Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Forogh Foroghi | -- | 2856 | 2022-06-08 06:10:51 | | | |
| 2 | Camila Xu | Meta information modification | 2856 | 2022-06-08 08:28:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Foroghi, F.; Roberts, S.; Carter, N.; Kawasaki, Y.; , .; Elikaee, S.; Alabdulal, M. Polyamine Metabolism in Leishmania Parasites. Encyclopedia. Available online: https://encyclopedia.pub/entry/23810 (accessed on 07 February 2026).
Foroghi F, Roberts S, Carter N, Kawasaki Y, , Elikaee S, et al. Polyamine Metabolism in Leishmania Parasites. Encyclopedia. Available at: https://encyclopedia.pub/entry/23810. Accessed February 07, 2026.
Foroghi, Forogh, Sigrid Roberts, Nicola Carter, Yumena Kawasaki, , Samira Elikaee, Mohammed Alabdulal. "Polyamine Metabolism in Leishmania Parasites" Encyclopedia, https://encyclopedia.pub/entry/23810 (accessed February 07, 2026).
Foroghi, F., Roberts, S., Carter, N., Kawasaki, Y., , ., Elikaee, S., & Alabdulal, M. (2022, June 08). Polyamine Metabolism in Leishmania Parasites. In Encyclopedia. https://encyclopedia.pub/entry/23810
Foroghi, Forogh, et al. "Polyamine Metabolism in Leishmania Parasites." Encyclopedia. Web. 08 June, 2022.
Copy Citation
Parasites of the genus Leishmania cause a variety of devastating and often fatal diseases in humans and domestic animals worldwide. The need for new therapeutic strategies is urgent because no vaccine is available, and treatment options are limited due to a lack of specificity and the emergence of drug resistance. Polyamines are metabolites that play a central role in rapidly proliferating cells, and recent studies have highlighted their critical nature in Leishmania. Numerous studies using a variety of inhibitors as well as gene deletion mutants have elucidated the pathway and routes of transport, revealing unique aspects of polyamine metabolism in Leishmania parasites.
Leishmania
polyamines
putrescine
spermidine
1. Introduction to Leishmania
Leishmaniasis is a neglected tropical disease that is endemic in nearly 90 countries across Africa, Asia, the Middle East, Europe, and Central and South America. More than 1 billion people are impacted by leishmaniasis worldwide with approximately 1 million new cases and 70,000 deaths occurring each year [1][2][3]. Poverty is a major risk factor underlying this disease, with increased human migration, civil war, and unrest contributing to recent outbreaks [2][3][4][5]. Furthermore, environmental damage such as deforestation, urbanization, and climate change have also contributed to a worldwide increases in leishmaniasis [2][5].
Leishmania parasites undergo two major morphological changes as they shuttle between the sandfly and mammalian host. The flagellated promastigote form resides in the gut of the sand fly, and the non-motile amastigote form inhabits the acidic phagolysosome within macrophages in the mammalian host. Transmission to the mammalian host occurs when an infected sand fly takes a bloodmeal. Parasites are phagocytosed by neutrophils, macrophages, or other antigen-presenting cells, although macrophages are the ultimate host cell [6][7][8]. Phagosomes containing internalized parasites merge with lysosomes to form phagolysosomes. Here, changes in temperature between the macrophage and sandfly, as well as the acidic pH of the phagolysosome, trigger the conversion of promastigotes to amastigotes and adaptation to the hostile host environment [9][10][11].
There are more than 20 species of Leishmania that infect humans and cause a spectrum of clinical symptoms. Visceral leishmaniasis (VL) is caused by L. donovani and L. infantum (L. chagasi) and is nearly always fatal if left untreated. It affects internal organs such as the spleen, liver, and bone marrow, with symptoms that include hepatosplenomegaly, fever, and weight loss. L. major, L. tropica, and L. mexicana are the main Leishmania species that cause cutaneous leishmaniasis (CL), which forms ulcerative skin lesions. A less common manifestation is mucocutaneous leishmaniasis (MCL), which may be caused by L. amazonenis and L. braziliensis and results in both sores and the destruction of mucous membranes in the nose, mouth, or throat [3][12].
Vaccines for preventing leishmaniasis in humans are not available, although recent clinical trials have shown some promise [13]. While three canine vaccines have been approved, these only provide partial protection [13][14]. Thus, drug therapy is crucial, but the currently available drugs for treating leishmaniasis are limited in number, have severe adverse effects, and are becoming sidelined by drug resistance [13]. Pentavalent antimonials were introduced over six decades ago, and despite significant and severe side effects associated with their administration, and widespread resistance to their antileishmanial effects, they are still used as a first-line treatment in many countries. Their mechanism of action is complex, although the inhibition of trypanothione reductase and disruption of trypanothione metabolism appears to contribute significantly to their mode of action [13][15]. Amphotericin B, an antifungal medication, binds to ergosterol that is present in the membranes of Leishmania and related parasites, as well as susceptible fungi, but it is absent in the human host. Amphotericin B is available as a deoxycholate salt, which causes nephrotoxicity and other significant side effects, or as liposomal formulation that has fewer associated adverse effects and improved pharmacokinetic properties, but it is expensive unless an access price has been negotiated [16]. Miltefosine is the only orally administered treatment approved for leishmaniasis. It appears to have multiple antileishmanial effects, including interference with phospholipid metabolism and the disruption of mitochondrial and acidocalcisome function [13][15][17]. Widespread use of miltefosine for treating leishmaniasis is limited by its teratogenic properties (the drug is contraindicated for use in pregnant women or in those that may become pregnant) and due to the emergence of drug-resistant strains [13][15][18]. Paromomycin, an aminoglycoside antibiotic, has been utilized with some success against VL and CL, but again, side effects such as hepatotoxicity occur and drug resistance have been demonstrated in the laboratory [13][15]. Pentamidine, an aromatic diamidine, is a second-line treatment that appears to act by accumulating in the mitochondria and binding to kinetoplastid DNA and topoisomerase II [13]. Combination therapy to reduce drug resistance and treatment failure have recently been attempted and met with mixed success. Clinical studies combining miltefosine with paromomycin or amphotericin B or combinations of antimonials with allopurinol were promising, while others found little advantage compared to monotherapy [13][19][20]. Further complicating therapeutic management is that Leishmania parasites may never disappear after clinical treatment but remain dormant at low numbers and can be reactivated [21][22][23][24]. This observation has stimulated recent interest in understanding the mechanisms that allow parasites to persist [21][25][26][27].
Thus, there is an urgent need for the identification and validation of new therapeutic targets. The polyamine biosynthetic pathway may be such a target. Polyamines are essential for Leishmania, and the parasite metabolic pathway shows significant differences to that of the mammalian host [28][29][30][31][32][33]. Furthermore, the polyamine pathway has been validated as a clinical target in the related parasite, Trypanosoma brucei [32][34][35][36][37].
2. Significance of Polyamines
The polyamines putrescine, spermidine, and spermine are small organic cations containing several amine groups that are positively charged under physiological conditions (Figure 1). Polyamines are ubiquitous and play critical roles in a variety of key processes, including growth, differentiation and macromolecular synthesis. Because of their relevance in rapidly proliferating cells, they have long been of interest in cancer and parasite research [32][33][38][39][40][41][42]. Furthermore, polyamines have shown protective properties, including anti-oxidant, anti-aging, and cardio- and neuroprotective functions [42][43][44][45][46]. In contrast, their catabolic by-products have been associated with tissue damage and diseases such as cancer, heart disease, and kidney failure [38][39][42][47][48][49]. Polyamine homeostasis is intricately regulated in mammalian cells and imbalances, such as excess polyamine production, have been associated with cancer and inflammatory disorders [38][42][45][46]. While the function and metabolism of polyamines have been extensively studied, particularly in the mammalian system, questions still remain about the spectrum of their effects in cells [42][45][46][50]. Despite the simple chemical structure of polyamines, they engage in complex interactions with cellular components and processes, which can be both specific and non-specific in nature [51]. Even less is known about the function or regulation of polyamines in parasites, despite their significance in parasite biology. This is underscored by recent studies highlighting their importance as potential therapeutic targets in trypanosomatids, which include Leishmania spp., T. brucei, and Trypanosoma cruzi [30][32][33][52][53][54]. The polyamine pathway in T. brucei, the causative agent of African sleeping sickness, is the target of the drug D, L-α-difluoromethylornithine (DFMO, eflornithine, ornidyl), an inhibitor of ornithine decarboxylase (ODC), that has shown remarkable therapeutic efficacy in the treatment of African trypanosomiasis [32][34][35][36][37].
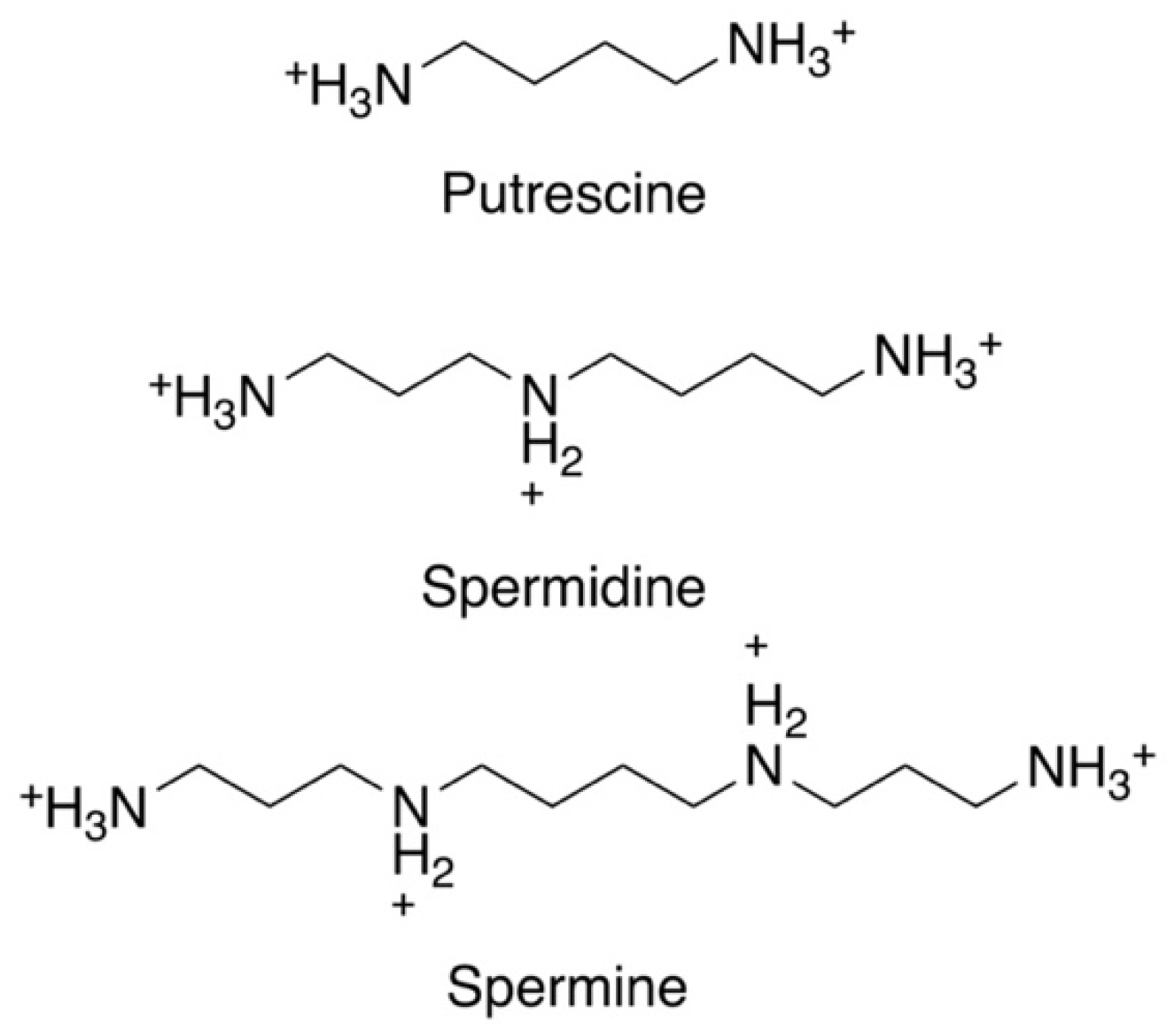 Figure 1. Chemical structures of the three main polyamines: putrescine, spermidine, and spermine.
Figure 1. Chemical structures of the three main polyamines: putrescine, spermidine, and spermine.The polyamine pathway of mammalian cells and Leishmania is depicted in Figure 2. The pathway can be primed by the enzyme arginase (ARG), which converts arginine to ornithine. In mammalian cells, ODC is considered to be the first and a rate-limiting enzyme, and it decarboxylates ornithine to form putrescine. Spermidine synthase (SPDSYN) and spermine synthase (SPMSYN) sequentially form spermidine and spermine by adding an aminopropyl group. The aminopropyl group is donated by decarboxylated S-adenosylmethionine, which is synthesized by S-adenosylmethionine decarboxylase (ADOMETDC). Spermine can be back-converted to spermidine and putrescine by the concerted action of spermidine/spermine N1-acetyltransferase and N1-acetylpolyamine oxidase. Spermine can also be converted to spermidine by spermine oxidase. In both scenarios, toxic side products such as hydrogen peroxide and reactive aldehydes are formed. A crucial downstream reaction of the polyamine pathway is the hypusination of eukaryotic translation initiation factor eIF5A, which requires spermidine for its activation.
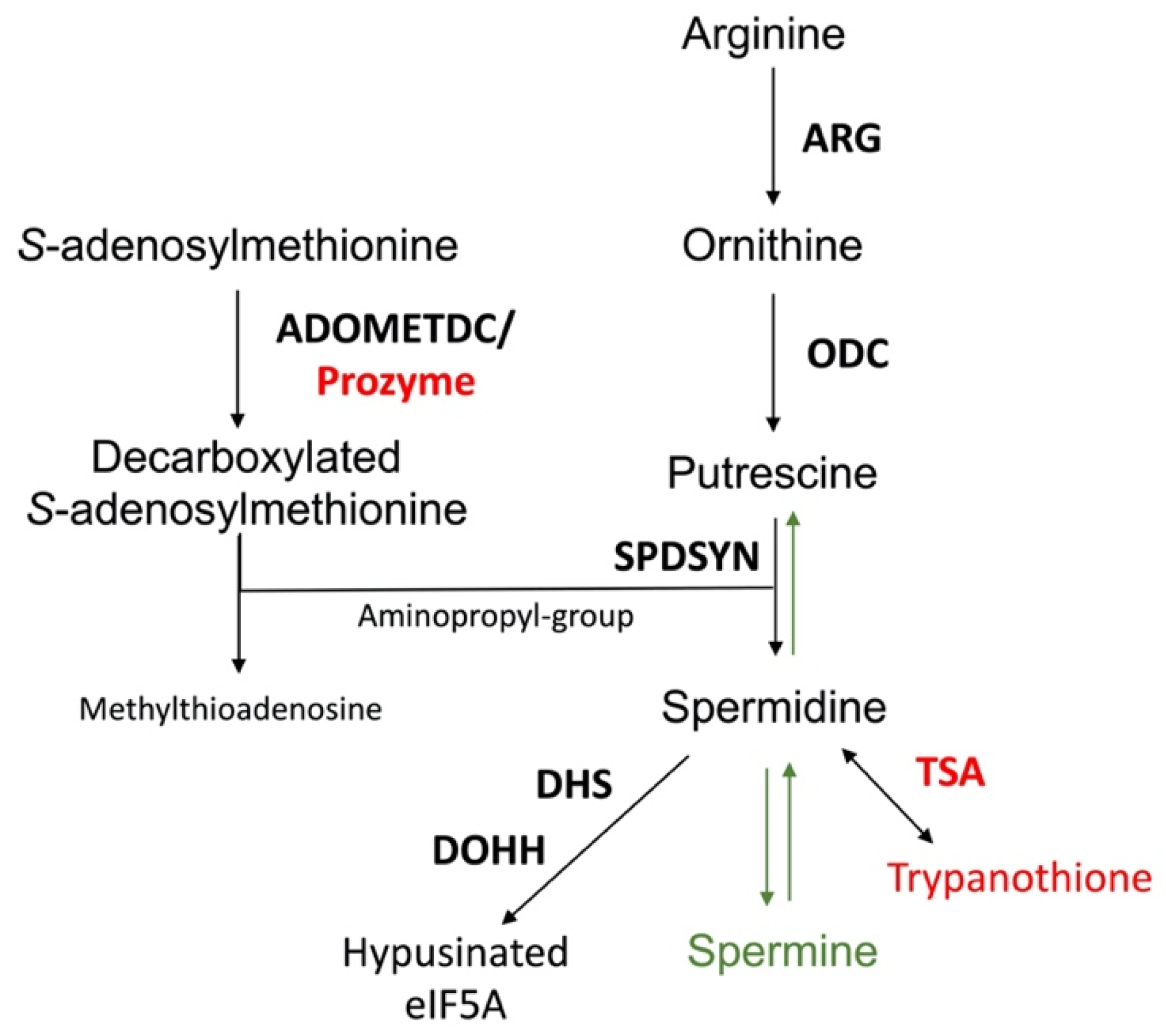 Figure 2. The polyamine biosynthetic pathway. Enzymes are shown in bold. The polyamine biosynthetic enzymes are arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC), the latter depicted with prozyme. Downstream reactions are catalyzed by trypanothione synthetase/amidase (TSA), a bifunctional enzyme that forms trypanothione, and by deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH) that in consecutive reactions catalyze the hypusination and activation of eIF5A. The enzymes and metabolites unique to trypanosomatids, prozyme, TSA, and trypanothione, are shown in red, while the synthesis of spermine and the back-conversion of spermidine to putrescine, present only in mammalian cells and not in Leishmania, are denoted in green.
Figure 2. The polyamine biosynthetic pathway. Enzymes are shown in bold. The polyamine biosynthetic enzymes are arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC), the latter depicted with prozyme. Downstream reactions are catalyzed by trypanothione synthetase/amidase (TSA), a bifunctional enzyme that forms trypanothione, and by deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH) that in consecutive reactions catalyze the hypusination and activation of eIF5A. The enzymes and metabolites unique to trypanosomatids, prozyme, TSA, and trypanothione, are shown in red, while the synthesis of spermine and the back-conversion of spermidine to putrescine, present only in mammalian cells and not in Leishmania, are denoted in green.The polyamine biosynthetic pathways of trypanosomatids are significantly different from that of the mammalian host [32][33][55] (Figure 2). For example, spermidine and spermine predominate in mammalian cells, while putrescine and spermidine are more abundant in trypanosomatids and other single cell organisms [56]. The polyamine spermine is neither produced nor utilized in trypanosomatids and the back-conversion of spermidine to putrescine does not exist [57][58][59]. Furthermore, while ODC and ADOMETDC are rapidly turned over in mammalian cells, these enzymes have a much longer half-life in trypanosomatid parasites [32][33][60][61][62]. Indeed, the selectivity of DFMO toward T. brucei is not due to disparate binding affinities to the parasite versus mammalian ODC enzymes, but rather that DFMO, as a covalently bound inhibitor, incapacitates the long-lived parasite ODC, while the human enzyme is rapidly replenished by resynthesis [61][63]. The trypanosomatid ADOMETDC is activated by the formation of a heterodimer with prozyme, which is a catalytically dead paralog of the parasite ADOMETDC. In a reaction unique to trypanosomatids, spermidine is conjugated to two glutathione molecules to produce trypanothione, which is the major intracellular thiol in Leishmania and other trypanosomatids and is essential for maintaining the intracellular redox balance and in oxidant defense [31][64][65]. The hypusination and activation of eIF5A occurs in both trypanosomatids and mammalian cells, although the enzyme deoxyhypusine synthase shows unique structural features in trypanosomatids [32][66][67][68].
Differences in the polyamine biosynthetic pathways also exist between the trypanosomatid parasites (Figure 3). In the mammalian host, Leishmania parasites live in phagolysosomes of macrophages, T. cruzi reside in the cytosol of host cells, and T. brucei are present in the bloodstream. In Leishmania parasites, the enzyme arginase (ARG) is considered to be the first enzyme of the polyamine biosynthetic pathway because the only essential role of ornithine is as precursor for polyamine biosynthesis [69][70][71][72]. Arginine is an essential amino acid and needs to be scavenged from the host for polyamine synthesis. Leishmania parasites contain ODC, SPDSYN, and ADOMETDC enzymes, while SPMSYN and back-conversion enzymes are absent. T. brucei, an extracellular parasite, contains an inactive ARG analog, and although the conversion of arginine to ornithine appears to occur, the majority of ornithine is salvaged from the host [32][73][74]. Because the levels of salvageable putrescine and spermidine in the bloodstream are too low (<1 µM) to meet the growth needs of T. brucei, the inhibition of ODC by DFMO is an effective therapeutic strategy for depleting putrescine in these parasites [32][75]. The polyamine pathway in T. cruzi parasites is substantially abbreviated. These parasites lack ARG and ODC [32][33][76], and they are dependent on putrescine salvage for growth and viability. They do, however, contain functional SPDSYN and ADOMETDC enzymes [77][78].
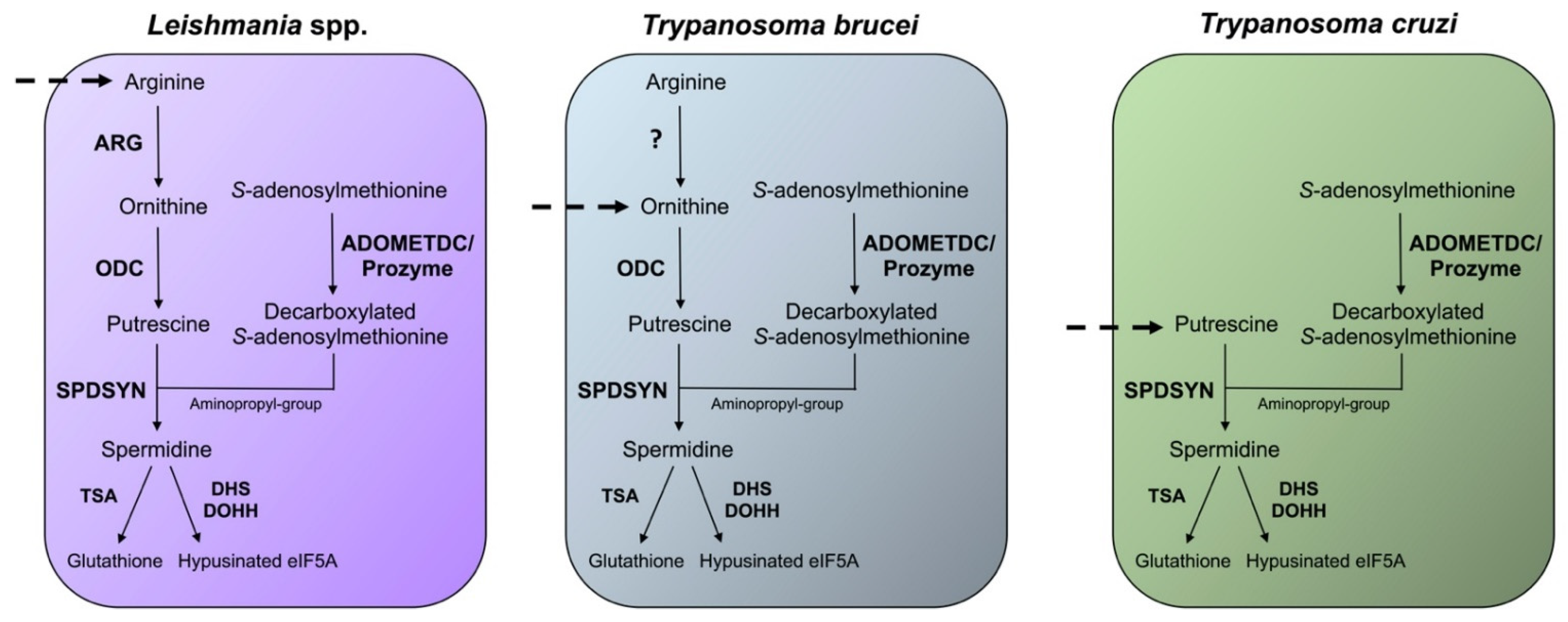 Figure 3. Differences in the polyamine biosynthetic pathways between trypanosomatids. The polyamine pathways of Leishmania spp., T. brucei, and T. cruzi are depicted. Enzymes are shown in bold. The polyamine biosynthetic enzymes spermidine synthase (SPDSYN), S-adenosylmethionine decarboxylase (ADOMETDC) with prozyme, trypanothione synthase (TSA), deoxyhypusine synthase (DHS), and deoxyhypusine hydroxylase (DOHH) are present in all three trypanosomatids. Ornithine decarboxylase (ODC) is missing in T. cruzi, and only Leishmania spp. appear to contain an active arginase (ARG). Dashed arrows represent the indispensable transport of polyamines or polyamine precursors.
Figure 3. Differences in the polyamine biosynthetic pathways between trypanosomatids. The polyamine pathways of Leishmania spp., T. brucei, and T. cruzi are depicted. Enzymes are shown in bold. The polyamine biosynthetic enzymes spermidine synthase (SPDSYN), S-adenosylmethionine decarboxylase (ADOMETDC) with prozyme, trypanothione synthase (TSA), deoxyhypusine synthase (DHS), and deoxyhypusine hydroxylase (DOHH) are present in all three trypanosomatids. Ornithine decarboxylase (ODC) is missing in T. cruzi, and only Leishmania spp. appear to contain an active arginase (ARG). Dashed arrows represent the indispensable transport of polyamines or polyamine precursors.3. Relevance of Polyamines for Host Parasite Interactions
Leishmania amastigotes reside within phagolysosomes in host macrophages. Studies with intracellular amastigotes, especially in intralesional macrophages, are difficult to perform, and thus, many questions remain about the host nutrient environment, as well as parasite nutrient requirements. It is well established that Leishmania are auxotrophic for purines, heme, certain vitamins, and several amino acids, and the supply of these essential nutrients is important for supporting parasite growth. Amino acids, sugars, and lipids are believed to be available in phagolysosomes, and it is probable that an array of host nutrients is delivered to the phagolysosome via fusion with phagocytic and endocytic vesicles [79][80][81][82][83]. Overall, a complex picture is emerging where the phagolysosome is both rich in some nutrients and limited in other metabolites [81].
Leishmania parasites modulate the host immune response and metabolism to favor their own survival [7][8][83][84][85]. Arginine, an essential amino acid, is required for polyamine biosynthesis, and Leishmania parasites compete with host macrophages for this metabolite [86][87][88]. In macrophages, arginine is a key substrate for two competing pathways: it can be converted to ornithine by ARG or alternatively into the potent antileishmanial agent nitric oxide by inducible nitric oxide synthase (iNOS) [89][90][91]. Two types of ARG are present in mammalian cells: type I, which is located in the cytosol, and type II, which is present in mitochondria. Numerous experiments and clinical observations have correlated an increased host ARG I expression and activity with augmented parasite loads and thus firmly established the mammalian ARG I as a key factor for Leishmania infections [89][92][93][94][95][96][97][98]. While it is not completely understood how increased levels of host ARG I contribute to disease exacerbation, one effect of higher ARG I activity may be the depletion of arginine levels, which would reduce the production of nitric oxide by iNOS. A local reduction in arginine has also been shown to impair the development of T cells, leading to suppression of the immune response and increased parasitemia [99][100][101]. It has furthermore been speculated that increased host ARG I activity leads to higher levels of host polyamines, which could be subsequently scavenged by intracellular parasites [82][90][91][95][102]. However, there is no direct evidence that Leishmania parasites salvage polyamines from the host, and murine infectivity studies with L. donovani polyamine pathway gene deletion mutants suggest otherwise (described below) [103][104].
There is only one ARG in Leishmania, and ARG gene deletion mutants have been generated in L. mexicana, L. major, L. amazonensis, and L. donovani [69][70][71][72]. ODC and SPDSYN gene deletion mutants have been created in L. donovani [103][104]. These mutants are valuable tools for assessing whether ornithine or polyamines are scavenged by intracellular parasites. Infectivity studies in mice demonstrated that the deletion of ARG caused reductions in infectivity compared to wild-type parasites in all four species investigated; however, infections were still established by these mutants [69][70][71]. In contrast, the L. donovani ODC and SPDSYN knockout parasites revealed profound reductions in infectivity [103][104]. The most dramatic drop of infectivity was observed with the L. donovani ODC gene deletion mutants, which showed an infectivity six orders of magnitude lower than wild-type parasites [103]. Similarly, the SPDSYN knockout strains exhibited a profound deficit in infectivity [104], and murine infectivity studies have also shown that L. donovani ADOMETDC gene deletion mutants exhibited a reduced infectivity phenotype (Buddy Ullman, personal communication).
The intriguing variations in intracellular survival across the various L. donovani mutants may be due to differences in the levels of salvageable ornithine versus polyamines in the phagolysosome. Accordingly, a model was proposed in which spermidine and especially putrescine availability is severely limited in macrophages under physiological conditions [69] (Figure 4). In support of this model, labeled arginine was rapidly converted to spermine (which parasites cannot utilize), and the levels of ornithine and spermine were increased in bone marrow-derived macrophages infected with L. major [95]. In contrast, only negligible amounts of putrescine and spermidine could be measured [95]. These results are consistent with other studies on differentiated mammalian cells (such as macrophages), which typically contain low levels of putrescine and spermidine [105][106].
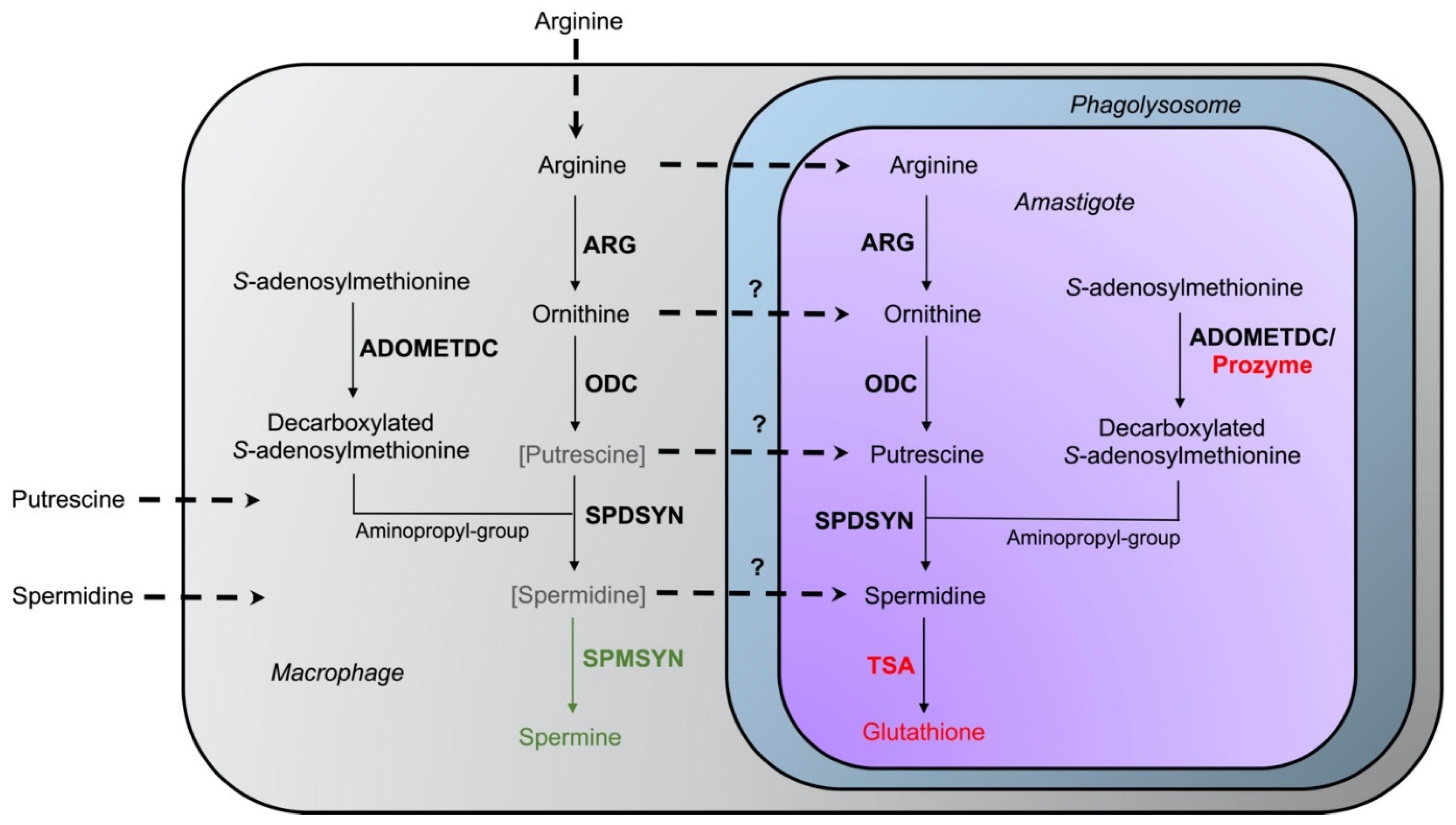 Figure 4. Interaction of host–parasite polyamine pathways. The enzymes arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC) are present in both host and parasite, while the enzyme spermine synthase (SPMSYN), displayed in green, can only be found in the host. The enzyme trypanothione synthetase/amidase (TSA) and prozyme, shown in red, are unique to the parasite. Putrescine and spermidine are bracketed and depicted in gray in the macrophage to indicate that only low amounts may be present in host cells. The uptake of arginine, ornithine, putrescine, and spermidine is represented in the dotted arrows. The question marks denote that the extent of transport of ornithine, putrescine, and spermidine into the phagolysosome and intracellular amastigote under physiological conditions is unclear.
Figure 4. Interaction of host–parasite polyamine pathways. The enzymes arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC) are present in both host and parasite, while the enzyme spermine synthase (SPMSYN), displayed in green, can only be found in the host. The enzyme trypanothione synthetase/amidase (TSA) and prozyme, shown in red, are unique to the parasite. Putrescine and spermidine are bracketed and depicted in gray in the macrophage to indicate that only low amounts may be present in host cells. The uptake of arginine, ornithine, putrescine, and spermidine is represented in the dotted arrows. The question marks denote that the extent of transport of ornithine, putrescine, and spermidine into the phagolysosome and intracellular amastigote under physiological conditions is unclear.Several studies indicate that when putrescine is supplied exogenously, it can be accessed by intracellular amastigotes within the phagolysosome. The addition of putrescine to the drinking water of mice infected with L. donovani ODC gene deletion mutants partially reversed the avirulent phenotype of the mutants [107]. Similarly, the addition of ornithine or putrescine to the media of infected macrophages increased the number of intracellular L. mexicana wild-type parasites and ARG gene deletion mutants [108]. These data suggest that it is possible that polyamines are normally limited under physiological conditions but that diets rich in polyamines may transiently increase their levels within infected cells. Additional research into determining polyamine levels in regular and stimulated macrophages, and particularly within the phagolysosome of infected cells, will shed light on the accessibility of host polyamines for Leishmania. Overall, however, the dramatically reduced infectivity phenotypes of L. donovani ODC, SPDSYN, and ADOMETDC gene deletion mutants bolsters these enzymes as promising therapeutic targets.
References
- World Health Organization. Leishmaniasis; World Health Organization: Geneva, Switzerland, 2020.
- Grifferty, G.; Shirley, H.; McGloin, J.; Kahn, J.; Orriols, A.; Wamai, R. Vulnerabilities to and the Socioeconomic and Psychosocial Impacts of the Leishmaniases: A Review. Res. Rep. Trop. Med. 2021, 12, 135–151.
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750.
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557.
- Hotez, P.J. The rise of leishmaniasis in the twenty-first century. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 421–422.
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2, 100041.
- Meira, C.D.S.; Gedamu, L. Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis. Microorganisms 2019, 7, 695.
- Kupani, M.; Pandey, R.K.; Mehrotra, S. Neutrophils and Visceral Leishmaniasis: Impact on innate immune response and cross-talks with macrophages and dendritic cells. J. Cell. Physiol. 2020, 236, 2255–2267.
- Tsigankov, P.; Gherardini, P.F.; Helmer-Citterich, M.; Späth, G.F.; Zilberstein, D. Phosphoproteomic Analysis of Differentiating Leishmania Parasites Reveals a Unique Stage-Specific Phosphorylation Motif. J. Proteome Res. 2013, 12, 3405–3412.
- Zilberstein, D. Lysosome Sensing Is a Key Mechanism in Leishmania Intracellular Development. Front. Microbiol. 2021, 12, 864.
- Zilberstein, D.; Shapira, M. The role of pH and temperature in the development of Leishmania parasites. Annu. Rev. Microbiol. 1994, 48, 449–470.
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132.
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554.
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557.
- Kumari, D.; Perveen, S.; Sharma, R.; Singh, K. Advancement in leishmaniasis diagnosis and therapeutics: An update. Eur. J. Pharmacol. 2021, 910, 174436.
- Berman, J. Amphotericin B Formulations and Other Drugs for Visceral Leishmaniasis. Am. J. Trop. Med. Hyg. 2015, 92, 471–473.
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P.M. Miltefosine Affects Lipid Metabolism in Leishmania donovani Promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430.
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; De Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597.
- Le Pape, P. Development of new antileishmanial drugs—Current knowledge and future prospects. J. Enzym. Inhib. Med. Chem. 2008, 23, 708–718.
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970.
- Bogdan, C. Mechanisms and consequences of persistence of intracellular pathogens: Leishmaniasis as an example. Cell. Microbiol. 2008, 10, 1221–1234.
- Conceição-Silva, F.; Leite-Silva, J.; Morgado, F. The Binomial Parasite-Host Immunity in the Healing Process and in Reactivation of Human Tegumentary Leishmaniasis. Front. Microbiol. 2018, 9, 1308.
- Gedda, M.R.; Singh, B.; Kumar, D.; Singh, A.K.; Madhukar, P.; Upadhyay, S.; Singh, O.P.; Sundar, S. Post kala-azar dermal leishmaniasis: A threat to elimination program. PLOS Neglected Trop. Dis. 2020, 14, e0008221.
- Mendonça, M.G.; De Brito, M.E.F.; Rodrigues, E.H.G.; Bandeira, V.; Jardim, M.L.; Abath, F.G.C. Persistence of Leishmania Parasites in Scars after Clinical Cure of American Cutaneous Leishmaniasis: Is There a Sterile Cure? J. Infect. Dis. 2004, 189, 1018–1023.
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Genet. 2019, 17, 607–620.
- Elmahallawy, E.K.; Alkhaldi, A.A.; Saleh, A.A. Host immune response against leishmaniasis and parasite persistence strategies: A review and assessment of recent research. Biomed. Pharmacother. 2021, 139, 111671.
- Mandell, M.A.; Beverley, S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA 2017, 114, E801–E810.
- Colotti, G.; Ilari, A. Polyamine metabolism in Leishmania: From arginine to trypanothione. Amino Acids 2010, 40, 269–285.
- Heby, O.; Persson, L.; Rentala, M. Targeting the polyamine biosynthetic enzymes: A promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids 2007, 33, 359–366.
- Ilari, A.; Fiorillo, A.; Baiocco, P.; Poser, E.; Angiulli, G.; Colotti, G. Targeting polyamine metabolism for finding new drugs against leishmaniasis: A review. Mini-Rev. Med. Chem. 2015, 15, 243–252.
- Ilari, A.; Fiorillo, A.; Genovese, I.; Colotti, G. Polyamine-trypanothione pathway: An update. Futur. Med. Chem. 2017, 9, 61–77.
- Phillips, M.A. Polyamines in protozoan pathogens. J. Biol. Chem. 2018, 293, 18746–18756.
- Roberts, S. Parasite Polyamines as Pharmaceutical Targets. Curr. Pharm. Des. 2017, 23, 3325–3341.
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252.
- Ebikeme, C. The Death and Life of the Resurrection Drug. PLOS Neglected Trop. Dis. 2014, 8, e2910.
- Eperon, G.; Balasegaram, M.; Potet, J.; Mowbray, C.; Valverde, O.; Chappuis, F. Treatment options for second-stage gambiense human African trypanosomiasis. Expert Rev. Anti-Infect. Ther. 2014, 12, 1407–1417.
- Logiudice, N.; Le, L.; Abuan, I.; Leizorek, Y.; Roberts, S.C. Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases. Med. Sci. 2018, 6, 12.
- Casero, R.A., Jr.; Stewart, T.M.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695.
- Damiani, E.; Wallace, H.M. Polyamines and Cancer. Methods Mol. Biol. 2018, 1694, 469–488.
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer pharmacoprevention: Targeting polyamine metabolism to manage risk factors for colon cancer. J. Biol. Chem. 2018, 293, 18770–18778.
- McNamara, K.M.; Gobert, A.P.; Wilson, K.T. The role of polyamines in gastric cancer. Oncogene 2021, 40, 4399–4412.
- Nakanishi, S.; Cleveland, J. Polyamine Homeostasis in Development and Disease. Med. Sci. 2021, 9, 28.
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168.
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788.
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406.
- Sagar, N.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44.
- Casero, R.; Stewart, T.; Devereux, W.; Hacker, A.; Wang, Y.; Smith, R.; Woster, P.; Woster, P. The role of polyamine catabolism in anti-tumour drug response. Biochem. Soc. Trans. 2003, 31, 361–365.
- Stewart, T.M.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745.
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamine Catabolism in Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 4790.
- Igarashi, K.; Kashiwagi, K. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 2018, 107, 104–115.
- Lightfoot, H.L.; Hall, J. Endogenous polyamine function—the RNA perspective. Nucleic Acids Res. 2014, 42, 11275–11290.
- Aoki, J.I.; Laranjeira-Silva, M.F.; Muxel, S.M.; Floeter-Winter, L.M. The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr. Opin. Microbiol. 2019, 52, 110–115.
- Bisceglia, J.; Mollo, M.C.; Gruber, N.; Orelli, L.R. Polyamines and Related Nitrogen Compounds in the Chemotherapy of Neglected Diseases Caused by Kinetoplastids. Curr. Top. Med. Chem. 2018, 18, 321–368.
- Carter, N.; Stamper, B.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267.
- Willert, E.; Phillips, M.A. Regulation and function of polyamines in African trypanosomes. Trends Parasitol. 2012, 28, 66–72.
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51.
- Jiang, Y.; Roberts, S.C.; Jardim, A.; Carter, N.S.; Shih, S.; Ariyanayagam, M.; Fairlamb, A.H.; Ullman, B. Ornithine Decarboxylase Gene Deletion Mutants of Leishmania donovani. J. Biol. Chem. 1999, 274, 3781–3788.
- Kaur, K.; Emmett, K.; McCANN, P.P.; Sjoerdsma, A.; Ullman, B. Effects of DL-alpha-difluoromethylornithine on Leishmania donovani promastigotes. J. Protozool. 1986, 33, 518–521.
- Singh, S.; Mukherjee, A.; Khomutov, A.R.; Persson, L.; Heby, O.; Chatterjee, M.; Madhubala, R. Antileishmanial Effect of 3-Aminooxy-1-Aminopropane Is Due to Polyamine Depletion. Antimicrob. Agents Chemother. 2007, 51, 528–534.
- Pegg, A.E.; Kameji, T.; Shirahata, A.; Stanley, B.; Madhubala, R.; Pajunen, A. Regulation of mammalian S-Adenosylmethionine decarboxylase. Adv. Enzym. Regul. 1988, 27, 31–39.
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal drugs: Mechanisms, resistance and new targets. Expert Rev. Mol. Med. 2009, 11, e31.
- Yerlikaya, A.; Stanley, B. S-Adenosylmethionine Decarboxylase Degradation by the 26 S Proteasome Is Accelerated by Substrate-mediated Transamination. J. Biol. Chem. 2004, 279, 12469–12478.
- Phillips, M.A.; Coffino, P.; Wang, C.C. Cloning and sequencing of the ornithine decarboxylase gene from Trypanosoma brucei. Implications for enzyme turnover and selective difluoromethylornithine inhibition. J. Biol. Chem. 1987, 262, 8721–8727.
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924.
- Singh, K.; Garg, G.; Ali, V. Current Therapeutics, Their Problems and Thiol Metabolism as Potential Drug Targets in Leishmaniasis. Curr. Drug Metab. 2016, 17, 897–919.
- Afanador, G.A.; Tomchick, D.; Phillips, M.A.; Afanador, G.A.; Tomchick, D.; Phillips, M.A.; Afanador, G.A.; Tomchick, D.; Phillips, M.A.; Afanador, G.A.; et al. Trypanosomatid Deoxyhypusine Synthase Activity Is Dependent on Shared Active-Site Complementation between Pseudoenzyme Paralogs. Structure 2018, 26, 1499–1512.
- Chawla, B.; Jhingran, A.; Singh, S.; Tyagi, N.; Park, M.H.; Srinivasan, N.; Roberts, S.C.; Madhubala, R. Identification and Characterization of a Novel Deoxyhypusine Synthase in Leishmania donovani. J. Biol. Chem. 2010, 285, 453–463.
- Nguyen, S.; Jones, D.C.; Wyllie, S.; Fairlamb, A.H.; Phillips, M.A. Allosteric Activation of Trypanosomatid Deoxyhypusine Synthase by a Catalytically Dead Paralog. J. Biol. Chem. 2013, 288, 15256–15267.
- Boitz, J.M.; Gilroy, C.A.; Olenyik, T.D.; Paradis, D.; Perdeh, J.; Dearman, K.; Davis, M.J.; Yates, P.A.; Li, Y.; Riscoe, M.K.; et al. Arginase Is Essential for Survival of Leishmania donovani Promastigotes but Not Intracellular Amastigotes. Infect. Immun. 2017, 85, e00554-16.
- Da Silva, M.F.L.; Zampieri, R.A.; Muxel, S.M.; Beverley, S.M.; Floeter-Winter, L.M. Leishmania amazonensis Arginase Compartmentalization in the Glycosome Is Important for Parasite Infectivity. PLoS ONE 2012, 7, e34022.
- Reguera, R.M.; Balaña-Fouce, R.; Showalter, M.; Hickerson, S.; Beverley, S.M. Leishmania major lacking arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol. Biochem. Parasitol. 2009, 165, 48–56.
- Roberts, S.C.; Tancer, M.J.; Polinsky, M.R.; Gibson, K.M.; Heby, O.; Ullman, B. Arginase Plays a Pivotal Role in Polyamine Precursor Metabolism in Leishmania. J. Biol. Chem. 2004, 279, 23668–23678.
- Hai, Y.; Kerkhoven, E.J.; Barrett, M.P.; Christianson, D.W. Crystal Structure of an Arginase-like Protein from Trypanosoma brucei That Evolved without a Binuclear Manganese Cluster. Biochemistry 2014, 54, 458–471.
- Vincent, I.M.; Creek, D.J.; Burgess, K.; Woods, D.J.; Burchmore, R.J.S.; Barrett, M.P. Untargeted Metabolomics Reveals a Lack Of Synergy between Nifurtimox and Eflornithine against Trypanosoma brucei. PLOS Negl. Trop. Dis. 2012, 6, e1618.
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149.
- Carrillo, C.; Cejas, S.; Cortés, M.; Ceriani, C.; Huber, A.; González, N.S.; Algranati, I.D. Sensitivity of Trypanosomatid Protozoa to DFMO and Metabolic Turnover of Ornithine Decarboxylase. Biochem. Biophys. Res. Commun. 2000, 279, 663–668.
- Amano, Y.; Namatame, I.; Tateishi, Y.; Honboh, K.; Tanabe, E.; Niimi, T.; Sakashita, H. Structural insights into the novel inhibition mechanism of Trypanosoma cruzi spermidine synthase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1879–1889.
- Persson, K.; Åslund, L.; Grahn, B.; Hanke, J.; Heby, O. Trypanosoma cruzi has not lost its S-adenosylmethionine decarboxylase: Characterization of the gene and the encoded enzyme. Biochem. J. 1998, 333, 527–537.
- McConville, M.J.; DE Souza, D.; Saunders, E.; Likic, V.A.; Naderer, T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007, 23, 368–375.
- McConville, M.J.; Naderer, T. Metabolic Pathways Required for the Intracellular Survival of Leishmania. Annu. Rev. Microbiol. 2011, 65, 543–561.
- McConville, M.J.; Saunders, E.C.; Kloehn, J.; Dagley, M.J. Leishmania carbon metabolism in the macrophage phagolysosome- feast or famine? F1000Research 2015, 4, 938.
- Naderer, T.; McConville, M.J. The Leishmania-macrophage interaction: A metabolic perspective. Cell. Microbiol. 2007, 10, 301–308.
- Saunders, E.C.; Sernee, M.F.; Ralton, J.E.; McConville, M.J. Metabolic stringent response in intracellular stages of Leishmania. Curr. Opin. Microbiol. 2021, 63, 126–132.
- Ferreira, C.; Estaquier, J.; Silvestre, R. Immune-metabolic interactions between Leishmania and macrophage host. Curr. Opin. Microbiol. 2021, 63, 231–237.
- Kima, P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007, 37, 1087–1096.
- Goldman-Pinkovich, A.; Kannan, S.; Nitzan-Koren, R.; Puri, M.; Pawar, H.; Bar-Avraham, Y.; McDonald, J.; Sur, A.; Zhang, W.-W.; Matlashewski, G.; et al. Sensing Host Arginine Is Essential for Leishmania Parasites’ Intracellular Development. mBio 2020, 11, e02023-20.
- Pawar, H.; Puri, M.; Weinberger, R.F.; Madhubala, R.; Zilberstein, D. The arginine sensing and transport binding sites are distinct in the human pathogen Leishmania. PLOS Negl. Trop. Dis. 2019, 13, e0007304.
- Zilberstein, D.; Myler, P.J. Arginine sensing in intracellular parasitism of Leishmania. Curr. Opin. Microbiol. 2021, 64, 41–46.
- Pessenda, G.; Da Silva, J.S. Arginase and its mechanisms in Leishmania persistence. Parasite Immunol. 2020, 42, e12722.
- Balaña-Fouce, R.; Calvo-Álvarez, E.; Álvarez-Velilla, R.; Prada, C.F.; Pérez-Pertejo, Y.; Reguera, R.M. Role of trypanosomatid’s arginase in polyamine biosynthesis and pathogenesis. Mol. Biochem. Parasitol. 2012, 181, 85–93.
- Wanasen, N.; Soong, L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 2007, 41, 15–25.
- Badirzadeh, A.; Taheri, T.; Taslimi, Y.; Abdossamadi, Z.; Heidari-Kharaji, M.; Gholami, E.; Sedaghat, B.; Niyyati, M.; Rafati, S. Arginase activity in pathogenic and non-pathogenic species of Leishmania parasites. PLOS Negl. Trop. Dis. 2017, 11, e0005774.
- Iniesta, V.; Carcelén, J.; Molano, I.; Peixoto, P.M.V.; Redondo, E.; Parra, P.; Mangas, M.; Monroy, I.; Campo, M.L.; Nieto, C.G.; et al. Arginase I Induction during Leishmania major Infection Mediates the Development of Disease. Infect. Immun. 2005, 73, 6085–6090.
- Iniesta, V.; Gomez-Nieto, L.C.; Molano, I.; Mohedano, A.; Carcelen, J.; Miron, C.; Alonso, C.; Corraliza, I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002, 24, 113–118.
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002.
- Mortazavi, H.; Sadeghipour, P.; Taslimi, Y.; Habibzadeh, S.; Zali, F.; Zahedifard, F.; Rahmati, J.; Kamyab, K.; Ghandi, N.; Zamanian, A.; et al. Comparing acute and chronic human cutaneous leishmaniasis caused byLeishmania major and Leishmania tropica focusing on arginase activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2118–2121.
- Müller, I.; Hailu, A.; Choi, B.-S.; Abebe, T.; Fuentes, J.M.; Munder, M.; Modolell, M.; Kropf, P. Age-Related Alteration of Arginase Activity Impacts on Severity of Leishmaniasis. PLOS Negl. Trop. Dis. 2008, 2, e235.
- Wilkins-Rodríguez, A.A.; Pérez-Torres, A.; Escalona-Montaño, A.R.; Gutiérrez-Kobeh, L. Differential Regulation of l -Arginine Metabolism through Arginase 1 during Infection with Leishmania mexicana Isolates Obtained from Patients with Localized and Diffuse Cutaneous Leishmaniasis. Infect. Immun. 2020, 88, e00963-19.
- Choi, B.-S.; Martinez-Falero, I.C.; Corset, C.; Munder, M.; Modolell, M.; Muller, I.; Kropf, P. Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages. J. Leukoc. Biol. 2008, 85, 268–277.
- Modolell, M.; Choi, B.-S.; Ryan, R.O.; Hancock, M.; Titus, R.G.; Abebe, T.; Hailu, A.; Müller, I.; Rogers, M.E.; Bangham, C.; et al. Local Suppression of T Cell Responses by Arginase-Induced L-Arginine Depletion in Nonhealing Leishmaniasis. PLOS Negl. Trop. Dis. 2009, 3, e480.
- Munder, M.; Choi, B.-S.; Rogers, M.; Kropf, P. L -Arginine deprivation impairs Leishmania major -specific T-cell responses. Eur. J. Immunol. 2009, 39, 2161–2172.
- Kropf, P.; Herath, S.; Weber, V.; Modolell, M.; Muller, I. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 2003, 25, 439–447.
- Boitz, J.M.; Yates, P.A.; Kline, C.; Gaur, U.; Wilson, M.E.; Ullman, B.; Roberts, S.C. Leishmania donovani Ornithine Decarboxylase Is Indispensable for Parasite Survival in the Mammalian Host. Infect. Immun. 2009, 77, 756–763.
- Gilroy, C.; Olenyik, T.; Roberts, S.C.; Ullman, B. Spermidine Synthase Is Required for Virulence of Leishmania donovani. Infect. Immun. 2011, 79, 2764–2769.
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732.
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894.
- Olenyik, T.; Gilroy, C.; Ullman, B. Oral putrescine restores virulence of ornithine decarboxylase-deficient Leishmania donovani in mice. Mol. Biochem. Parasitol. 2011, 176, 109–111.
- Gaur, U.; Roberts, S.C.; Dalvi, R.P.; Corraliza, I.; Ullman, B.; Wilson, M. An Effect of Parasite-Encoded Arginase on the Outcome of Murine Cutaneous Leishmaniasis. J. Immunol. 2007, 179, 8446–8453.
More
Information
Subjects:
Parasitology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
890
Revisions:
2 times
(View History)
Update Date:
10 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




