1. Introduction to Leishmania
Leishmaniasis is a neglected tropical disease that is endemic in nearly 90 countries across Africa, Asia, the Middle East, Europe, and Central and South America. More than 1 billion people are impacted by leishmaniasis worldwide with approximately 1 million new cases and 70,000 deaths occurring each year [
1,
2,
3]. Poverty is a major risk factor underlying this disease, with increased human migration, civil war, and unrest contributing to recent outbreaks [
2,
3,
4,
5]. Furthermore, environmental damage such as deforestation, urbanization, and climate change have also contributed to a worldwide increases in leishmaniasis [
2,
5].
Leishmania parasites undergo two major morphological changes as they shuttle between the sandfly and mammalian host. The flagellated promastigote form resides in the gut of the sand fly, and the non-motile amastigote form inhabits the acidic phagolysosome within macrophages in the mammalian host. Transmission to the mammalian host occurs when an infected sand fly takes a bloodmeal. Parasites are phagocytosed by neutrophils, macrophages, or other antigen-presenting cells, although macrophages are the ultimate host cell [
6,
7,
8]. Phagosomes containing internalized parasites merge with lysosomes to form phagolysosomes. Here, changes in temperature between the macrophage and sandfly, as well as the acidic pH of the phagolysosome, trigger the conversion of promastigotes to amastigotes and adaptation to the hostile host environment [
9,
10,
11].
There are more than 20 species of
Leishmania that infect humans and cause a spectrum of clinical symptoms. Visceral leishmaniasis (VL) is caused by
L. donovani and
L. infantum (
L. chagasi) and is nearly always fatal if left untreated. It affects internal organs such as the spleen, liver, and bone marrow, with symptoms that include hepatosplenomegaly, fever, and weight loss.
L. major,
L. tropica, and
L. mexicana are the main
Leishmania species that cause cutaneous leishmaniasis (CL), which forms ulcerative skin lesions. A less common manifestation is mucocutaneous leishmaniasis (MCL), which may be caused by
L. amazonenis and
L. braziliensis and results in both sores and the destruction of mucous membranes in the nose, mouth, or throat [
3,
12].
Vaccines for preventing leishmaniasis in humans are not available, although recent clinical trials have shown some promise [
13]. While three canine vaccines have been approved, these only provide partial protection [
13,
14]. Thus, drug therapy is crucial, but the currently available drugs for treating leishmaniasis are limited in number, have severe adverse effects, and are becoming sidelined by drug resistance [
13]. Pentavalent antimonials were introduced over six decades ago, and despite significant and severe side effects associated with their administration, and widespread resistance to their antileishmanial effects, they are still used as a first-line treatment in many countries. Their mechanism of action is complex, although the inhibition of trypanothione reductase and disruption of trypanothione metabolism appears to contribute significantly to their mode of action [
13,
15]. Amphotericin B, an antifungal medication, binds to ergosterol that is present in the membranes of
Leishmania and related parasites, as well as susceptible fungi, but it is absent in the human host. Amphotericin B is available as a deoxycholate salt, which causes nephrotoxicity and other significant side effects, or as liposomal formulation that has fewer associated adverse effects and improved pharmacokinetic properties, but it is expensive unless an access price has been negotiated [
16]. Miltefosine is the only orally administered treatment approved for leishmaniasis. It appears to have multiple antileishmanial effects, including interference with phospholipid metabolism and the disruption of mitochondrial and acidocalcisome function [
13,
15,
17]. Widespread use of miltefosine for treating leishmaniasis is limited by its teratogenic properties (the drug is contraindicated for use in pregnant women or in those that may become pregnant) and due to the emergence of drug-resistant strains [
13,
15,
18]. Paromomycin, an aminoglycoside antibiotic, has been utilized with some success against VL and CL, but again, side effects such as hepatotoxicity occur and drug resistance have been demonstrated in the laboratory [
13,
15]. Pentamidine, an aromatic diamidine, is a second-line treatment that appears to act by accumulating in the mitochondria and binding to kinetoplastid DNA and topoisomerase II [
13]. Combination therapy to reduce drug resistance and treatment failure have recently been attempted and met with mixed success. Clinical studies combining miltefosine with paromomycin or amphotericin B or combinations of antimonials with allopurinol were promising, while others found little advantage compared to monotherapy [
13,
19,
20]. Further complicating therapeutic management is that
Leishmania parasites may never disappear after clinical treatment but remain dormant at low numbers and can be reactivated [
21,
22,
23,
24]. This observation has stimulated recent interest in understanding the mechanisms that allow parasites to persist [
21,
25,
26,
27].
Thus, there is an urgent need for the identification and validation of new therapeutic targets. The polyamine biosynthetic pathway may be such a target. Polyamines are essential for
Leishmania, and the parasite metabolic pathway shows significant differences to that of the mammalian host [
28,
29,
30,
31,
32,
33]. Furthermore, the polyamine pathway has been validated as a clinical target in the related parasite,
Trypanosoma brucei [
32,
34,
35,
36,
37].
2. Significance of Polyamines
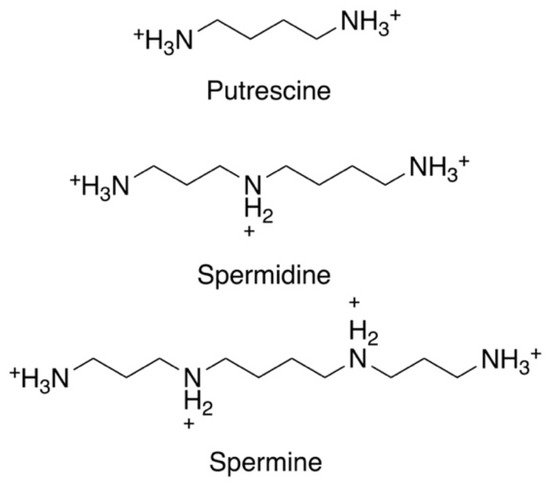
The polyamines putrescine, spermidine, and spermine are small organic cations containing several amine groups that are positively charged under physiological conditions (
Figure 1). Polyamines are ubiquitous and play critical roles in a variety of key processes, including growth, differentiation and macromolecular synthesis. Because of their relevance in rapidly proliferating cells, they have long been of interest in cancer and parasite research [
32,
33,
38,
39,
40,
41,
42]. Furthermore, polyamines have shown protective properties, including anti-oxidant, anti-aging, and cardio- and neuroprotective functions [
42,
43,
44,
45,
46]. In contrast, their catabolic by-products have been associated with tissue damage and diseases such as cancer, heart disease, and kidney failure [
38,
39,
42,
47,
48,
49]. Polyamine homeostasis is intricately regulated in mammalian cells and imbalances, such as excess polyamine production, have been associated with cancer and inflammatory disorders [
38,
42,
45,
46]. While the function and metabolism of polyamines have been extensively studied, particularly in the mammalian system, questions still remain about the spectrum of their effects in cells [
42,
45,
46,
50]. Despite the simple chemical structure of polyamines, they engage in complex interactions with cellular components and processes, which can be both specific and non-specific in nature [
51]. Even less is known about the function or regulation of polyamines in parasites, despite their significance in parasite biology. This is underscored by recent studies highlighting their importance as potential therapeutic targets in trypanosomatids, which include
Leishmania spp.,
T. brucei, and
Trypanosoma cruzi [
30,
32,
33,
52,
53,
54]. The polyamine pathway in
T. brucei, the causative agent of African sleeping sickness, is the target of the drug D, L-α-difluoromethylornithine (DFMO, eflornithine, ornidyl), an inhibitor of ornithine decarboxylase (ODC), that has shown remarkable therapeutic efficacy in the treatment of African trypanosomiasis [
32,
34,
35,
36,
37].
Figure 1. Chemical structures of the three main polyamines: putrescine, spermidine, and spermine.
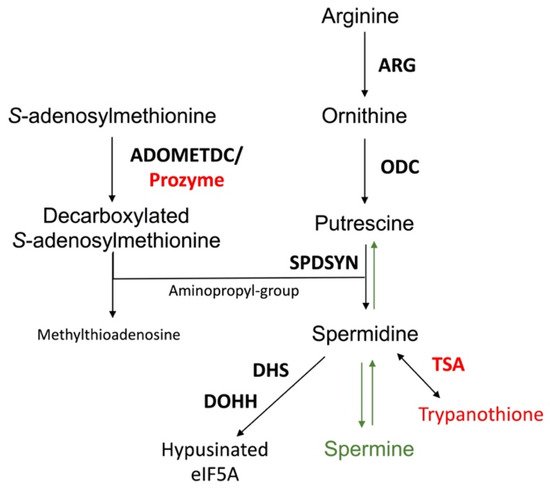
The polyamine pathway of mammalian cells and Leishmania is depicted in Figure 2. The pathway can be primed by the enzyme arginase (ARG), which converts arginine to ornithine. In mammalian cells, ODC is considered to be the first and a rate-limiting enzyme, and it decarboxylates ornithine to form putrescine. Spermidine synthase (SPDSYN) and spermine synthase (SPMSYN) sequentially form spermidine and spermine by adding an aminopropyl group. The aminopropyl group is donated by decarboxylated S-adenosylmethionine, which is synthesized by S-adenosylmethionine decarboxylase (ADOMETDC). Spermine can be back-converted to spermidine and putrescine by the concerted action of spermidine/spermine N1-acetyltransferase and N1-acetylpolyamine oxidase. Spermine can also be converted to spermidine by spermine oxidase. In both scenarios, toxic side products such as hydrogen peroxide and reactive aldehydes are formed. A crucial downstream reaction of the polyamine pathway is the hypusination of eukaryotic translation initiation factor eIF5A, which requires spermidine for its activation.
Figure 2. The polyamine biosynthetic pathway. Enzymes are shown in bold. The polyamine biosynthetic enzymes are arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC), the latter depicted with prozyme. Downstream reactions are catalyzed by trypanothione synthetase/amidase (TSA), a bifunctional enzyme that forms trypanothione, and by deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH) that in consecutive reactions catalyze the hypusination and activation of eIF5A. The enzymes and metabolites unique to trypanosomatids, prozyme, TSA, and trypanothione, are shown in red, while the synthesis of spermine and the back-conversion of spermidine to putrescine, present only in mammalian cells and not in Leishmania, are denoted in green.
The polyamine biosynthetic pathways of trypanosomatids are significantly different from that of the mammalian host [
32,
33,
55] (
Figure 2). For example, spermidine and spermine predominate in mammalian cells, while putrescine and spermidine are more abundant in trypanosomatids and other single cell organisms [
56]. The polyamine spermine is neither produced nor utilized in trypanosomatids and the back-conversion of spermidine to putrescine does not exist [
57,
58,
59]. Furthermore, while ODC and ADOMETDC are rapidly turned over in mammalian cells, these enzymes have a much longer half-life in trypanosomatid parasites [
32,
33,
60,
61,
62]. Indeed, the selectivity of DFMO toward
T. brucei is not due to disparate binding affinities to the parasite versus mammalian ODC enzymes, but rather that DFMO, as a covalently bound inhibitor, incapacitates the long-lived parasite ODC, while the human enzyme is rapidly replenished by resynthesis [
61,
63]. The trypanosomatid ADOMETDC is activated by the formation of a heterodimer with prozyme, which is a catalytically dead paralog of the parasite ADOMETDC. In a reaction unique to trypanosomatids, spermidine is conjugated to two glutathione molecules to produce trypanothione, which is the major intracellular thiol in
Leishmania and other trypanosomatids and is essential for maintaining the intracellular redox balance and in oxidant defense [
31,
64,
65]. The hypusination and activation of eIF5A occurs in both trypanosomatids and mammalian cells, although the enzyme deoxyhypusine synthase shows unique structural features in trypanosomatids [
32,
66,
67,
68].
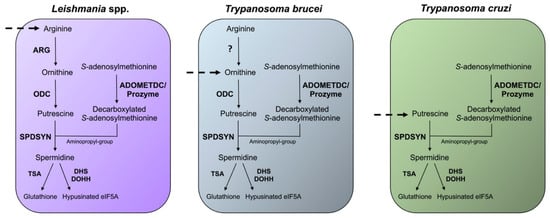
Differences in the polyamine biosynthetic pathways also exist between the trypanosomatid parasites (
Figure 3). In the mammalian host,
Leishmania parasites live in phagolysosomes of macrophages,
T. cruzi reside in the cytosol of host cells, and
T. brucei are present in the bloodstream. In
Leishmania parasites, the enzyme arginase (ARG) is considered to be the first enzyme of the polyamine biosynthetic pathway because the only essential role of ornithine is as precursor for polyamine biosynthesis [
69,
70,
71,
72]. Arginine is an essential amino acid and needs to be scavenged from the host for polyamine synthesis.
Leishmania parasites contain ODC, SPDSYN, and ADOMETDC enzymes, while SPMSYN and back-conversion enzymes are absent.
T. brucei, an extracellular parasite, contains an inactive ARG analog, and although the conversion of arginine to ornithine appears to occur, the majority of ornithine is salvaged from the host [
32,
73,
74]. Because the levels of salvageable putrescine and spermidine in the bloodstream are too low (<1 µM) to meet the growth needs of
T. brucei, the inhibition of ODC by DFMO is an effective therapeutic strategy for depleting putrescine in these parasites [
32,
75]. The polyamine pathway in
T. cruzi parasites is substantially abbreviated. These parasites lack ARG and ODC [
32,
33,
76], and they are dependent on putrescine salvage for growth and viability. They do, however, contain functional SPDSYN and ADOMETDC enzymes [
77,
78].
Figure 3. Differences in the polyamine biosynthetic pathways between trypanosomatids. The polyamine pathways of Leishmania spp., T. brucei, and T. cruzi are depicted. Enzymes are shown in bold. The polyamine biosynthetic enzymes spermidine synthase (SPDSYN), S-adenosylmethionine decarboxylase (ADOMETDC) with prozyme, trypanothione synthase (TSA), deoxyhypusine synthase (DHS), and deoxyhypusine hydroxylase (DOHH) are present in all three trypanosomatids. Ornithine decarboxylase (ODC) is missing in T. cruzi, and only Leishmania spp. appear to contain an active arginase (ARG). Dashed arrows represent the indispensable transport of polyamines or polyamine precursors.
3. Relevance of Polyamines for Host Parasite Interactions
Leishmania amastigotes reside within phagolysosomes in host macrophages. Studies with intracellular amastigotes, especially in intralesional macrophages, are difficult to perform, and thus, many questions remain about the host nutrient environment, as well as parasite nutrient requirements. It is well established that
Leishmania are auxotrophic for purines, heme, certain vitamins, and several amino acids, and the supply of these essential nutrients is important for supporting parasite growth. Amino acids, sugars, and lipids are believed to be available in phagolysosomes, and it is probable that an array of host nutrients is delivered to the phagolysosome via fusion with phagocytic and endocytic vesicles [
84,
85,
86,
87,
88]. Overall, a complex picture is emerging where the phagolysosome is both rich in some nutrients and limited in other metabolites [
86].
Leishmania parasites modulate the host immune response and metabolism to favor their own survival [
7,
8,
88,
89,
90]. Arginine, an essential amino acid, is required for polyamine biosynthesis, and
Leishmania parasites compete with host macrophages for this metabolite [
91,
92,
93]. In macrophages, arginine is a key substrate for two competing pathways: it can be converted to ornithine by ARG or alternatively into the potent antileishmanial agent nitric oxide by inducible nitric oxide synthase (iNOS) [
80,
94,
95]. Two types of ARG are present in mammalian cells: type I, which is located in the cytosol, and type II, which is present in mitochondria. Numerous experiments and clinical observations have correlated an increased host ARG I expression and activity with augmented parasite loads and thus firmly established the mammalian ARG I as a key factor for
Leishmania infections [
80,
96,
97,
98,
99,
100,
101,
102]. While it is not completely understood how increased levels of host ARG I contribute to disease exacerbation, one effect of higher ARG I activity may be the depletion of arginine levels, which would reduce the production of nitric oxide by iNOS. A local reduction in arginine has also been shown to impair the development of T cells, leading to suppression of the immune response and increased parasitemia [
103,
104,
105]. It has furthermore been speculated that increased host ARG I activity leads to higher levels of host polyamines, which could be subsequently scavenged by intracellular parasites [
87,
94,
95,
99,
106]. However, there is no direct evidence that
Leishmania parasites salvage polyamines from the host, and murine infectivity studies with
L. donovani polyamine pathway gene deletion mutants suggest otherwise (described below) [
107,
108].
There is only one ARG in
Leishmania, and
ARG gene deletion mutants have been generated in
L. mexicana,
L. major,
L. amazonensis, and
L. donovani [
69,
70,
71,
72].
ODC and
SPDSYN gene deletion mutants have been created in
L. donovani [
107,
108]. These mutants are valuable tools for assessing whether ornithine or polyamines are scavenged by intracellular parasites. Infectivity studies in mice demonstrated that the deletion of
ARG caused reductions in infectivity compared to wild-type parasites in all four species investigated; however, infections were still established by these mutants [
69,
70,
71]. In contrast, the
L. donovani ODC and
SPDSYN knockout parasites revealed profound reductions in infectivity [
107,
108]. The most dramatic drop of infectivity was observed with the
L. donovani ODC gene deletion mutants, which showed an infectivity six orders of magnitude lower than wild-type parasites [
107]. Similarly, the
SPDSYN knockout strains exhibited a profound deficit in infectivity [
108], and murine infectivity studies have also shown that
L. donovani ADOMETDC gene deletion mutants exhibited a reduced infectivity phenotype (Buddy Ullman, personal communication).
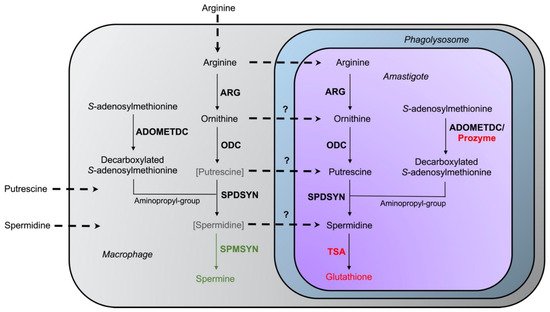
The intriguing variations in intracellular survival across the various
L. donovani mutants may be due to differences in the levels of salvageable ornithine versus polyamines in the phagolysosome. Accordingly, a model was proposed in which spermidine and especially putrescine availability is severely limited in macrophages under physiological conditions [
69] (
Figure 4). In support of this model, labeled arginine was rapidly converted to spermine (which parasites cannot utilize), and the levels of ornithine and spermine were increased in bone marrow-derived macrophages infected with
L. major [
99]. In contrast, only negligible amounts of putrescine and spermidine could be measured [
99]. These results are consistent with other studies on differentiated mammalian cells (such as macrophages), which typically contain low levels of putrescine and spermidine [
109,
110].
Figure 4. Interaction of host–parasite polyamine pathways. The enzymes arginase (ARG), ornithine decarboxylase (ODC), spermidine synthase (SPDSYN), and S-adenosylmethionine decarboxylase (ADOMETDC) are present in both host and parasite, while the enzyme spermine synthase (SPMSYN), displayed in green, can only be found in the host. The enzyme trypanothione synthetase/amidase (TSA) and prozyme, shown in red, are unique to the parasite. Putrescine and spermidine are bracketed and depicted in gray in the macrophage to indicate that only low amounts may be present in host cells. The uptake of arginine, ornithine, putrescine, and spermidine is represented in the dotted arrows. The question marks denote that the extent of transport of ornithine, putrescine, and spermidine into the phagolysosome and intracellular amastigote under physiological conditions is unclear.
Several studies indicate that when putrescine is supplied exogenously, it can be accessed by intracellular amastigotes within the phagolysosome. The addition of putrescine to the drinking water of mice infected with
L. donovani ODC gene deletion mutants partially reversed the avirulent phenotype of the mutants [
111]. Similarly, the addition of ornithine or putrescine to the media of infected macrophages increased the number of intracellular
L. mexicana wild-type parasites and
ARG gene deletion mutants [
112]. These data suggest that it is possible that polyamines are normally limited under physiological conditions but that diets rich in polyamines may transiently increase their levels within infected cells. Additional research into determining polyamine levels in regular and stimulated macrophages, and particularly within the phagolysosome of infected cells, will shed light on the accessibility of host polyamines for
Leishmania. Overall, however, the dramatically reduced infectivity phenotypes of
L. donovani ODC,
SPDSYN, and
ADOMETDC gene deletion mutants bolsters these enzymes as promising therapeutic targets.
This entry is adapted from the peer-reviewed paper 10.3390/medsci10020024