Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sayyar Shah | -- | 1019 | 2022-06-09 13:33:56 | | | |
| 2 | Sirius Huang | -5 word(s) | 1014 | 2022-06-10 03:18:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shah, S.; , .; Yuan, A. Synthesis of MoS2. Encyclopedia. Available online: https://encyclopedia.pub/entry/23882 (accessed on 07 February 2026).
Shah S, , Yuan A. Synthesis of MoS2. Encyclopedia. Available at: https://encyclopedia.pub/entry/23882. Accessed February 07, 2026.
Shah, Sayyar, , Aihua Yuan. "Synthesis of MoS2" Encyclopedia, https://encyclopedia.pub/entry/23882 (accessed February 07, 2026).
Shah, S., , ., & Yuan, A. (2022, June 09). Synthesis of MoS2. In Encyclopedia. https://encyclopedia.pub/entry/23882
Shah, Sayyar, et al. "Synthesis of MoS2." Encyclopedia. Web. 09 June, 2022.
Copy Citation
Molybdenum disulfide (MoS2), with a two-dimensional (2D) structure, has attracted huge research interest due to its unique electrical, optical, and physicochemical properties. MoS2 has been used as a co-catalyst for the synthesis of novel heterojunction composites with enhanced photocatalytic hydrogen production under solar light irradiation. Nanostructured MoS2 can be fabricated via both top-down and bottom-up approaches.
photocatalysis
heterojunction
layers structure materials
hydrogen production
1. Introduction
Molybdenum disulfide (MoS2), with a 2D nanostructure, has attracted huge attention due to its outstanding optical and electronic properties and promising applications [1][2][3][4][5]. MoS2 nanomaterials as co-catalysts are promising photocatalysts for hydrogen evolution reaction (HER) [1][6]. It is reported that the exposed edges of layers of MoS2 contain active sites for catalytic activity while its basal planes are mostly inactive [2][7].
Nanostructured MoS2 can be fabricated via both top-down and bottom-up approaches. In the case of the top-down method, the commercially available bulk crystal of MoS2 is physically downsized into MoS2 nanomaterials (Figure 1) [8][9][10], while in the bottom-up approach, MoS2 nanomaterials are synthesized via chemical reaction with small molecules using chemical vapor deposition (CVD) and hydrothermal or solvothermal methods, etc. [11][12][13]. Single layers, multilayers, nanoparticles, and quantum dots of MoS2 have also been reported [14][15][16][17]. Continued efforts have been reported for the fabrication of MoS2 nanomaterials via the top-down and bottom-up strategies [8][9][10][11][12][13][18][19][20][21][22][23][24].
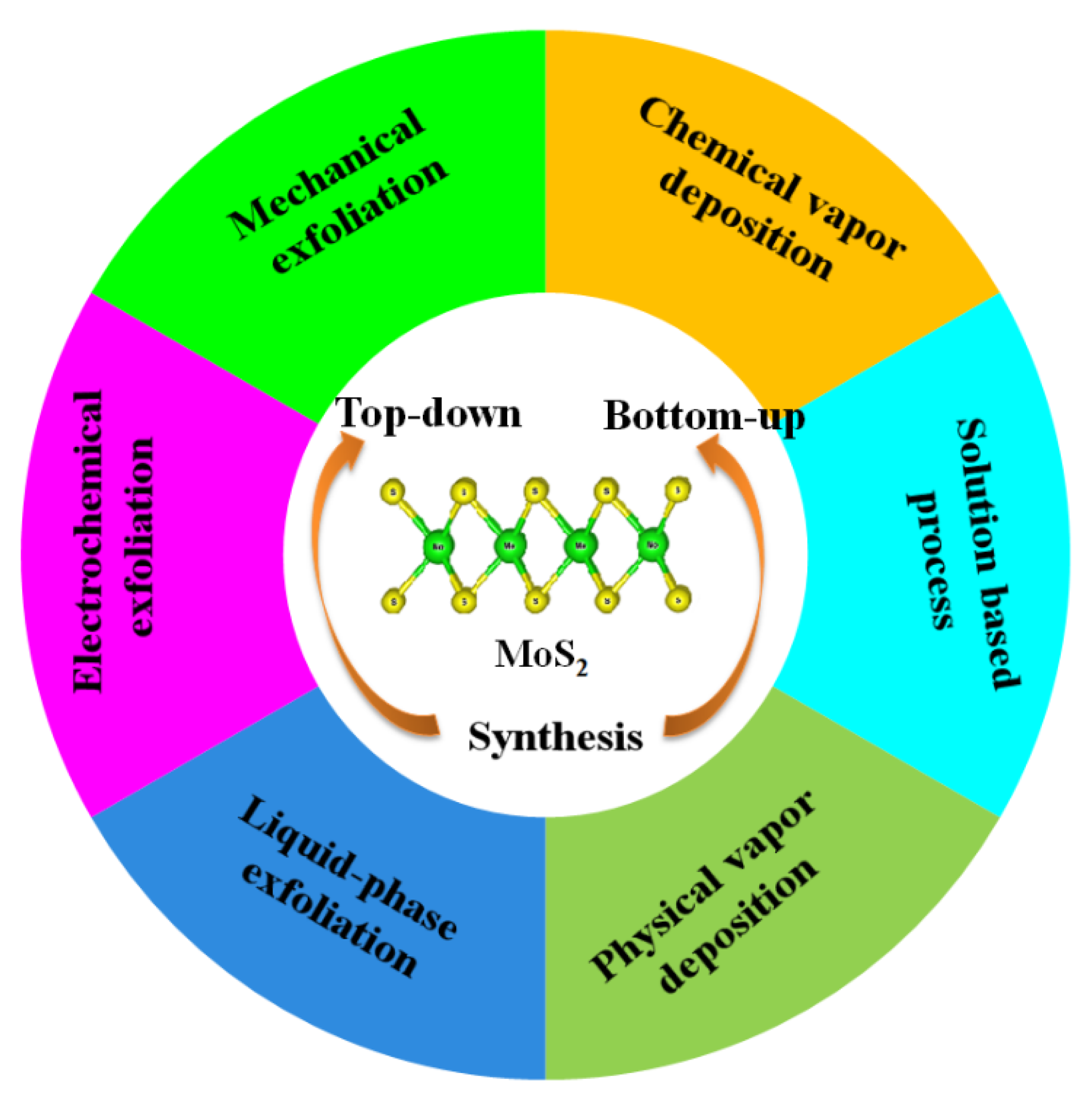
Figure 1. Various synthetic methods for MoS2 preparation.
2. Top-Down Approach
Exfoliation of MoS2
Due to the layered structure and van der Waals interactions, MoS2 nanosheets can be easily prepared through the exfoliation method. Mechanical, chemical, electrochemical, and liquid-phase exfoliation processes have been reported for the synthesis of MoS2 nanosheets [1][2][3][4][5][9][10][11][12][13][14][15][16][17]. For example, in the mechanical exfoliation technique, the suitable MoS2 flakes are peeled off from the bulk crystal of MoS2 using adhesive tape and shifted onto a specific substrate [6][15]. When the scotch tape is detached, some parts of MoS2 remain on the substrate. As result, single- or few-layer MoS2 nanosheets with random shapes and sizes are obtained. The 2D materials prepared by the exfoliation method have good quality and allow to study the pristine properties of materials and device performance. However, during this process, the thickness and size of the MoS2 are difficult to control, and the resulting materials are inappropriate for large-scale production and scaled-up applications [6][7]. Li et al., mechanically exfoliated single- and multilayer MoS2 nanosheets from SiO2/Si with the adhesive tape method [10]. The flakes of MoS2 were mechanically stripped on Si/SiO2 substrate. The obtained single-layer and multilayer MoS2 materials were characterized using a bright-field optical microscope and an atomic force microscope (AFM). From the AFM measurements, the height of a single MoS2 sheet was found to be 0.8 nm, while the thickness of two, three, and four layers of MoS2 nanosheets was 1.5, 2.1, and 2.9 nm, respectively. The MoS2 nanosheet monolayers showed an enhanced optical performance, especially single-layer MoS2 nanosheets. It was observed that the van der Waals interactions between MoS2 to SiO2 were much weaker. For this purpose, gold can be used as a substrate to exfoliate the MoS2 nanosheets due to its strong affinity for sulfur. It can exfoliate the MoS2 monolayer from the bulk because of the strong van der Waals interactions between Au and MoS2 layers [25][26][27]. Huang et al. prepared large-area MoS2 nanosheets using a Au-assisted exfoliation strategy [3]. In a typical synthesis, a Au thin layer was deposited on a Ti or Cr adhesion-covered substrate. To develop good contact between a MoS2 bulk crystal on tape and a Au-covered substrate, it should be passed under high pressure. The monolayer sheets with a large area were collected from the surface of the Au after peeling off the tape.
In the top-down approaches, single- and multilayer MoS2 nanosheets are prepared, which have been used to study some fundamental properties of MoS2 nanosheets.
3. Bottom-Up Approach
3.1. Chemical Vapor Deposition
The CVD technique has a long history and is commonly used for the synthesis of high-quality semiconductor materials. In a typical CVD process of MoS2 nanosheets, the Mo sources are solid precursors of Mo or MoO3 powder, and the S sources are H2S gas or solid S powder [28][29][30][31]. The solid MoO3 and vaporized S react with each other in a low-pressure chamber, forming nuclei for the growth of MoS2 [28]. Then, MoS2 slowly grows and enlarges its size on the substrates under carrier gas flow. The temperatures at which MoS2 grows during the CVD process are usually between 700 and 1000 °C, with a metal catalyst such as Au [31]. Plasma-enhanced CVD requires a low temperature (150–300 °C) for the growth of MoS2 nanosheets, and MoS2 can even be directly deposited on the plastic substrate [32]. Recently, metal organic CVD has been reported for the synthesis of MoS2 nanosheets [33][34], where organometallic precursors were used as starting materials.
3.2. Physical Vapor Deposition
Advanced technology such as molecular beam epitaxy (MBE) can be used to prepare single-crystal semiconductor thin films. However, its applications are limited to the synthesis of 2D materials [35]. Ordinary physical vapor deposition is rarely reported for 2D materials. A MoS2–Ti composite was prepared by direct current magnetron sputtering, using Ti and MoS2 materials [36]. In this process, the MoS2 was amorphous.
3.3. Solution-Based Process
Solution-based processes are commonly used to synthesize MoS2 nanosheets. Hydrothermal and solvothermal methods are the most interesting for the preparation of MoS2 nanosheets [37][38]. In these methods, the Mo source is commonly a molybdate, such as Na2MoO4 or (NH4)6Mo7O24, and the S source is thiourea and thioacetamide and L-cysteine [39][40][41][42][43]. The molybdate reacts with the S or S compound in a stainless steel autoclave. The physicochemical reaction takes place at high temperatures (160–200 °C) and pressure for at least a few hours. In the solvothermal method, organic solvents such as 1-methyl-2-pyrrolidinone, N,N-dimethylformamide, and polyethylene glycol-600 are used to proceed with the reaction, while in the hydrothermal method, water is used as a solvent. The MoS2 powders obtained from these methods have different sizes and shapes. The sizes and shapes of the products can be adjusted by altering the experimental conditions. To improve the crystalline quality of MoS2, the products are usually post-annealed at high temperature.
The MoS2 nanomaterials prepared through different bottom-up approaches have various sizes, shapes, morphologies, and thicknesses and can be used for many applications.
References
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578.
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2015, 164, 1–9.
- Shah, S.A.; Xu, L.; Sayyar, R.; Bian, T.; Liu, Z.; Yuan, A.; Shen, X.; Khan, I.; Tahir, A.A.; Ullah, H. Growth of MoS2 nanosheets on carbon particles (M = Co, Fe or CoFe Alloy) as an efficient electrocatalyst toward hydrogen evolution reaction. Chem. Eng. J. 2022, 428, 132126.
- Wilcoxon, J.P.; Newcomer, P.P.; Samara, G.A. Synthesis and optical properties of MoS2 and isomorphous nanoclusters in the quantum confinement regime. J. Appl. Phys. 1997, 81, 7934.
- Thurston, T.R.; Wilcoxon, J.P. Photooxidation of organic chemicals catalyzed by nanoscale MoS2. J. Phys. Chem. B 1999, 103, 11–17.
- Liu, H.; Li, Y.; Xiang, M.; Zeng, H.; Shao, X. Single-layered MoS2 directly grown on rutile TiO2 (110) for enhanced interfacial charge transfer. ACS Nano 2019, 13, 6083–6089.
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C 2018, 35, 39–55.
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075.
- Jeon, J.; Lee, J.; Yoo, G.; Park, J.H.; Yeom, G.Y.; Jang, Y.H.; Lee, S. Size-tunable synthesis of monolayer MoS2 nanoparticles and their applications in non-volatile memory devices. Nanoscale 2016, 8, 16995–17003.
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 quantum dots growth induced by S vacancy in ZnIn2S4 monolayer: Atomic-level heterostructure for photocatalytic hydrogen production. ACS Nano 2018, 12, 751–758.
- Sun, K.; Liu, M.; Pei, J.; Li, D.; Ding, C.; Wu, K.; Jiang, H. Incorporating transition-metal phosphides into metal-organic frameworks for enhanced photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 22749.
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating benzothiadiazole-based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting. Angew. Chem. Int. Ed. 2020, 59, 16902.
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578.
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2015, 164, 1–9.
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712.
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318.
- Cheng, P.; Sun, K.; Hu, Y.H. Memristive Behavior and Ideal Memristor of 1T Phase MoS2 Nanosheets. Nano Lett. 2016, 16, 572–576.
- Jeong, S.Y.; Song, J.; Lee, S. Photoelectrochemical device designs toward practical solar water splitting: A review on the recent progress of BiVO4 and BiFeO3 photoanodes. Appl. Sci. 2018, 8, 1388.
- Huang, Y.; Pan, Y.H.; Yang, R.; Bao, L.H.; Meng, L.; Luo, H.L.; Cai, Y.Q.; Liu, G.D.; Zhao, W.J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453.
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116.
- Ambrosi, A.; Pumera, M. Electrochemical exfoliation of MoS2 crystal for hydrogen electrogeneration. Chem. Eur. J. 2018, 24, 18551–18555.
- Liu, Y.; He, X.; Hanlon, D.; Harvey, A.; Coleman, J.N.; Li, Y. Liquid phase exfoliated MoS2 nanosheets percolated with carbon nanotubes for high volumetric/areal capacity sodium-ion batteries. ACS Nano 2016, 10, 8821–8828.
- Ottaviano, L.; Palleschi, S.; Perrozzi, F.; DOlimpio, G.; Priante, F.; Donarelli, M.; Benassi, P.; Nardone, M.; Gonchigsuren, M.; Gombosuren, M.; et al. Mechanical exfoliation and layer number identification of MoS2 revisited. 2D Mater. 2017, 4, 045013.
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469.
- Magda, G.; Peto, J.; Dobrik, G.; Hwang, C.; Biro, L.P.; Tapaszto, L. Exfoliation of large-area transition metal chalcogenide single layers. Sci. Rep. 2015, 5, 14714.
- Desai, S.B.; Madhvapathy, S.R.; Amani, M.; Kiriya, D.; Hettick, M.; Tosun, M.; Zhou, Y.; Dubey, M.; Ager, J.W.; Chrzan, D.; et al. Gold-mediated exfoliation of ultralarge optoelectronically-perfect monolayers. Adv. Mater. 2016, 28, 4053–4058.
- Velicky, M.; Donnelly, G.E.; Hendren, W.R.; McFarland, S.; Scullion, D.; DeBenedetti, W.J.I.; Correa, G.C.; Han, Y.; Wain, A.J.; Hines, M.A.; et al. Mechanism of Gold-assisted exfoliation of centimeter-sized transition-metal dichalcogenide monolayers. ACS Nano 2018, 12, 10463–10472.
- Lin, Y.C.; Zhang, W.; Huang, J.K.; Liu, K.K.; Lee, Y.H.; Liang, C.T.; Chu, C.W.; Li, L.J. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 2012, 4, 6637–6641.
- Cai, J.; Jian, J.; Chen, X.; Lei, M.; Wang, W. Regular hexagonal MoS2 microflakes grown from MoO3 precursor. Appl. Phys. A 2007, 89, 783–788.
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2014, 8, 966–971.
- Song, I.; Park, C.; Hong, M.; Baik, J.; Shin, H.-J.; Choi, H. Patternable large-scale molybdenium disulfide atomic layers grown by gold-assisted chemical vapor deposition. Angew. Chem. Int. Ed. 2014, 53, 1266–1269.
- Ahn, C.; Lee, J.; Kim, H.-U.; Bark, H.; Jeon, M.; Ryu, G.; Lee, Z.; Yeom, G.; Kim, K.; Jung, J.; et al. Low-temperature synthesis of large-scale molybdenum disulfide thin films directly on a plastic substrate using plasma-enhanced chemical vapor deposition. Adv. Mater. 2015, 27, 5223–5229.
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.; Mak, K.; Kim, C.-J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660.
- Kumar, V.; Dhar, S.; Choudhury, T.; Shivashankar, S.; Raghavan, S. A predictive approach to CVD of crystalline layers of TMDs: The case of MoS2. Nanoscale 2015, 7, 7802–7810.
- Vishwanath, S.; Liu, X.; Rouvimov, S.; Mende, P.; Azcatl, A.; McDonnell, S.; Wallace, R.; Feenstra, R.; Furdyna, J.; Jena, D.; et al. Comprehensive structural and optical characterization of MBE grown MoSe2 on graphite, CaF2 and graphene. 2D Mater. 2015, 2, 024007.
- Qin, X.; Ke, P.; Wang, A.; Kim, K. Microstructure, mechanical and tribological behaviors of MoS2-Ti composite coatings deposited by a hybrid HIPIMS method. Surf. Coat. Technol. 2013, 228, 275–281.
- Feng, X.; Tang, Q.; Zhou, J.; Fang, J.; Ding, P.; Sun, L.; Shi, L. Novel mixed–solvothermal synthesis of MoS2 nanosheets with controllable morphologies. Cryst. Res. Technol. 2013, 48, 363–368.
- Liu, Y.; Xu, X.; Li, H.; Si, Z.; Wu, X.; Ra, R.; Duan, W. A facile one step synthesis of MoS2/g-C3N4 photocatalyst with enhanced visible light photocatalytic hydrogen production. Catal. Lett. 2022, 152, 972–979.
- Deng, Z.; Hu, Y.; Ren, D.; Lin, S.; Jiang, H.; Li, C. Reciprocal hybridization of MoO2 nanoparticles and few-layer MoS2 for stable lithium-ion batteries. Chem. Commun. 2015, 51, 13838–13841.
- Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavicius, S.; Kurtinaitiene, M.; Trusovas, R. Substrate impact on the structure and electrocatalyst properties of molybdenum disulfide for HER from water. Metals 2020, 10, 1251.
- Guo, X.; Wang, Z.; Zhu, W.; Yang, H. The novel and facile preparation of multilayer MoS2 crystals by a chelation-assisted sol-gel method and their electrochemical performance. RSC Adv. 2017, 7, 9009–9014.
- Shah, S.A.; Shen, X.; Xie, M.; Zhu, G.; Ji, Z.; Zhou, H.; Xu, K.; Yue, X.; Yuan, A.; Zhu, J.; et al. 2 nanosheets: An efficient electrocatalyst for hydrogen evolution reaction. Small 2019, 15, 1804545.
- Shah, S.A.; Zhu, G.; Shen, X.; Kong, L.; Ji, Z.; Xu, K.; Zhou, H.; Zhu, J.; Song, P.; Song, C.; et al. Controllable sandwiching of reduced graphene oxide in hierarchical defect-rich MoS2 ultrathin nanosheets with expanded interlayer spacing for electrocatalytic hydrogen evolution reaction. Adv. Mater. Interfaces 2018, 5, 1801093.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




