.M.; Metwally, E.; Siyam, T. Gamma radiation-induced preparation of poly(1-vinyl-2-pyrrolidone-co-sodium acrylate) for effective removal of Co(II), Ni(II), and Cu(II). Polym. Bull. 2019, 76, 303–322.
- Omondi, B.A.; Okabe, H.; Hidaka, Y.; Hara, K. Poly (1,4-diazocane-5,8-dione) macrocyclic-functionalized hydrogel for high selectivity transition metal ion adsorption. React. Funct. Polym. 2018, 125, 11–19.
- Omondi, B.A.; Okabe, H.; Hidaka, Y.; Hara, K. Fabrication of poly (1,4-dioxa-7,12-diazacyclotetradecane-8,11-dione) macrocyclic functionalized hydrogel for high selective adsorption of Cr, Cu and Ni. React. Funct. Polym. 2018, 130, 90–97.
- Samaddar, P.; Kumar, S.; Kim, K.-H. Polymer Hydrogels and Their Applications Toward Sorptive Removal of Potential Aqueous Pollutants. Polym. Rev. 2019, 59, 418–464.
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221.
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891.
- Cavallaro, G.; Lazzara, G.; Rozhina, E.; Konnova, S.; Kryuchkova, M.; Khaertdinov, N.; Fakhrullin, R. Organic-nanoclay composite materials as removal agents for environmental decontamination. RSC Adv. 2019, 9, 40553–40564.
- Basu, H.; Saha, S.; Kailasa, S.K.; Singhal, R.K. Present status of hybrid materials for potable water decontamination: A review. Environ. Sci. Water Res. Technol. 2020, 6, 3214–3248.
- Shalla, A.H.; Bhat, M.A.; Yaseen, Z. Hydrogels for removal of recalcitrant organic dyes: A conceptual overview. J. Environ. Chem. Eng. 2018, 6, 5938–5949.
- Kanazawa, H.; Matsushima, Y.; Okano, T. Temperature-responsive chromatography. TrAC Trends Anal. Chem. 1998, 17, 435–440.
- Seida, Y.; Nakano, Y. Concept to control the phase behavior of stimulisensitive polymer gel. J. Chem. Eng. Jpn. 1995, 28, 425–428.
- Weiss, R.G. Molecular Gels: Structure and Dynamics (Monographs in Supramolecular Chemistry) (GLD); The Royal Society of Chemistry: London, UK, 2018.
- Kanazawa, R.; Yoshida, T.; Gotoh, T.; Sakohara, S. Preparation of Molecular Imprinted Thermosensitive Gel Adsorbents and Adsorption/Desorption Properties of Heavy Metal Ions by Temperature Swing. J. Chem. Eng. Jpn. 2004, 37, 59–66.
- Kubo, T. Focusing Review, Development and Applications of Fragment Imprinting Technique. Chromatography 2008, 29, 9–17.
- Ahmad, I.; Siddiqi, W.A.; Qadir, S.; Ahmad, T. Synthesis and characterization of molecular imprinted nanomaterials for the removal of heavy metals from water. J. Mater. Res. Technol. 2018, 7, 270–282.
- Tominaga, Y.; Kubo, T.; Kaya, K.; Hosoya, K. Effective Recognition on the Surface of a Polymer Prepared by Molecular Imprinting Using Ionic Complex. Macromolecules 2009, 42, 2911–2915.
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals by using ion-imprinted polymers. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404.
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537.
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2000, 35, 496–500.
- Nakano, Y.; Seida, Y.; Uchida, M.; Yamamoto, S. Behavior of ions within hydrogel and its swelling properties. J. Chem. Eng. Jpn. 1990, 23, 574–579.
- Gotoh, T.; Ogawa, A.; Nakata, D.; Iizawa, T.; Nakai, S. Metal Hydroxide Formation in DMAPAA Hydrogel and Novel Metal Ion Removal Method. Kagaku Kogaku Ronbunshu 2017, 43, 199–206.
- Yang, J.; Li, Z.; Zhu, H. Adsorption and photocatalytic degradation of sulfamethoxazole by a novel composite hydrogel with visible light irradiation. Appl. Catal. B Environ. 2017, 217, 603–614.
- Zhu, J.; Gotoh, T.; Nakai, S.; Tsunoji, N.; Sadakane, M. Poly(triethylene glycol methyl ether methacrylate) hydrogel as a carrier of phosphotungstic acid for acid catalytic reaction in water. Mater. Adv. 2021, 2, 3556–3559.
- Tokuyama, H.; Hamaguchi, R. TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol. Gels 2022, 8, 137.
- Gotoh, T.; Tanaka, K.; Arase, T.; Sakohara, S. A Novel Thermosensitive Gel Adsorbent for Phosphate Ions. Macromol. Symp. 2010, 295, 81–87.
- Honda, R.; Hamasaki, A.; Miura, Y.; Hoshino, Y. Thermoresponsive CO2 absorbent for various CO2 concentrations: Tuning the pKa of ammonium ions for effective carbon capture. Polym. J. 2020, 53, 157–167.
- Nagasawa, Y.; Seida, Y.; Gotoh, T.; Furuya, E. Influence of Hydrophobicity of Backbone Polymer in Thermo-Responsive Hydrogel with Immobilized Amine on Cycle Capacity for Absorption and Recovery of CO2. Polymers 2019, 11, 1024.
- Rehman, S.U.; Siddiq, M.; Al-Lohedan, H.; Aktas, N.; Sahiner, M.; Demirci, S.; Sahiner, N. Fast removal of high quantities of toxic arsenate via cationic p(APTMACl) microgels. J. Environ. Manag. 2016, 166, 217–226.
- Xu, Z.; Jiang, X.; Zhou, H.; Li, J. Preparation of magnetic hydrophobic polyvinyl alcohol (PVA)–cellulose nanofiber (CNF) aerogels as effective oil absorbents. Cellulose 2017, 25, 1217–1227.
- Xiao, Q.; Fan, J.; Li, L.; Xu, T.; Yuan, W. Solar thermal energy storage based on sodium acetate trihydrate phase change hydrogels with excellent light-to-thermal conversion performance. Energy 2018, 165, 1240–1247.
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5(4), 22.
- Madden, B. Gels in Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017.
- Hayakawa, N.; Fujii, Y.; Yamamoto, N.; Sano, C. On-site Surface Cleaning of Japanese Architecture Using Gels Incorporating Organic Solvents. Stud. Conserv. 2020, 65, P139–P141.
- Shirakawa, Y. Practical Use of Gels. In Seminor on Restoration Treatments for Cultural Property; Tokyo National Research Institute for Cultural Properties: Tokyo, Japan, 2021; pp. 25–31. Available online: http://id.nii.ac.jp/1440/00008990/ (accessed on 26 March 2022).
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
15 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





