Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Navarro Jiménez | -- | 2910 | 2022-06-09 14:45:27 | | | |
| 2 | Jessie Wu | -1 word(s) | 2909 | 2022-06-10 03:15:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Navarro Jiménez, E.; Clemente-Suárez, V.J.; Redondo, L.; Rubio Zarapuz, A.; Martínez Guardado, I.; , . Exercise Interventions in Cancer-Related Cachexia. Encyclopedia. Available online: https://encyclopedia.pub/entry/23886 (accessed on 08 February 2026).
Navarro Jiménez E, Clemente-Suárez VJ, Redondo L, Rubio Zarapuz A, Martínez Guardado I, . Exercise Interventions in Cancer-Related Cachexia. Encyclopedia. Available at: https://encyclopedia.pub/entry/23886. Accessed February 08, 2026.
Navarro Jiménez, Eduardo, Vicente Javier Clemente-Suárez, Laura Redondo, Alejandro Rubio Zarapuz, Ismael Martínez Guardado, . "Exercise Interventions in Cancer-Related Cachexia" Encyclopedia, https://encyclopedia.pub/entry/23886 (accessed February 08, 2026).
Navarro Jiménez, E., Clemente-Suárez, V.J., Redondo, L., Rubio Zarapuz, A., Martínez Guardado, I., & , . (2022, June 09). Exercise Interventions in Cancer-Related Cachexia. In Encyclopedia. https://encyclopedia.pub/entry/23886
Navarro Jiménez, Eduardo, et al. "Exercise Interventions in Cancer-Related Cachexia." Encyclopedia. Web. 09 June, 2022.
Copy Citation
Cancer-related cachexia is a complex multifactorial phenomenon in which systemic inflammation plays a key role in the development and maintenance of the symptomatology. Pharmacological interventions seem to produce a positive effect on inflammatory state and cachexia. Nutritional interventions are focused on a high-energy diet with high-density foods and the supplementation with antioxidants, while physical activity is focused on strength-based training. The implementation of multidisciplinary non-pharmacological interventions in cancer-related cachexia could be an important tool to improve traditional treatments and improve patients’ quality of life.
cancer
cachexia
exercise
1. Cancer Related Cachexia
Cachexia is a multifactorial syndrome characterized by its affection in different tissues, organs, and metabolic pathways. Its axis of pathological action begins with a process of systemic inflammation, which results in a progressive loss of the patient’s weight. In this loss, the main characteristic is negative body recomposition, increasing adipose tissue, and decreasing metabolically active tissue and lean muscle mass. This process can adversely affect patients during cancer treatment, reducing their tolerance and response to treatments, resulting in a loss of quality of life and increased mortality in patients with advanced cancer [1].
Clinically, cachexia is defined as a weight loss greater than 5% in the previous 6 months or corresponding to 2–5% for patients with a body mass index (BMI) ≤ 20 kg/m2 or with reduced muscle mass (sarcopenia) [2]. According to the authors of the recent reviews, its prevalence is estimated at 15.8/10,000 in Europe (2013), 16.5/10,000 in the United States (2014), and 13.4/10,000 in Japan [3]. Indeed, its epidemiology varies depending on the type of cancer. The prevalence is 80% in patients with pancreatic and gastric cancer; 60% in patients with colon cancer, prostate cancer, lung cancer, and non-Hodgkin’s lymphoma; and about 40% in patients with breast cancer, sarcoma, and leukemia. Moreover, it is considered to be indirectly associated with 20% of all cancer-related deaths [4].
The main pathological feature is the deterioration of lean muscle mass, sarcopenia. However, there are a greater number of factors which are related to the appearance of sarcopenia. For this, Figure 1 offers a quick overview of them. However, the affection is multi-organic, affecting the intestine, heart, kidneys, and liver function and morphology. With its progression, related pathologies may arise, such as cardiac arrhythmias, hypoventilation, thromboembolic events, and cardiorenal disorders [1]. Indeed, cardiac dysfunction is present in a high percentage of cancer cachexia-induced patients [5]. Thus, understanding the relationship between the heart and skeletal muscles in the clinical course of the patient with cancer cachexia is essential, linking established measures for its treatment and prevention.
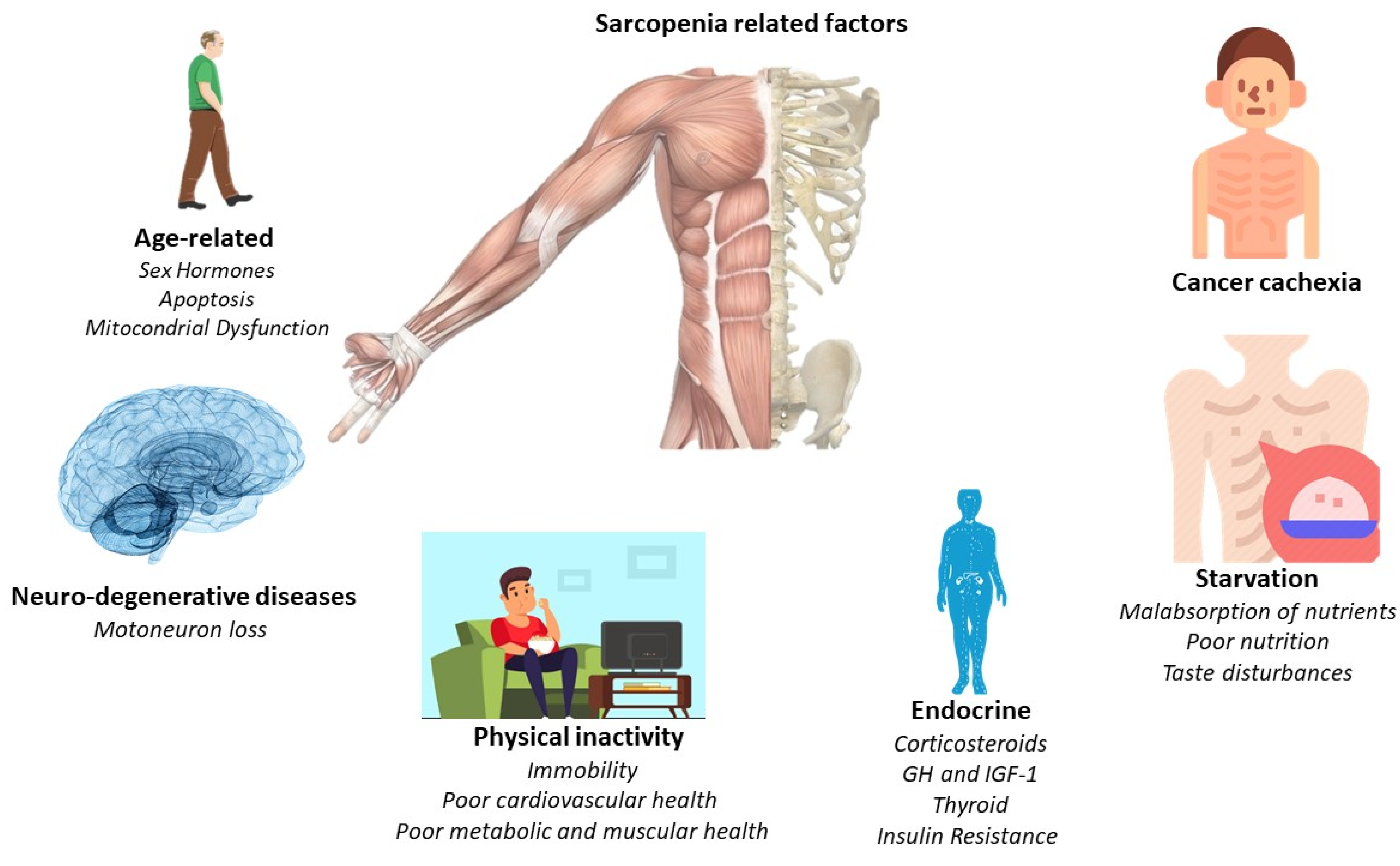
Figure 1. Principal sarcopenia-related factors.
1.1. Pathological Changes Underlying Muscle Atrophy in Cancer Cachexia
The subjacent mechanisms of cancer cachexia are multiple and intertwined. Factors related to skeletal and cardiac muscles (myokines and cardiokines) or factors secreted by cancer and cancer-associated immune cells (TGF-β, DAMPSs, and LIF) trigger a cascade of processes that ultimately result in cachexia. Circulating factors, intracellular signaling pathways, and atrophic end effectors lead to cardiac and skeletal muscle atrophy. Although the biological processes underlying cardiac and skeletal muscle atrophy are similar, their relative contribution and the specific molecular players involved differ slightly: damage-associated molecular patterns (DAMPs), growth differentiation factor 15 (GDF15), IL interleukins, leukemia inhibitory factor LIF, tumor necrosis factor alpha TNF-α, and transforming growth factor beta TGF-β [4].
1.2. Signaling Pathways Involved in Muscle Atrophy
The activation of inflammatory cytokines, specifically TNF, IL-1, and IL-6, leads to activation of NF-κB and FOXO (Figure 2). Binding of IL-6 to its receptor induces STAT3 expression, leading to the activation of the NF-κB pathway. The autophagy–lysosome system is activated by the transcription factor FOXO. Activation of p38 and JAK/MAPK leads to caspase-mediated apoptosis. Myostatin also activates protein degradation through FOXO and can decrease protein synthesis by inhibiting AKT through SMAD. Levels of insulin-like growth factor-1 (IGF-1) decrease during muscle atrophy, suppressing the IGF-1 pathway, thus inhibiting protein synthesis. The ubiquitin–proteasome system (UPS) is initiated by transcription of the E3 ubiquitin ligases MuRF-1 and MAFbx/atrogin-1: ActRIIB, activin receptor type IIB; TNFα, tumor necrosis factor-α; IL, interleukin; JAK, Janus kinase; STAT, signal transducers and activators of transcription; FOXO, Forkhead box transcription factors; mTOR, mammalian target of rapamycin; NF κB, nuclear factor-κB; MAPK, mitogen-activated protein kinase; MuRF-1, muscle RING finger protein-1; IGF-1, insulin-like growth factor-1; and MAFbx, muscle atrophy F-box protein 1 [1].
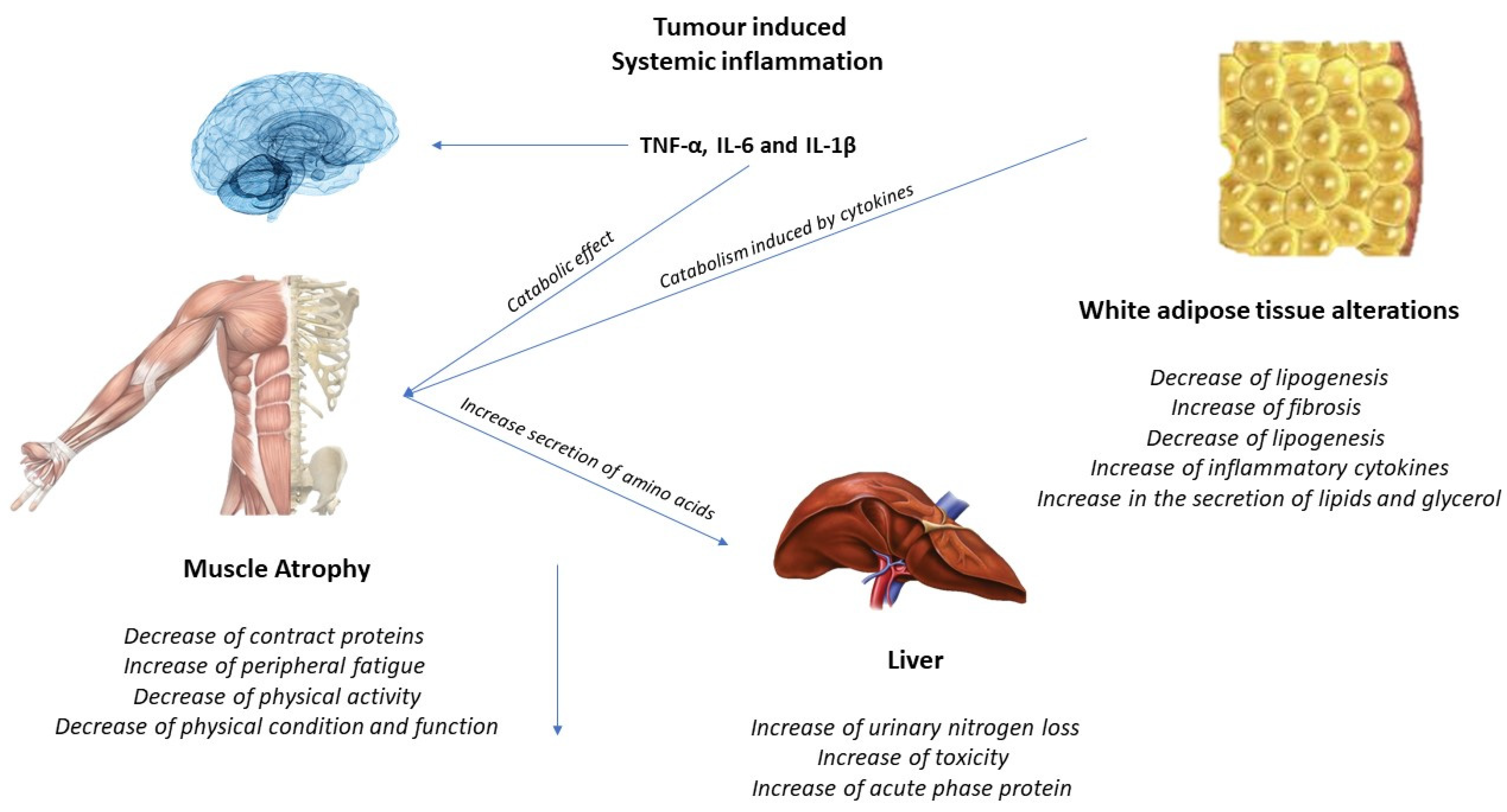
Figure 2. Tumor-induced systemic inflammation.
1.3. Effects of Exercise on the Muscle and Possible Mechanisms for the Treatment of Cachexia
Increased IL-6 may induce an anti-inflammatory effect, increasing IL-10 and IL-1ra and reducing TNF-alpha. A higher production of antioxidants than pro-oxidants may be responsible for the restoration of redox homeostasis during exercise. Increased activity of the PI3K/ALT/mTOR pathway and reduced activity of the ubiquitin proteasome and lysosomal autophagy pathways may induce protein homeostasis, increasing muscle synthesis and decreasing muscle degradation (Figure 3). PGC-1alpha activation can regulate genes involved in mitochondrial biogenesis and redox homeostasis (nuclear respiratory factors 1 and 2 and mitochondrial transcription factor A); increase GLUT-4 expression; regulate glucose metabolism; and decrease glucose metabolism, FOXO function, and proteolysis [6][7].
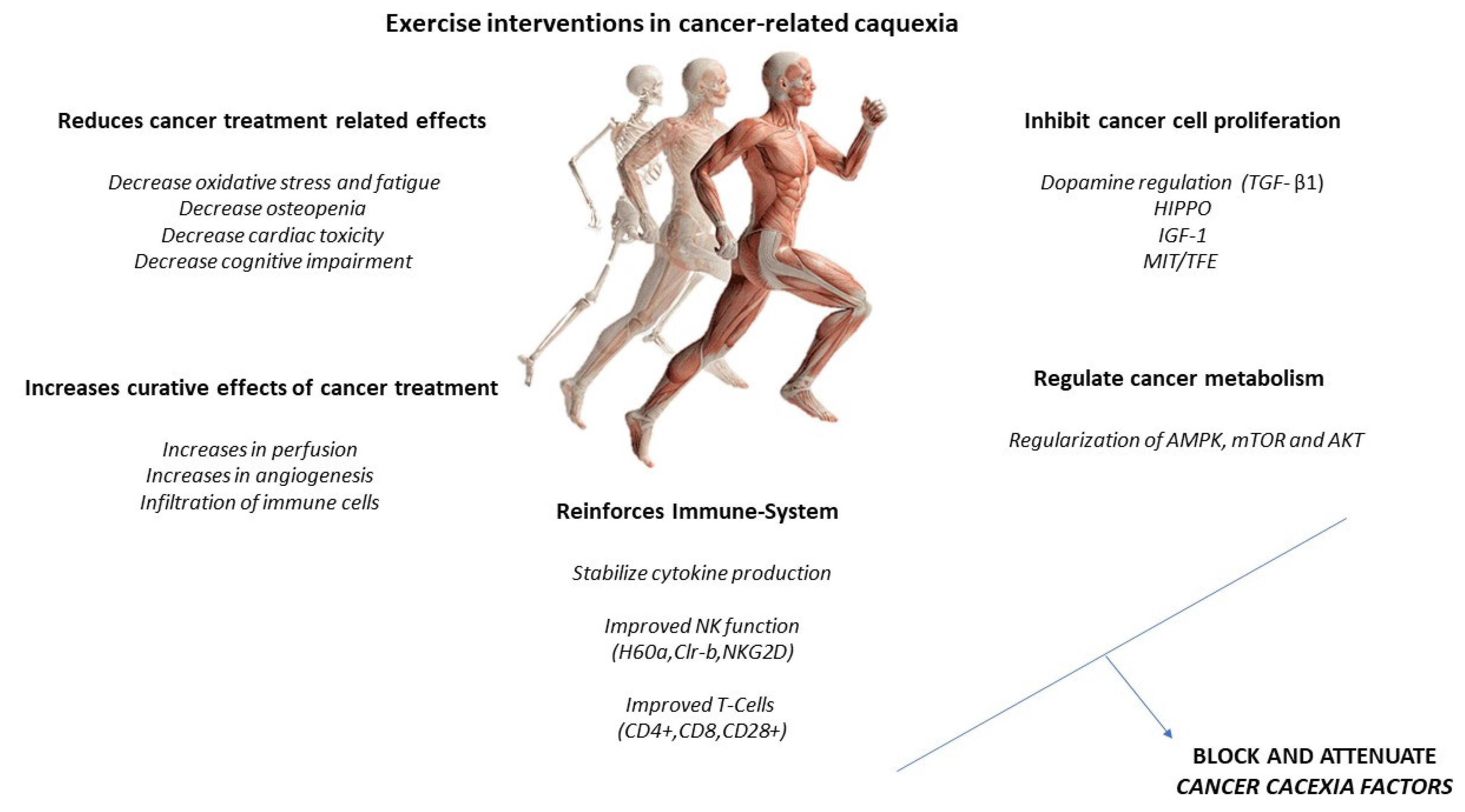
Figure 3. Exercise intervention in cancer-related cachexia.
However, treatment and guidelines for patients with cachectic cancer have yet to be fully developed. Systemic alterations common to cancer remain viable therapeutic targets for both preventing and treating the cachectic state. Focusing on skeletal muscle maintenance has the potential to reduce systemic inflammation and improve patients’ metabolism and overall physical function. Along these lines, metabolic health and muscle mass are dramatically affected by physical activity and exercise. It is clear that physical activity and exercise are beneficial during cancer treatment and survivorship and have clear potential as a non-pharmacological treatment for muscle-wasting conditions. However, successful randomized controlled trials in patients with cachectic cancer on muscle mass, metabolism, and physical function are lacking. Further research is needed to determine the mechanistic basis for these improvements and whether these benefits can be achieved.
2. Physical Exercise Interventions in the Cancer Patient: Cardiovascular Exercise
It is well documented that physical activity, specifically aerobic exercise, leads to an increase in aerobic capacity, cardiovascular function, and metabolic regulation in humans [8]. This modality is carried out continuously with an intensity that resides between values of maximal oxygen consumption, ranging from 40% to 70%; an activity duration between 30 to 60 min; and a frequency of 3 to 5 times for a week [9]. Despite that aerobic exercise increases the same degree of skeletal muscle hypertrophy as resistance exercise [10], it has been shown that it can also produce skeletal muscle hypertrophy through different physiological events, such as reduction in catabolic mRNA expression, mitochondrial biogenesis, and increased muscle protein synthesis [11][12][13]. In this line, it could be postulated as an interesting non-pharmacological strategy in older adults and other clinical populations, such as cancer patients, who are experiencing muscle loss [14]. In this line, several studies have reported that aerobic exercise produces the activation of AKT/mTOR pathway, reducing protein degradation [15][16] and decreasing intracellular ROS production by protecting muscles through antioxidant effect [17].
According to the progression of cancer cachexia, Fearon et al. [2] reported that it should be understood as a continuous process starting with pre-cachexia, moving toward cachexia, and ending with refractory cachexia. In this line, the practice of physical exercise evokes an anti-inflammatory response, which is a direct biomarker of muscle wasting [18][19][20][21][22][23][24][25][26][27] and one of the main problems associated with cachexia. However, most studies that have implemented an aerobic-exercise intervention to observe the effect on muscle loss during cachexia have been performed in animal models. In this line, a recent meta-analysis [28] established that the main cause of this fact is the late but rapid development of cachexia in the last stages of the disease, making it difficult to carry out RT or RCT in human models.
2.1. Aerobic Exercise Interventions in Animal Models
In the majority of studies using animal models, the development of cachexia was induced through tumor inoculation. In this line, Khamoui et al. [29] reported that 60 min/5 days per week in motorized wheels did not prevent weight loss but contributed to maintaining body function and recovering a percentage of muscle mass after tumor-induced cachexia in murine rodents. Moreover, Ballarò et al. [17] conducted a preclinical study in colon-carcinoma-bearing mice and informed that moderate exercise, 3-days at 11 m/min for 45 min in motorized wheels, significantly decreased body weight loss, increasing muscle mass and appetite compared to the control mice. Moreover, Gholamian et al. [30] reported that the group of female Balb/c mice that started aerobic physical exercise before breast-cancer induction and continued after it prevented muscle dysfunction and atrophy caused by tumor cachexia. Similar results were found by Shamsi et al. [31] after 12 weeks (6 weeks prior to breast cancer tumor injection and 6 weeks after it) aerobic training on a treadmill for 5 days/week in female Balb/c mice. In this line, Moreira et al. [32] reported that aerobic training performed during 8 weeks in treadmill running, 3 days/week, 44 min/day, at 55–65% VO2max, significantly decreased cachexia in adult male Wistar rats with an induced tumor. Furthermore, Alves et al. [33] showed that aerobic interval exercise, three bouts at 85% at maximal speed for 4 min, with intervals at 60% of maximal speed for 3 min, attenuated muscle loss in the plantaris muscle of rats bearing bone cancer. Despite the results, Niels et al. [28] established in a meta-analysis that, due to significant heterogeneity among designs (20 preclinical trials) and training modalities used, no real differences were observed between control and intervention (exercised mice) regarding body mass or muscle mass. Moreover, certain studies implemented training before inoculating the tumor in rodents and others after inoculation, and this may have affected the conclusions obtained.
2.2. Aerobic Exercise Interventions in Human Models
As mentioned above, due to the developmental characteristics of cachexia, it is difficult to establish the role of physical exercise in cancer patients. In this line, no RT or RCT has been carried out to evaluate the application of an aerobic exercise program in isolation in cancer patients with cachexia. In this sense, a recent meta-analysis [34] included only four RCTs that evaluated the effect of physical exercise on cancer cachexia. However, the authors reported that the evidence is very low, due to the low number of studies carried out, to establish an improvement in muscle mass loss after the application of an exercise program compared to traditional treatment. In addition, it seems that multimodal training (the combination of different physical training modalities as aerobic and strength exercises with medication and/or nutritional supplementation) could be a much more interesting strategy to obtain better results in this type of clinical population [35].
2.3. High-Intensity Interval Interventions
Despite the positive adaptations associated with aerobic-type strategies in cancer patients, high-intensity interval training emerged as a much more time-efficient strategy that is capable of promoting metabolic and brain adaptations to a greater extent than aerobic exercise [36][37]. In addition, HIIT has been shown to be a safe [38] and effective strategy to increase parameters related to cardiovascular fitness (VO2max) in cancer patients [39]. Due to these positive adaptations, it could be an effective strategy to reduce the loss of muscle mass caused by cancer [40]. In this line, Ahmadabadi et al. reported that 4 weeks of HIIT (six intervals of 3 min and 20 s at 80–95% VO2max, with one-minute recovery between each interval at 30–35% VO2max) reduced muscle wasting during tumor progression in breast-cancer-bearing mice with cachexia [41][42]. However, Alves et al. observed an improvement in running capacity and muscle contractility without changes in muscle mass after 16 days of five intervals of 3 min running at 18 m.min1, followed by 4 min running at 25 m.min−1, in mice with tumor induced [43]. In humans, Niels et al. showed a maintenance of weight and BMI and improvements in muscular strength during 7 months of high-intensity training (a combination of resistance and endurance training) in a 46-year-old male with a stage IV pancreatic cancer [44]. Although more studies are needed to assess the effect of a HIIT protocol on cachexia-induced muscle loss, current guidelines state that HIIT is safe, tolerable, and effective as part of a comprehensive cancer rehabilitation program [45].
3. Physical Exercise Interventions in the Cancer Patient: Strength Exercise
It is known that strength training (ST) improves strength and promotes skeletal muscle hypertrophy by progressively overloading the muscles through external loads [46]. In this line, this modality is associated with performing different exercises by using the bodyweight or other equipment developed with variable intensities commonly expressed as a percentage of a maximum repetition (RM) [9]. During cancer cachexia, signaling and metabolic pathways that increase protein synthesis are suppressed [47], leading to loss of muscle mass. However, it is well established that TS can increase protein synthesis and activate the mTORC1 pathway [48], which appears to be an important mechanism for hypertrophy and a potential strategy to mitigate cachexia-induced muscle loss [1][49]. Concretely, this kinase activates downstream substrates such as p70S6k, which is directly involved during the signaling pathway for muscle protein synthesis [18]. However, a recent review established that aerobic exercise could be more beneficial than resistance training in counteracting muscle loss during cachexia [19]. However, this conclusion is based on studies in animal models, due to the difficulty of finding patients who engage in long-term regular exercise during the development of the disease. In addition, aerobic training sessions may be too long to be carried out on a daily basis in conjunction with cancer treatment per se, which also consumes too much time of patients during and after treatment [20]. Thus, strength training sessions could be postulated as a good alternative to reduce the negative effects of cachexia in skeletal muscle to be able to be carried out in less time and in an indoor way. In this vein, Capozzi et al. [21] implemented a 12-week lifestyle intervention and progressive ST program in a cancer patient in radiation treatment or immediately after completion. Despite that intervention while undergoing treatment did not reduce the loss of lean body mass, the patients who carried out the training program until the end of the treatment showed greater adherence to physical exercise, a fact which may have important clinical implications.
Several studies have implemented a combination training program, such as concurrent training, in a patient with cancer. In this line, Newton et al. [22] reported that strength training on its own provided greater increases in appendicular muscle mass than strength training accompanied by more than 20–30 min of low-intensity aerobic training in prostate cancer patients. These results could be logical, taking into account the interference phenomenon that occurs when both modalities are combined [23]. However, 10 weeks of supervised aerobic and resistance training (60 min/day) in cancer patients showed an improvement and increase of antioxidant capacity and muscular strength, along with a reduction of protein markers and DNA oxidation [24]. In this line, Galvāo et al. [25] carried out a 12-week combination program of aerobic (15 to 20 min of cardiovascular exercises at 65 to 80% of patients’ max heart rate and a rated perceived exertion scale of 11 to 13) and strength (full-body workout with intensity progress from 12RM to 6RM and two to four sets per exercise). The results showed that patients undergoing exercise showed an increase in lean mass and strength level than patients treated with usual care. Moreover, 12 weeks of strength training in combination with electrical muscle stimulation performed twice a week significantly improved muscle mass and body weight in patients with advanced lung cancer [26]. In another study with 41 head-and-neck patients, the patients were divided into two experimental groups that carried out 12 weeks of strength training and 12 weeks of self-selected physical activity, but in a different order. After the intervention, both groups increased lean body mass and muscle strength at a similar level [27]. Furthermore, Donatto et al. [20] reported that mice that had a tumor induced but underwent eight sessions of resistance training exhibited a minor loss in total muscle weight and in the amount of glycogen in the gastrocnemius. These events are related to the main consequences during cachexia. Conversely, Khamoui et al. [29] established that neither mode of training (cardiovascular or strength training) was able to prevent bodyweight loss in rats. Furthermore, cardiovascular training appeared to be more beneficial than strength training, since the latter induced the expression of genes associated with muscle damage. However, Kamel et al. [48] reported improvements in muscle mass in both the upper and lower limbs of patients with pancreatic cachexia after 12 weeks of whole-body ST with an intensity of 50–80% of 1RM, three sets, and 8–12 repetitions than group control.
According to training intensity, HIIT can be a very interesting strategy to obtain positive adaptations in skeletal muscle in cancer patients. In this line, a recent meta-analysis has established that incorporating resistance exercises into this type of strategy can reduce the muscle wasting caused by cachexia by regulating the deficiencies in skeletal muscle and adipose tissue that occur during this phenomenon [49]. However, there is not much evidence of HIIT programs with resistance exercises, because this type of training has been shown to produce much lower exercise adherence rates than aerobic exercise [50]. In this line, only Cormie et al. performed a HIIT protocol with high- or low-intensity resistance exercises in women with lymphedema related to breast cancer. The training sessions for the group that trained with the highest intensity included six upper-body exercises and two lower-body exercises with high loads (75–85% of 1RM). Results showed that moderate-to-high-intensity resistance exercise significantly improves muscular strength and muscular endurance in this population than low-intensity exercise [51].
Although the results of the studies are very heterogeneous, as well as the methodologies used, it seems that strength training can increase or at least maintain the loss of muscle mass experienced during cachexia. However, it is recommended that this type of strategy be carried out in combination with other techniques (other training modality, nutrition, or supplementation control) to address it from a multimodal point of view, since cachexia comes from multiple metabolic pathways that contribute to this condition [35].
References
- World Health Organization WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available online: https://www.who.int/publications/i/item/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (accessed on 14 February 2022).
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896.
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174.
- Shpata, V.; Prendushi, X.; Kreka, M.; Kola, I.; Kurti, F.; Ohri, I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med. Arch. 2014, 68, 263–267.
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015, 162, 1229–1241.
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259.
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. 2), S51–S63.
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61.
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359.
- Grgic, J.; Mcllvenna, L.C.; Fyfe, J.J.; Sabol, F.; Bishop, D.J.; Schoenfeld, B.J.; Pedisic, Z. Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta-analysis. Sport. Med. 2019, 49, 233–254.
- Konopka, A.R.; Douglass, M.D.; Kaminsky, L.A.; Jemiolo, B.; Trappe, T.A.; Trappe, S.; Harber, M.P. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65A, 1201–1207.
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of human skeletal muscle mitochondrial biogenesis and quality control: Effects of age and aerobic exercise training. J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, 371–378.
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101.
- Morinaga, M.; Sako, N.; Isobe, M.; Lee-Hotta, S.; Sugiura, H.; Kametaka, S. Aerobic exercise ameliorates cancer cachexia-induced muscle wasting through adiponectin signaling. Int. J. Mol. Sci. 2021, 22, 3110.
- Hardee, J.P.; Montalvo, R.N.; Carson, J.A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid. Med. Cell. Longev. 2017, 2017, 8018197.
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991.
- Ballarò, R.; Penna, F.; Pin, F.; Gómez-Cabrera, M.C.; Viña, J.; Costelli, P. Moderate exercise improves experimental cancer cachexia by modulating the redox homeostasis. Cancers 2019, 11, 285.
- Goodman, C.A.; Frey, J.W.; Mabrey, D.M.; Jacobs, B.L.; Lincoln, H.C.; You, J.S.; Hornberger, T.A. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J. Physiol. 2011, 589 Pt 22, 5485–5501.
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and exercise against muscle wasting during cancer cachexia. Cells 2020, 9, 2536.
- Donatto, F.F.; Neves, R.X.; Rosa, F.O.; Camargo, R.G.; Ribeiro, H.; Matos-Neto, E.M.; Seelaender, M. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour-bearing rats. Cytokine 2013, 61, 426–432.
- Capozzi, L.C.; McNeely, M.L.; Lau, H.Y.; Reimer, R.A.; Giese-Davis, J.; Fung, T.S.; Culos-Reed, S.N. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer 2016, 122, 1185–1200.
- Newton, R.U.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Wall, B.A.; Bolam, K.A.; Taaffe, D.R. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med. Sci. Sports Exerc. 2019, 51, 607–614.
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.C.; Loenneke, J.P.; Anderson, J.C. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 2012, 26, 2293–2307.
- Repka, C.P.; Hayward, R. Oxidative stress and fitness changes in cancer patients after exercise training. Med. Sci. Sports Exerc. 2016, 48, 607–614.
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J. Clin. Oncol. 2010, 28, 340–347.
- Schink, K.; Gaßner, H.; Reljic, D.; Herrmann, H.J.; Kemmler, W.; Schwappacher, R.; Meyer, J.; Eskofier, B.M.; Winkler, J.; Neurath, M.F.; et al. Assessment of gait parameters and physical function in patients with advanced cancer participating in a 12-week exercise and nutrition programme: A controlled clinical trial. Eur. J. Cancer Care 2020, 29, e13199.
- Lønbro, S.; Dalgas, U.; Primdahl, H.; Johansen, J.; Nielsen, J.L.; Aagaard, P.; Hermann, A.P.; Overgaard, J.; Overgaard, K. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—Results from the randomized DAHANCA 25B trial. Radiother. Oncol. 2013, 108, 314–319.
- Niels, T.; Tomanek, A.; Freitag, N.; Schumann, M. Can Exercise Counteract Cancer Cachexia? A Systematic Literature Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19, 1–14.
- Khamoui, A.V.; Park, B.S.; Kim, D.H.; Yeh, M.C.; Oh, S.L.; Elam, M.L.; Jo, E.; Arjmandi, B.H.; Salazar, G.; Grant, S.C.; et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism 2016, 65, 685–698.
- Gholamian, S.; Hosseini, S.R.A.; Rashidlamir, A.; Aghaalinejad, H. The effects of interval aerobic training on mesenchymal biomarker gene expression, the rate of tumor volume, and cachexia in mice with breast cancer. Iran. J. Basic Med. Sci. 2020, 23, 244–250.
- Molanouri Shamsi, M.; Chekachak, S.; Soudi, S.; Quinn, L.S.; Rangbar, K.; Chenari, J.; Yazdi, M.H.; Mahdavi, M. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine 2017, 90, 100–108.
- Moreira, V.M.; da Silva Franco, C.C.; Prates, K.V.; Gomes, R.M.; de Moraes, A.M.P.; Ribeiro, T.A.; Martins, I.P.; Previate, C.; Pavanello, A.; Matiusso, C.C.I.; et al. Aerobic exercise training attenuates tumor growth and reduces insulin secretion in walker 256 tumor-bearing rats. Front. Physiol. 2018, 9, 465.
- Alves, C.R.R.; Neves, W.D.; de Almeida, N.R.; Eichelberger, E.J.; Jannig, P.R.; Voltarelli, V.A.; Tobias, G.C.; Bechara, L.R.G.; de Paula Faria, D.; Alves, M.J.N.; et al. Exercise training reverses cancer-induced oxidative stress and decrease in muscle COPS2/TRIP15/ALIEN. Mol. Metab. 2020, 39, 101012.
- Grande, A.J.; Silva, V.; Sawaris Neto, L.; Teixeira Basmage, J.P.; Peccin, M.S.; Maddocks, M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2021, 2021, CD010804.
- Bordignon, C.; Dos Santos, B.S.; Rosa, D.D. Impact of cancer cachexia on cardiac and skeletal muscle: Role of exercise training. Cancers 2022, 14, 342.
- Karlsen, T.; Aamot, I.L.; Haykowsky, M.; Rognmo, Ø. High intensity interval training for maximizing health outcomes. Prog. Cardiovasc. Dis. 2017, 60, 67–77.
- Rodriguez, A.L.; Whitehurst, M.; Fico, B.G.; Dodge, K.M.; Ferrandi, P.J.; Pena, G.; Adelman, A.; Huang, C.J. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. 2018, 243, 1153–1160.
- Papadopoulos, E.; Santa Mina, D. Can we HIIT cancer if we attack inflammation? Cancer Causes Control 2018, 29, 7–11.
- O’Donovan, G.; Lee, I.M.; Hamer, M.; Stamatakis, E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern. Med. 2017, 177, 335–342.
- Callahan, M.J.; Parr, E.B.; Hawley, J.A.; Camera, D.M. Can High-Intensity Interval Training Promote Skeletal Muscle Anabolism? Sports Med. 2021, 51, 405–421.
- Ahmadabadi, F.; Saghebjoo, M.; Huang, C.J.; Saffari, I.; Zardast, M. The effects of high-intensity interval training and saffron aqueous extract supplementation on alterations of body weight and apoptotic indices in skeletal muscle of 4T1 breast cancer-bearing mice with cachexia. Appl. Physiol. Nutr. Metab. 2020, 45, 555–563.
- Ahmadabadi, F.; Saghebjoo, M.; Hoshyar, R. Decreased Liver Tissue Wasting following High-Intensity Interval Training through Apoptosis Signaling Suppression in Breast Tumor–Bearing Female Mice. Iran. Q. J. Breast Dis. 2020, 13, 49–58.
- Alves, C.R.R.; da Cunha, T.F.; da Paixão, N.A.; Brum, P.C. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015, 125, 9–14.
- Niels, T.; Tomanek, A.; Schneider, L.; Hasan, I.; Hallek, M.; Baumann, F.T. Exercise improves patient outcomes in advanced pancreatic cancer patient during medical treatment. Pancreat. Disord. Ther. 2018, 8, 193.
- Klika, R.J.; Stafford, L.H. Exercise Oncology: High-Intensity Interval Training for Cancer Survivors. ACSM’s Health Fit. J. 2021, 25, 44–53.
- Phillips, S.M.; Winett, R.A. Uncomplicated resistance training and health-related outcomes: Evidence for a public health mandate. Curr. Sports Med. Rep. 2010, 9, 208–213.
- Mavropalias, G.; Sim, M.; Taaffe, D.R.; Galvão, D.A.; Spry, N.; Kraemer, W.J.; Häkkinen, K.; Newton, R.U. Exercise medicine for cancer cachexia: Targeted exercise to counteract mechanisms and treatment side effects. J. Cancer Res. Clin. Oncol. 2022.
- Kamel, F.A.H.; Basha, M.A.; Alsharidah, A.S.; Salama, A.B. Resistance training impact on mobility, muscle strength and lean mass in pancreatic cancer cachexia: A randomized controlled trial. Clin. Rehabil. 2020, 34, 1391–1399.
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Mayo, X.; Liguori, G.; Humphreys, L.; Copeland, R.J.; Jiménez, A. Effects of high-intensity training on the quality of life of cancer patients and survivors: A systematic review with meta-analysis. Sci. Rep. 2021, 11, 15089.
- Plotnikoff, R.C.; Courneya, K.S.; Trinh, L.; Karunamuni, N.; Sigal, R.J. Aerobic physical activity and resistance training: An application of the theory of planned behavior among adults with type 2 diabetes in a random, national sample of Canadians. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 61.
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.; Saunders, C.; Zissiadis, Y.; Newton, R.U. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J. Cancer Surviv. 2013, 7, 413–424.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
14 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




