
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco De Francesco | -- | 2883 | 2022-06-13 10:38:46 | | | |
| 2 | Dean Liu | Meta information modification | 2883 | 2022-06-13 11:17:04 | | |
Video Upload Options
Osteoarthritis (OA) is a chronic debilitating disorder causing pain and gradual degeneration of weight-bearing joints with detrimental effects on cartilage volume as well as cartilage damage, generating inflammation in the joint structure. The etiology of OA is multifactorial. Currently, therapies are mainly addressing the physical and occupational aspects of osteoarthritis using pharmacologic pain treatment and/or surgery to manage the symptomatology of the disease with no specific regard to disease progression or prevention.
1. Introduction
OA is an idiopathic disorder, and the management reflects the lack of understanding of the disease. The non-surgical approach involves the usage of treatment such as physiotherapy, kinesitherapy, weight control, drugs. The main drugs used are hormones (parathyroid hormones, calcitonin, leptin), which act as regulating molecular pathways of cartilage metabolism; bisphosphonates (zoledronic acid, alendronate) which act as improving bone metabolism, reducing bone reabsorption; monoclonal antibodies (bevacizumab, adalimumab,) which act on articular cartilage promoting collagen production; statins (atorvastatin, which reduces cartilage degradation); supplements (glucosamine, chondroitin sulfate, vitamin C, vitamin D, Selenium, Zinc, Magnesium), which act as a cartilage nourishment [1][2].
2. Regenerative Treatment for Osteoarthritis Disease
2.1. Platelet-Rich Plasma (PRP)
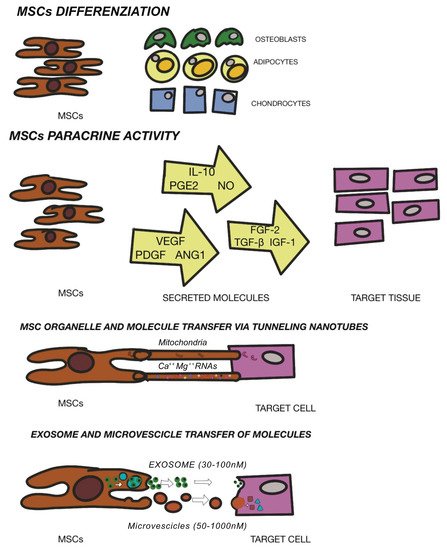
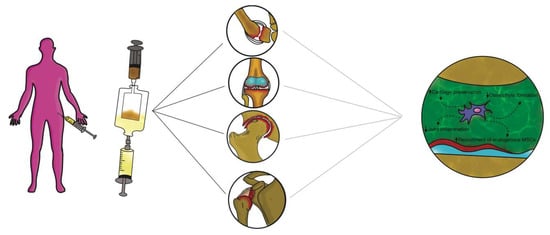
2.2. Mesenchymal Stem Cells Therapy
2.3. Intra-articular Application of Autologous Microfragmented Adipose Tissue with Stromal Vascular Fraction
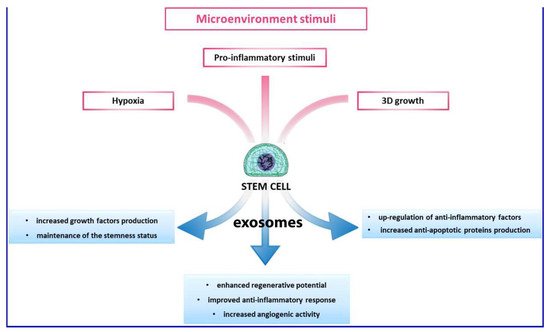
2.4. Exosome and Extracellular Vescicles (EVs)
References
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Baciut, M.; Baciut, G.; Bran, S.; Onisor, F.; Piciu, A.; Pasca, R.D.; et al. Systemic drugs with impact on osteoarthritis. Drug Metab. Rev. 2019, 51, 498–523.
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Gheban, D.; Benea, H.R.C. Tibolone, alendronate, and simvastatin enhance implant osseointegration in a preclinical in vivo model. Clin. Oral Implant. Res. 2020, 31, 655–668.
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. IJMS 2020, 21, 7794.
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272.
- Russell, R.P.; Apostolakos, J.; Hirose, T.; Cote, M.P.; Mazzocca, A.D. Variability of platelet-rich plasma preparations. Sports Med. Arthrosc. Rev. 2013, 21, 186–190.
- Xu, Z.; Yin, W.; Zhang, Y. Comparative evaluation of leukocyte and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301.
- Jiang, G.; Wu, Y.; Meng, J.; Wu, F.; Li, S.; Lin, M.; Gao, X.; Hong, J.; Chen, W.; Yan, S.; et al. Comparison of leukocyte-rich platelet -rich plasma and leukocyte-poor platelet-rich plasma on Achilles Tendinopathy at an early stage in a rabbit model. Am. J. Sports Med. 2020, 48, 1189–1199.
- Kobayashi, Y.; Saita, Y.; Nishio, H.; Ikeda, H.; Takazawa, Y.; Nagao, M.; Takaku, T.; Komatsu, N.; Kaneko, K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J. Orthop. Sci. 2016, 21, 683–689.
- Kenmochi, M. Clinical outcomes following injections of leukocyte-rich platelet-rich plasma in osteoarthritis patients. J. Orthop. 2020, 18, 143–149.
- Marmotti, A.; Rossi, R.; Castoldi, F.; Roveda, E.; Michielon, G.; Peretti, G.M. PRP and articular cartilage: A clinical update. Biomed. Res. Int. 2015, 2015, 542502.
- Mariani, E.; Canella, V.; Cattini, L.; Kon, E.; Marcacci, M.; Di Matteo, B.; Pulsatelli, L.; Filardo, G. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS ONE 2016, 11, e015613753V.
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16.
- Paterson, K.L.; Hunter, D.J.; Metcalf, B.R.; Eyles, J.; Duong, V.; Kazsa, J.; Wang, Y.; Buchbinder, R.; Cicuttini, F.; Forbes, A.; et al. Efficacy of intra-articular injections of platelet-rich plasma as a symptom- and disease-modifying treatment for knee osteoarthritis–the RESTORE trial protocol. BMC Musculoskelet. Disord. 2018, 19, 272.
- Delgado, D.; Garate, A.; Vincent, H.; Bilbao, A.M.; Patel, R.; Fiz, N.; Sampson, S.; Sanchez, M. Current concepts in intraosseous platelet-rich plasma injections for knee osteoarthritis. J. Clin. Orthop. Trauma. 2019, 10, 36–41.
- Sanchez, M.; Delgado, D.; Pompei, O.; Perez, J.C.; Sanchez, P.; Garate, A.; Bilbao, A.M.; Fiz, N.; Padilla, S. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: An observational study. Cartilage 2019, 10, 245–253.
- Vyas, C.; Mishbak, H.; Cooper, G.; Peach, C.; Pereira, R.F.; Bartolo, P. Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanuf. Rev. 2020, 5, 2.
- Bianco, P. “Mesenchymal” Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The international Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22.
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5.
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic properties of mesenchymal stromal/Stem Cells: The need of cell priming for cell-free therapies in regenerative medicine. Int. J. Mol. Sci. 2021, 22, 763.
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449.
- Matula, Z.; Nemeth, A.; Lorincz, P.; Szepesi, A.; Brozik, A.; Buzas, E.I.; Low, P.; Nemet, K.; Uher, F.; Urban, V.S. The role of extracellular vesicle and tunneling nanotubes-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cell. Stem Cell Dev. 2016, 25, 1818–1832.
- Matta, C.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells and their potential for microengineering the chondrocyte niche. EBioMedicine 2015, 2, 1560–1561.
- Jones, D.L.; Wagers, A.J. No place like home: Anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21.
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056.
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584.
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768.
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152.
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front. Genet. 2016, 7, 213.
- Harrel, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326.
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539.
- De Francesco, F.; Tirino, V.; Desiderio, V.; Ferraro, G.; D’Andrea, F.; Giuliano, M.; Libondi, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE 2009, 4, e6537.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): An overview. Int. J. Mol. Sci. 2018, 19, 1897.
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived m esenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752.
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose CD34+CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissue. J. Cell Biochem. 2013, 114, 1039–1049.
- D’Andrea, F.; De Francesco, F.; Ferraro, G.A.; Desiderio, V.; Tirino, V.; De Rosa, A.; Papaccio, G. Large-scale production of human adipose tissue from stem cells: A new tool for regenerative medicine and tissue banking. Tissue Eng. Part C Methods 2008, 14, 233–242.
- Nicoletti, G.F.; De Francesco, F.; D’Andrea, F.; Ferraro, G.A. Methods and procedures in adipose stem cells: State of the art and perspective for translation medicine. J. Cell Physiol. 2015, 230, 489–495.
- Pagani, S.; Veronesi, F.; Giavaresi, G.; Filardo, G.; Papio, T.; Romandini, I.; Fini, M. Autologous protein soluction effect on chondrogenic differentiation of mesenchymal stem cells from adipose tissue and bone marrow in an osteoarthritic environment. Cartilage 2021, 15, 1947603521993217.
- Gaut, C.; Sugaya, K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen. Med. 2015, 10, 665–679.
- Trumbull, A.; Subramanian, G.; Yildirim-Ayan, E. Mechanoresponsive musculoskeletal tissue differentiation of adipose-derived stem cells. Biomed. Eng. Online 2016, 15, 43.
- De Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.A.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal Stem/Stromal Cells: A new “cells as drugs” paradigm. Effic. Crit. Asp. Cell Ther. Curr. Pharm. Design 2013, 19, 13.
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A Non-enzymatic method to obtain a fat tissue derivatite highly enriched in adipose stem cells (ASCs) from human lipoaspirates: Preliminary results. Int. J. Mol. Sci. 2018, 19, 2061.
- Yano, K.; Speidel, A.T.; Yamato, M. Four Food and Drug Administration draft guidance documents and the REGROW Act: A litmus test for future changes in human cell- and tissue-based products regulatory policy in the United States? J. Tissue Eng. Regen. Med. 2018, 12, 1579–1593.
- Raposio, E.; Ciliberti, R.G. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64.
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus mechanical centrifugation. Int. J. Mol. Sci. 2019, 20, 5471.
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7.
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713.
- Tremolada, C.; Colombo, C.; Ventura, C. Adipose tissue and mesenchymal stem cells: State of the art and lipogems technology development. Curr. Stem. Cell. Rep. 2016, 2, 304–312.
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant. 2015, 24, 1233–1252.
- Condè-Green, A.; Kotamarti, V.S.; Sherman, L.S.; Keith, J.D.; Lee, E.S.; Granick, M.S.; Rameshwar, P. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: Review of upcoming techniques. Plast. Reconstr. Surg. Global Open 2016, 4, e1017.
- Ferguson, R.E.H.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L.Q. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast. Surg. 2008, 60, 594–597.
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Yu, J.C.; Arm, D.M.; Fraser, J.K. Comparison of three different fat graft preparation methods: Gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast. Reconstr. Surg. 2013, 131, 873–880.
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast. Reconstr. Surg. Global Open 2020, 8, e3010.
- De Fazio, D.; Cingozoglu, C.A.C. Combined mastopexy and augmentation with autologous fat grafting: First results with lipopexy. Plast. Reconstr. Surg. Global Open 2020, 8, e1957.
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raddaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077.
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Peault, B. Higher Perycite content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl. Med. 2018, 7, 876–886.
- Randelli, P.; Menon, A.; Ragone, V.; Creo, P.; Bergante, S.; Randelli, F.; De Girolamo, L.; Montrasio, U.A.; Banfi, G.; Cabitza, P.; et al. Lipogems product treatment increases the proliferation rate of human tendon stem cells without affecting their stemness and differentiation capability. Stem Cells Int. 2016, 2016, 4373410.
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Thomas Vangsness, C., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: A study protocol. BMC Musculoskelet. Disord. 2018, 19, 383.
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New medical device rigeneracons allows to obtain viable micrografts from mechanical disaggregation of human tissues. J. Cell Physiol. 2015, 230, 2299–2303.
- Dai Prè, E.; Busato, A.; Mannucci, S.; Vurro, F.; De Francesco, F.; Riccio, V.; Solito, S.; Biswas, R.; Bernardi, P.; Riccio, M.; et al. In Vitro characterization of adipose stem cells non-enzymatically extracted from the thigh and abdomen. Int. J. Mol. Sci. 2020, 21, 3081.
- Raposio, E.; Caruana, G.; Petrella, M.; Bonomini, M.P.; Grieco, A. A standardized method of isolating adipose-derived stem cells for clinical application. Ann. Plast. Surg. 2016, 76, 124–126.
- Raposio, E.; Simonacci, F.; Perrotta, R.E. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann. Med. Surg. 2017, 20, 87–91.
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2.
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: Preliminary results. Front. Cell Dev. Biol. 2019, 7, 88.
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and Rapid Non-enzymatic procedure allows the isolation of structurally preserved connective tissue micro-fragments enriched with SVF. Cells 2020, 10, 36.
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8.
- Isola, A.; Chen, S. Exosomes: The messengers of health and disease. Curr. Neuropharmacol. 2016, 15, 157–165.
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98.
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214.
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140.
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017, 9, 617–631.
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagn, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340.
- Ruiz, M.; Cosenza, S.; Maumus, M.; Jorgensen, C.; Noel, D. Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin. Biol. Ther. 2016, 16, 33–42.
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Castejon, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 7197598.
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249.
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; De Girolamo, L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109.




