2. In Vitro Evidence Demonstrating the Direct Effects of Statins on Macrophages
An abundance of in vitro studies have reported paradoxical statin-mediated effects on inflammation (Table 2, Figure 2 and Figure 3), resulting from either blunting or enhancing pro-inflammatory signalling cascades. However, a limited number of studies have also reported that statins may alter the differentiation of macrophages rather than simply acting as regulators of inflammatory signalling pathways.
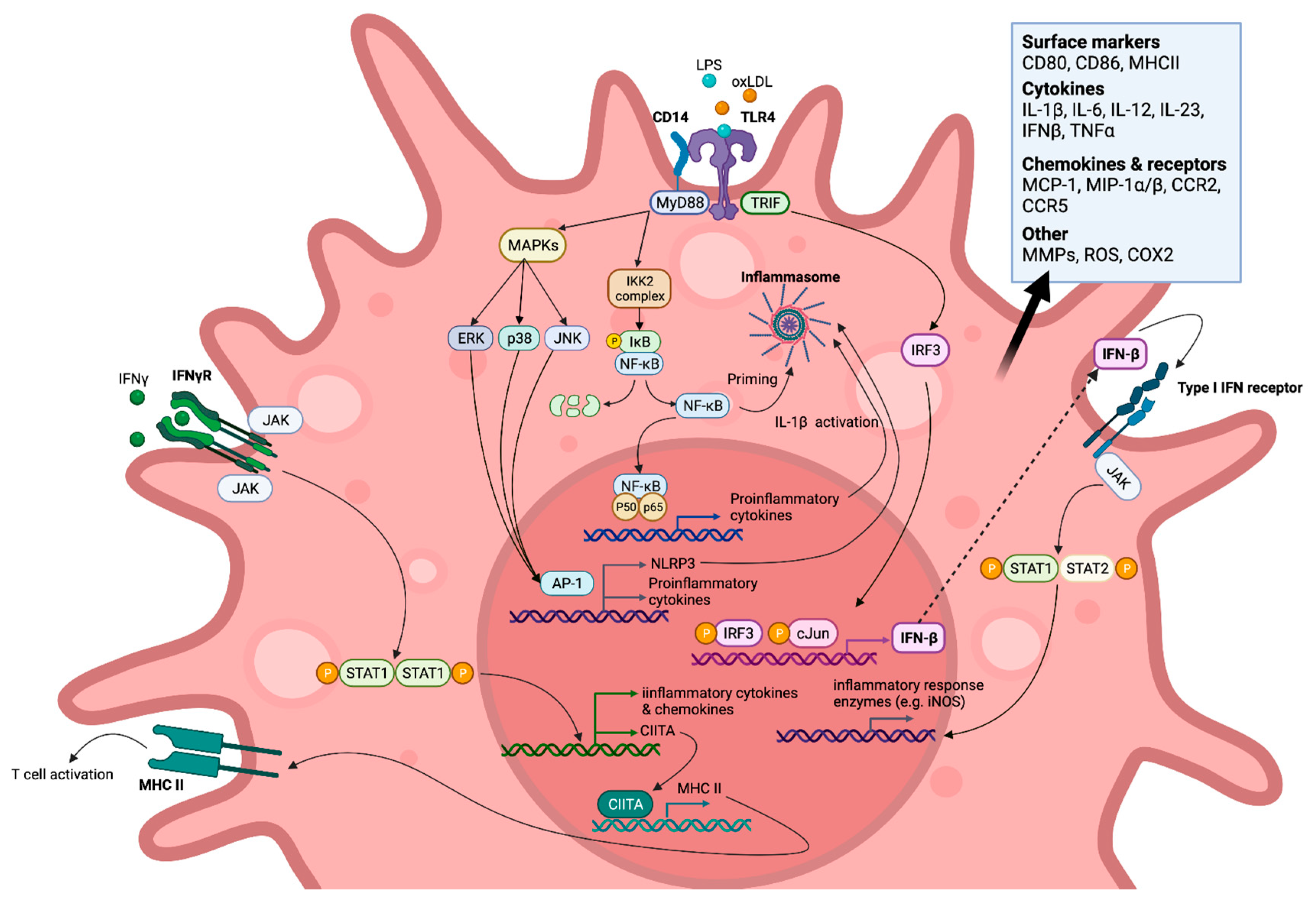
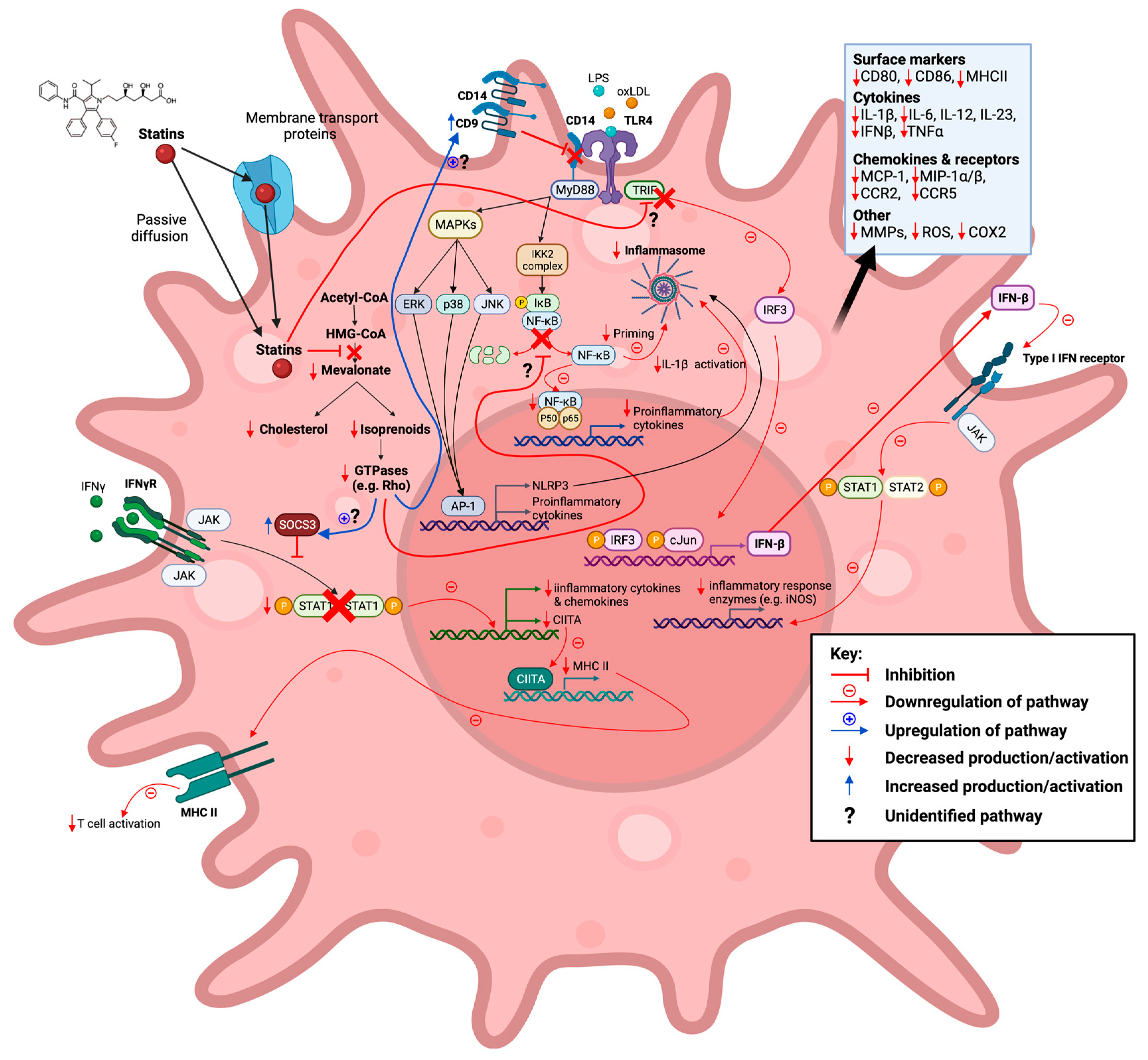
Figure 2. Statins inhibit the mevalonate pathway leading to both reduced cholesterol and isoprenoid biosynthesis, thereby also blocking farnesylation and geranylgeranylation of GTPases. Reduction in these downstream mevalonate intermediates is demonstrated to affect M1-associated macrophage inflammatory signalling pathways in vitro in an anti-inflammatory manner. This action of statins is seen in response to exogenous lipopolysaccharide (LPS), endogenous (interferon gamma (IFN-γ), and oxidized low-density lipoprotein (oxLDL) ligands. Created with
BioRender.com, accessed on 7 March 2022.
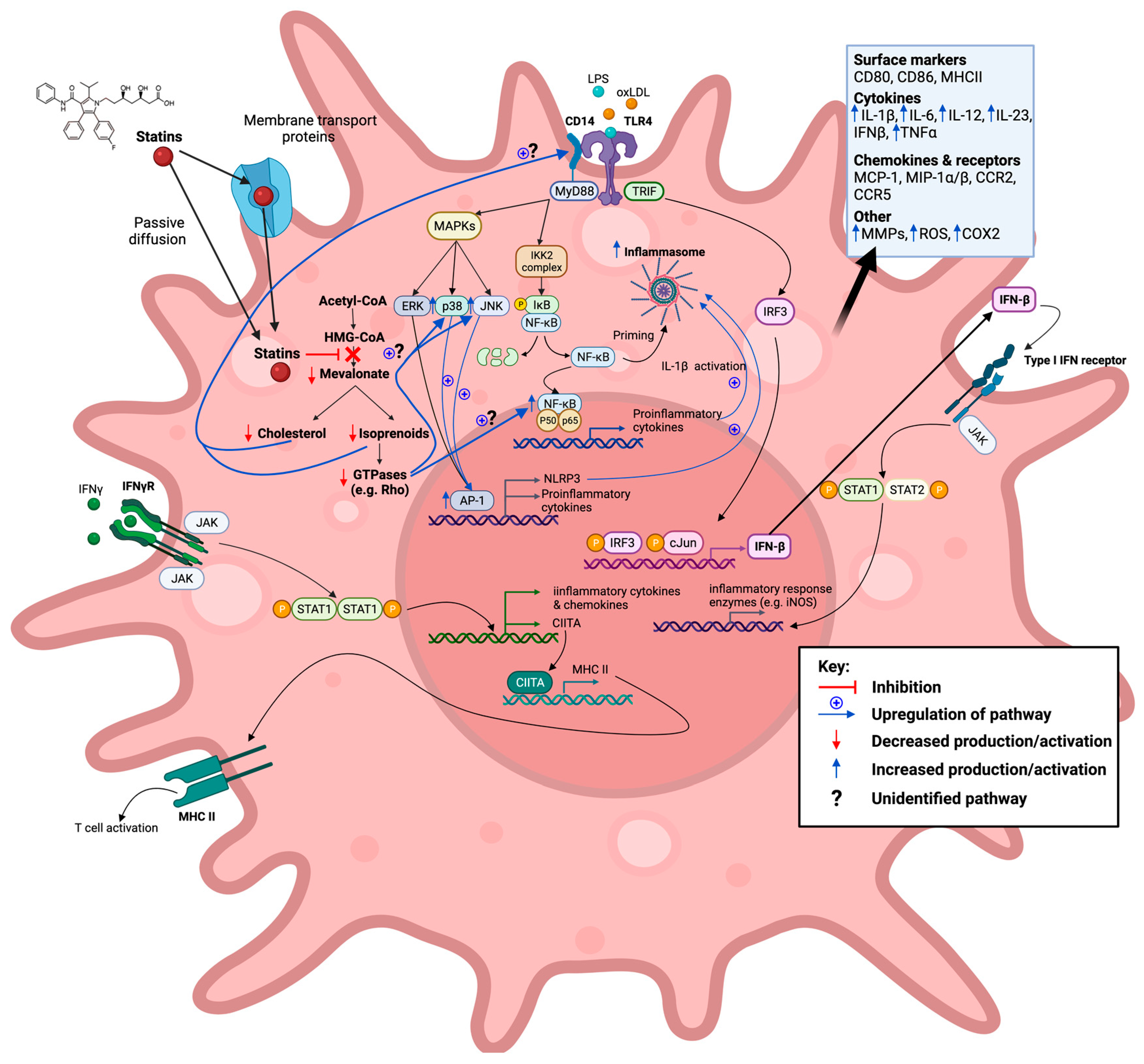
Figure 3. Statins inhibit the mevalonate pathway leading to both reduced cholesterol and isoprenoid biosynthesis, thereby also blocking farnesylation and geranylgeranylation of GTPases. Reduction in these downstream mevalonate intermediates is demonstrated to affect M1-associated macrophage inflammatory signalling pathways in vitro in a pro-inflammatory manner. This action of statins is seen in response to exogenous lipopolysaccharide (LPS) and oxidized low-density lipoprotein (oxLDL) ligands. Created with
BioRender.com, accessed on 7 March 2022.
Table 2. The effects of statins on macrophages in vitro. Abbreviations: PBMC, peripheral blood mononuclear cells; BMDMs, bone-marrow derived macrophages; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; uPAR, urokinase plasminogen activator receptor; AdipoR, adiponectin receptors; mTOR, mechanistic target of rapamycin; mRNA, messenger ribonucleic acid; MWCNT, multi-walled carbon nanotubes; TF, tissue factor; GILZ, glucocorticoid-induced leucine zipper; TPA, 12-O-tetradecanoyl-phorbol-13-acetate; PMA, phorbol myristate acetate; acLDL, acetylated LDL; M-CSF, macrophage colony-stimulating factor; CC, cholesterol crystal; MSU, monosodium urate; SOD1, superoxide dismutase-1; AGE-RAGE, advanced glycation endproducts-receptor for advanced glycation endproducts; C/EBP, CCAAT/enhancer binding proteins; IP-10, interferon gamma-induced protein-10; agLDL, aggregated LDL; ETS-1, erythroblast transformation specific-1; KLF-2, Krüppel-like factor-2; ICAM-1, intercellular adhesion molecule-1.
| Statin |
Model |
Summary |
Inflammatory
Effect |
Ref. |
| Pro |
Anti |
| Atorvastatin |
Human PBMC derived macrophages |
Statins acted as inhibitors of the induction of MHC-II expression by IFN-γ due to suppression of CIITA transcription. Statins repressed MHC-II mediated T-cell activation. |
|
✓ |
[57] |
| |
Primary macrophages from B10.PL mice |
Atorvastatin prevented IFN-γ induced MHC-II, CD40, CD80, and CD86 expression. |
|
✓ |
[58] |
| |
RAW 264.7 macrophages |
Atorvastatin inhibited LPS and IFN-γ-induced NO formation and iNOS induction—thought to be mediated through suppression of NF-κB activation and IFN-γ through STAT1. |
|
✓ |
[59] |
| |
Murine peritoneal macrophages |
Atorvastatin pretreatment enhanced TLR2 and TLR4 ligand-stimulated IL-6 and TNF production. |
✓ |
|
[60] |
| |
RAW 264.7 macrophages |
Enhanced LPS-mediated MMP-9 gene expression. |
✓ |
|
[61] |
| |
RAW 264.7 macrophages |
Atorvastatin pretreatment inhibited oxLDL-induced increase in COX-2, TNFα, and MCP-1 secretion. |
|
✓ |
[62] |
| |
Murine BMDMs |
Atorvastatin pretreatment exacerbated LPS-induced upregulation of Il-1b, IL-6, and NLRP3 transcript levels. |
✓ |
|
[63] |
| |
Human PBMC derived macrophages |
Statin treatment in combination with IL-4 during the macrophage differentiation phase led to increased M2 polarization via PPARγ activation. |
|
✓ |
[64] |
| |
RAW 264.7 macrophages |
Atorvastatin pretreatment inhibited LPS-induced IL-1β and TNFα production in RAW 264.7 macrophages through the enhancement of autophagy. Statin treatment was seen to attenuate NLRP3 inflammasome induction in response to LPS stimulation. Atorvastatin pretreatment inhibited the expression of IL-1β in response to LPS stimulation in peritoneal murine macrophages through autophagy activation, but not that of TNFα. |
|
✓ |
[65] |
| |
Human PBMC derived macrophages |
Atorvastatin reduced matrix degradation capability via reduced MMP-14 activation and uPAR localization to filipodia in LPS and IFN-γ stimulated macrophages. |
|
✓ |
[66] |
| |
RAW 264.7 macrophages and J774 macrophages |
Atorvastatin increased Rac1 GTP-loading in LPS stimulated macrophages, enhancing production of the proinflammatory cytokines IL-1β, TNFα, and IL-6. |
✓ |
|
[67] |
| |
Human monocyte derived macrophages |
Statin treatment during macrophage differentiation phase led to enhanced LPS-induced IL-1β and IL-6 secretion. |
✓ |
|
[68] |
| |
THP1 derived macrophages |
Statin treatment led to increased pro-inflammatory cytokine (IL-1β, TNFα, and IL-6) and AdipoR expression (also seen in combination with oxLDL stimulation); 24 h statin treatment resulted in increased IL-10 mRNA levels, whilst 72 h treatment resulted in decreased expression. |
✓ |
|
[69] |
| |
Murine BMDMs |
Statin-treated macrophages exhibited increased LPS-induced activation of NF-κB and IL-1β protein secretion in response to inflammasome stimulation. |
✓ |
✓ |
[70] |
| |
Murine BMDMs |
Statin pretreatment exacerbated LPS-induced upregulation of IL-1β and NLRP3 transcript levels via p38 and mTOR. |
✓ |
|
[71] |
| |
THP1 derived macrophages |
Impaired MWCNT-elicited IL-1β secretion. |
|
✓ |
[72] |
| Cerivastatin |
Human PBMC derived macrophages |
Cerivastatin treatment suppressed growth of macrophages expressing MMPs and TFs. |
|
✓ |
[73] |
| |
Rabbit foamy macrophages |
Decreased protein expression and activity of MMP-1, MMP-2, and MMP-9. |
|
✓ |
[74] |
| |
RAW-Blue™ cells and Murine BMDMs |
Cerivastatin increased NF-κB/AP-1 activation in unstimulated and LPS-activated macrophages. LPS-induced TNF, IL-1β, and IL-6 expression was amplified. Expression of arginase-1 and GILZ was enhanced in unstimulated, LPS- and IL-4-activated macrophages. |
✓ |
✓ |
[75] |
| Fluvastatin |
human PBMC derived macrophages |
Fluvastatin decreased TF activity in both unstimulated and LPS-, or ac-LDL-stimulated macrophages, but enhanced IL-1β cytokine release. |
✓ |
✓ |
[76] |
| |
Murine peritoneal macrophages and human PBMC derived macrophages |
Simvastatin decreased MMP-9 protein secretion and inhibited TPA-induced enhanced MMP-9 release. |
|
✓ |
[77] |
| |
RAW 264.7 macrophages |
Fluvastatin inhibited LPS and IFN-γ-induced NO formation and iNOS induction.Thought to be mediated through suppression of NF-κB activation and IFN-γ through STAT1. |
|
✓ |
[59] |
| |
RAW 264.7 macrophages |
Fluvastatin upregulated macrophage Socs3 expression, resulting in low responsiveness to inflammatory signals (IFN-γ, IL-6, and M-CSF) due to lower activation of STAT1, STAT3, and STAT5. |
|
✓ |
[78] |
| |
THP1 derived macrophages and THP1 derived acLDL loaded macrophages |
Fluvastatin reduced both the expression, secretion, and proportion of active MMP-9 in PMA stimulated and acLDL-loaded THP1 derived macrophages. |
|
✓ |
[79] |
| |
RAW 264.7 macrophages and murine BMDMs |
Fluvastatin inhibited LPS-induced suppression of CD9, leading to reduced formation of CD14/TLR4 complexes and TNFα and MMP-9 release. |
|
✓ |
[80] |
| |
Murine BMDMs |
Fluvastatin pre-treatment exacerbated LPS-induced upregulation of IL-1b, IL-6, and NLRP3 transcript levels. Statin and LPS treatment of BMDMs harvested from NLRP3−/− mice synergistically enhanced IL-6 but did not affect IL-1β secretion. Statin treatment alone had no effect on the production of inflammatory mediators. |
✓ |
|
[63] |
| |
Human monocyte derived macrophages |
Statin treatment during macrophage differentiation phase led to enhanced LPS-induced IL-1β and IL-6 secretion. |
✓ |
|
[68] |
| |
Murine BMDMs |
Statin pretreatment exacerbated LPS-induced upregulation of IL-1b and NLRP3 transcript levels via p38 and mTOR. |
✓ |
|
[71] |
| |
THP1 derived macrophages |
Impaired MWCNT-elicited IL-1β secretion. |
|
✓ |
[72] |
| |
Human PBMC derived macrophages |
Decreased the activity of iNOS in M1 macrophages. |
|
✓ |
[81] |
| Lovastatin |
Rat peritoneal macrophages and microglia |
Inhibited LPS-induced production of NO, TNFα, IL-1β, and IL-6 in rat primary microglia and macrophages. |
|
✓ |
[82] |
| |
Human PBMC derived macrophages |
Statins acted as inhibitors of the induction of MHC-II expression by IFN-γ due to suppression of CIITA transcription. Statins repressed MHC-II mediated T-cell activation. |
|
✓ |
[57] |
| |
RAW 264.7 macrophages |
Lovastatin inhibited LPS and IFN-γ-induced NO formation and iNOS induction—thought to be mediated through suppression of NF-κB activation and IFN-γ through STAT1. |
|
✓ |
[59] |
| |
RAW 264.7 macrophages |
Lovastatin upregulated macrophage Socs3 expression, resulting in low responsiveness to inflammatory signals (IFN-γ, IL-6, and M-CSF) due to lower activation of STAT1, STAT3, and STAT5. |
|
✓ |
[78] |
| |
Rabbit foamy macrophages |
Decreased protein expression and activity of MMP-1, MMP-2, and MMP-9. |
|
✓ |
[74] |
| |
RAW 264.7 macrophages |
Lovastatin increased LPS-induced TNFα production. |
✓ |
|
[83] |
| |
P388D1 macrophages |
Statins increased production of MMP-12 in activated macrophage. |
✓ |
|
[84] |
| |
RAW 264.7 macrophages |
Lovastatin increased CD14 expression and enhanced LPS-induced membrane levels leading to greater TNFα production, but simultaneously suppressed soluble CD14. |
✓ |
|
[85] |
| |
BMDMs from C57BL/6J mice and RAW 264.7 macrophages |
Lovastatin blocked IFN-γ-induced Citta gene expression by inhibiting transcriptional events at Citta pIV, thereby suppressing MHC-II expression. |
|
✓ |
[86] |
| |
RAW 264.7 macrophages |
Lovastatin treatment induced NO release but did not affect pro-inflammatory cytokine levels in unstimulated cells. However, with LPS it synergistically enhanced IL-6, IL-12p40, IL-1β, and NO release. |
✓ |
|
[87] |
| |
Murine BMDMs |
Lovastatin pretreatment exacerbated LPS-induced upregulation of IL-1b, IL-6, and NLRP3 transcript levels. |
✓ |
|
[63] |
| |
THP1 derived macrophages |
Impaired MWCNT-elicited IL-1β secretion. |
|
✓ |
[72] |
| Metavastatin |
P388D1 cell line |
Statins increased production of MMP-12 in activated macrophages. |
✓ |
|
[84] |
| |
U937 derived macrophages and RAW 264.7 macrophages |
Metavastatin pretreatment significantly increased bacterial clearance, despite reducing oxidative burst and phagocytosis due to increased induction of extracellular traps. |
✓ |
✓ |
[88] |
| |
J774A.1 mouse macrophages |
Increased levels of iNOS and killing of internalized S. pneumoniae. |
✓ |
|
[89] |
| Pitavastatin |
RAW 264.7 macrophages |
Suppressed LPS-induced upregulation of MCP-1, iNOS, and IL-6 gene expression. |
|
✓ |
[90] |
| |
THP1 derived macrophages, and murine peritoneal macrophages and BMDMs (BALB/cCrSlc mice) |
Pravastatin repressed mature IL-1β release elicited by MWCNT/CC/MSU exposure in THP1-derived macrophages, and LPS + MWCNT induced mature IL-1β release in peritoneal macrophages. Pravastatin pretreatment strongly enhanced mature IL-1β release in LPS + MWCNT exposed BMDMs. |
✓ |
✓ |
[72] |
| Pravastatin |
Human PBMC derived macrophages |
Statins acted as inhibitors of the induction of MHC-II expression by IFN-γ due to suppression of CIITA transcription. Statins repressed MHC-II mediated T-cell activation. |
|
✓ |
[57] |
| |
RAW 264.7 macrophages |
Pravastatin inhibited LPS and IFN-γ-induced NO formation and iNOS induction—thought to be mediated through suppression of NF-κB activation and IFN-γ through STAT1. |
|
✓ |
[59] |
| |
RAW 264.7 macrophages |
Pravastatin upregulated macrophage Socs3 expression, resulting in low responsiveness to inflammatory signals (IFN-γ, IL-6, and M-CSF) due to lower activation of STAT1, STAT3, and STAT5. |
|
✓ |
[78] |
| |
RAW 264.7 macrophages |
Suppressed LPS-induced upregulation of MCP-1, iNOS, and IL-6 gene expression. |
|
✓ |
[90] |
| Rosuvastatin |
Human monocyte derived macrophages |
Rosuvastatin reduced MMP-7 and MMP-9 production. |
|
✓ |
[91] |
| |
oxLDL induced THP1 foam cells |
Rosuvastatin inhibited ox-LDL-induced reduction of SOD1 expression. |
|
✓ |
[92] |
| |
THP1 derived macrophages |
Rosuvastatin inhibited the AGE-RAGE axis and ROS production. |
|
✓ |
[93] |
| |
RAW 264.7 macrophages and J774 macrophages |
Rosuvastatin increased Rac1 GTP-loading in LPS-stimulated macrophages, enhancing production of the proinflammatory cytokines IL-1β, TNFα, and IL-6. |
✓ |
|
[67] |
| |
Human monocyte derived macrophages |
Statin treatment during macrophage differentiation phase led to enhanced LPS-induced IL-1β and IL-6 secretion |
✓ |
|
[68] |
| |
THP1 derived macrophages |
Statin treatment led to increased pro-inflammatory cytokine (IL-1β, TNFα, and IL-6) and AdipoR expression (also seen in combination with oxLDL stimulation); 24 h statin treatment resulted in increased IL-10 mRNA levels, whilst 72 h treatment resulted in decreased expression. |
✓ |
|
[69] |
| |
THP1 derived macrophages |
Inhibited foam cell formation and lessened the secretion of inflammatory cytokines (e.g., TNFα, IL-1β, and IL-6) from oxLDL-treated macrophages |
|
✓ |
[94] |
| Simvastatin |
Human monocyte derived macrophages |
Simvastatin decreased superoxide production and therefore LDL oxidation |
|
✓ |
[95] |
| |
human PBMC derived macrophages |
Simvastatin decreased TF activity in both unstimulated and LPS-stimulated/ac-LDL-stimulated macrophages. The suppression of TF activity induced by statin treatment was accompanied by a diminution in TF mRNA expression. |
|
✓ |
[76] |
| |
Murine peritoneal macrophages |
Simvastatin decreased MMP-9 protein secretion and inhibited TPA-induced enhanced MMP-9 release. |
|
✓ |
[77] |
| |
Rabbit foamy macrophages |
Decreased protein expression and activity of MMP-1, MMP-2, and MMP-9. |
|
✓ |
[74] |
| |
Peritoneal murine macrophages and RAW 264.7 macrophages |
Simvastatin pretreatment enhanced both IL-12p40 and TNFα LPS-induced mRNA expression and protein production by a mechanism involving the AP-1 and C/EBP transcription factors, but IP-10 levels were reduced. |
✓ |
✓ |
[96] |
| |
PBMC derived human macrophages |
Simvastatin inhibited IFN-γ-induced upregulated mRNA expression of the chemokines MCP-1, MIP-1a, and MIP-1b and the chemokine receptors CCR1, CCR2, and CCR5. MCP-1 protein expression was also notably reduced. |
|
✓ |
[97] |
| |
human primary monocyte derived macrophages |
Statin administration significantly increased the secretion of IL-1β but had no significant effect on IL-8 or IL-6 and inhibited the secretion of TNFα. In combination with agLDL loading, statin treatment enhanced secretion of IL-1β and IL-8, but had no effect on TNFα or IL-6 secretion. |
✓ |
✓ |
[98] |
| |
BMDMs from C57BL/6J mice and RAW 264.7 macrophages |
Simvastatin blocked IFN-γ-induced Citta gene expression by inhibiting transcriptional events at Citta pIV, thereby suppressing MHC-II expression. |
|
✓ |
[86] |
| |
PBMC derived human macrophages and THP1 derived macrophages |
Simvastatin treatment led to the downregulation of inflammatory signalling pathways, marked by a reduction in the gene expression of proinflammatory associated chemokines (MCP-1, MIP-1, and tissue factor) and transcription factors (NF-κB and ETS-1). The anti-inflammatory associated transcription factor KLF-2 had upregulated gene and protein expression. |
|
✓ |
[99] |
| |
Murine peritoneal macrophages |
Simvastatin pretreatment enhanced TLR2 and TLR4 ligand-stimulated IL-6 and TNF production. |
✓ |
|
[60] |
| |
RAW 264.7 macrophages |
Enhanced LPS-mediated MMP-9 gene expression. |
✓ |
|
[61] |
| |
PBMC derived human macrophages, HL-60 derived macrophages and murine peritoneal macrophages (treated with simvastatin in vivo) |
Simvastatin reduced phagocytosis and oxidative burst of IgG opsonized bacteria but enhanced the production of inflammatory mediators (TNFα and COX-2). No effect was seen on inflammatory mediators in response to non-opsonized bacteria, but impairment of phagocytosis remained. |
✓ |
✓ |
[100] |
| |
RAW 264.7 macrophages |
Simvastatin pretreatment reduced basal and S. aureus-stimulated levels of C5aR and dampened macrophage sensitivity to membrane vesicles released from infected cells, decreasing TNFα production. |
|
✓ |
[101] |
| |
RAW 264.7 macrophages and murine BMDMs |
Simvastatin inhibited LPS induced suppression of CD9, leading to reduced formation of CD14/TLR4 complexes and TNFα and MMP-9 release. |
|
✓ |
[80] |
| |
RAW 264.7 macrophages and murine BMDMs |
Simvastatin pretreatment enhanced IL-12p40 and TNFα production in IFN-γ and L. monocytogenes stimulated macrophages. Statins suppressed MHC-II surface expression on IFN-γ-activated macrophages |
✓ |
✓ |
[102] |
| |
THP1 derived macrophages |
Simvastatin pretreatment inhibited IFN-γ induced expression of MCP-1 and ICAM-1. |
|
✓ |
[103] |
| |
Murine BMDMs and human PBMCs |
Simvastatin enhanced LPS-stimulated pro-IL-1β (28 kDa form), which disrupted mature IL-1β inflammatory actions. |
|
✓ |
[104] |
| |
Murine BMDMs |
Simvastatin pretreatment exacerbated LPS-induced upregulation of IL-1b, IL-6, and NLRP3 transcript levels. |
✓ |
|
[63] |
| |
Murine BMDMs |
Simvastatin reduced parasite burden by enhancing oxidative burst and phagosome maturation. |
✓ |
|
[105] |
| |
Raw 264.7 macrophages |
Simvastatin repressed IL-1β secretion in response to H. pylori infection and increased autophagy. |
|
✓ |
[106] |
| |
Human monocyte derived macrophages |
Statin treatment during macrophage differentiation phase led to enhanced LPS-induced IL-1β and IL-6 secretion |
✓ |
|
[68] |
| |
RAW-Blue™ cells and Murine BMDMs |
Simvastatin increased NF-κB/AP-1 activation in unstimulated and LPS-activated macrophages. LPS-induced TNF, IL-1β, and IL-6 expression was amplified. Expression of arginase-1 and GILZ was enhanced in unstimulated, LPS-, and IL-4-activated macrophages. |
✓ |
✓ |
[75] |
2.1. Statins Modulate TLR Inflammatory Signalling Pathways
Cell surface TLRs, such as TLR1, TLR2, TLR4, TLR5, and TLR6, are key initiators of innate immune responses. They are predominantly involved in host defence mechanisms through their recognition of a diverse array of stimulatory signals related to microbial membrane components, such as lipids, lipoproteins, proteins, and LPS
[107]. TLR engagement triggers a range of antimicrobial responses, including the production of reactive nitrogen and oxygen species, inflammatory cytokines, and matrix metalloproteinases (MMPs). However, alongside their responsiveness to exogenous ligands, TLRs also recognise endogenous ligands (e.g., oxLDL) released from damaged tissues or dead cells, thereby regulating sterile inflammatory processes
[108]. Indeed, prolonged TLR activation has been associated with uncontrolled chronic inflammatory diseases, including atherosclerosis
[109][110][111]. TLR4, in particular, is upregulated in atherosclerotic plaques and demonstrates increased expression as a result of ox-LDL exposure
[112][113]. TLR4 signalling is mediated by the adaptor proteins myeloid differentiation primary response 88 (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF), which initiate two separate signal transduction pathways that culminate in the activation of a multitude of transcription factors
[114][115], including members of the NF-κB
[116] and IRF
[117] families. MyD88-dependent signalling cascades include the activation of NF-κB and mitogen-activated protein kinase (MAPK) family members, such as extracellular signal-regulated kinase1/2, p38, and c-Jun N-terminal kinase (JNK), which, in turn, mediate the activation of AP-1 family transcription factors or the stabilization of mRNA to regulate inflammatory responses
[107]. In contrast, TRIF-mediated TLR4 signalling occurs through the activation of IFN3 and STAT1, which induce the expression of IFN genes (e.g.,
IFN-
B) and are also involved in late-phase NF-κB activation
[116][118]. A number of accessory proteins, such as CD14 and CD36, are also suggested to play a role in macrophage inflammation cascades through their association with TLR4
[38].
2.1.1. Anti-Inflammatory Modulation of TLR Signalling Pathways
As noted, NF-κB, through its activation in the TLR4 signalling pathways, is a key regulator of both macrophage inflammatory responses to pathogens and their role in sterile inflammatory diseases. Multiple statins (atorvastatin
[59], fluvastatin
[59][76], lovastatin
[59][82], pravastatin
[59], and simvastatin
[76][99]) have been shown to inhibit NF-κB activation. The effects of statins on NF-κB activation are suggested to be the result of statins’ inhibition of the mevalonate pathway, specifically the isoprenoid branch, as various studies have reported that the addition of mevalonate, FPP, and GGPP reverses their action on NF-κB
[76][99]. The exact links between statins’ inhibitory action on both protein prenylation and NF-κB activation have yet to be fully elucidated, although it has recently been reported that statins attenuate the degradation of the NF- κB inhibitor protein IκB
[119]. IκB degradation is reliant on the phosphorylation of the IKK2 complex, which may be regulated by Rac1 in macrophages
[120]. The upregulated gene and protein expression of Krüppel-like factor 2
[99] (a potent regulator of pro-inflammatory activation) and SOD1
[92] (associated with increased antioxidant enzyme activity and decreased ROS production
[121]) have also been reported to occur in statin-treated macrophages and may contribute to the suppression of NF-κB-driven signalling pathways. Statin-mediated inhibition of the IκB/NF-κB pathway has been shown to result in a global anti-inflammatory effect on macrophages, with mRNA and protein analysis revealing the attenuated expression of many pro-inflammatory associated mediators, including cytokines (TNFα, IL-1β, and IL-6)
[82][99], chemokines (MCP-1 and MIP-1α/β)
[99], and tissue factor (a membrane-bound glycoprotein that plays a prominent role in the extrinsic pathway of blood coagulation and fibrin deposition)
[76], and NO production
[59][81][82]. Importantly, the inhibitory effects of statin treatment on NF-κB-induced cytokine synthesis have also been seen when using the CVD-relevant endogenous ligand oxLDL and are associated with reduced macrophage oxLDL loading and foam cell formation
[62][92][94][95].
Interestingly, statin-mediated inhibition of the MyD88/NF-κB pathway has also been implicated in reducing inflammatory responses through enhancing autophagy
[65][106][122][123] via the Akt-mTORC1 axis
[65][122], but there are conflicting thoughts on whether this results from the inhibition of the cholesterol or isoprenoid biosynthesis branch of the mevalonate pathway
[106][122][123]. The increased autophagy resulting from statin treatment has been noted to restrict NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome activation and thus reduce pro-inflammatory cytokine release
[65][106].
In addition to signalling through NF-κB-dependent pathways, which are thought to be induced predominantly by MyD88-signalling, it has been proposed that statins’ inhibitory effects on macrophage inflammatory responses result from a downstream suppression of TRIF-mediated signalling
[90]. Pravastatin and pitavastatin treatment of TLR4-stimulated RAW264 macrophages have a strong inhibitory effect on the TRIF/IRF3/IFN-β pathway in macrophages. The reduction in IFN-β expression resulting from statin treatment led to decreased STAT1 phosphorylation and the attenuation of pro-inflammatory gene expression in macrophages, evidenced by the reduced secretion of MCP-1, NO, and IL-6. Unlike previous studies, the researchers could not identify whether this action was the result of mevalonate or isoprenoid inhibition by statins, as they noted that mevalonate itself also suppressed LPS-induced expression of IFN-β
[90].
Statin treatment has also been reported to reduce the matrix degrading capacity of M1-like polarized macrophages through the modulation of matrix metalloproteinase (MMP) expression
[66][74][77][79]. This is particularly relevant to CVD, as atherosclerotic lesions show enhanced MMP expression, and this is thought to contribute to the weakening of the vascular wall, aiding plaque rupture
[124]. Atorvastatin co-incubation during the polarization of classically activated macrophages was found to reduce MMP-14 activation
[66], which is thought to mediate the expression of other MMPs, such as MMP-9. MMP-9 is one of the most widely investigated MMPs and is known to be involved in inflammation (e.g., extracellular processing of IL-1β
[125]) and fibrosis in CVD
[126]. In line with this, various studies have reported that statin treatment decreases MMP-9 protein secretion, thereby reducing its activity
[77][79]. Importantly, this effect was also seen in in vitro studies of foamy macrophages
[74], which are abundant in atherosclerotic plaques. This effect of statins is thought to be dependent on their action as mevalonate inhibitors
[66][77], and there is evidence that the uncoupling of JAK/STAT signalling plays a role
[79]. However, it should be noted that most of the studies examining statin-mediated effects on MMP expression in macrophages have not investigated the potential underlying mechanisms, and the exact point in the TLR-signalling pathway that is impacted awaits clarification. Macrophage production of MMPs in the absence of statin treatment is regulated via both the NF-κB
[127][128] and MAPK
[129] pathways.
A final means by which statins are thought to blunt TLR4-induced macrophage inflammation is not via inhibition of its signalling cascade but rather via the enhancement of anti-inflammatory response elements. In this respect, it has been reported that fluvastatin and simvastatin upregulate CD9 expression in both RAW264.7 cells and murine bone-marrow derived macrophages (BMDMs) treated with LPS
[80], consequently leading to reduced TNFα and MMP-9 production. CD9 is a recognised anti-inflammatory marker of macrophages
[130] and negatively regulates LPS-induced macrophage activation by preventing the formation of CD14/TLR4 complexes
[131]. Indeed, statin treatment no longer resulted in significant inhibition of TNFα and MMP-9 in BMDMs from CD9 knock-out mice, suggesting that statins’ anti-inflammatory effects are, to a degree, dependent on CD9
[80]. The upregulation of CD9 observed following statin treatment appears to be dependent on their inhibitory action on protein prenylation (
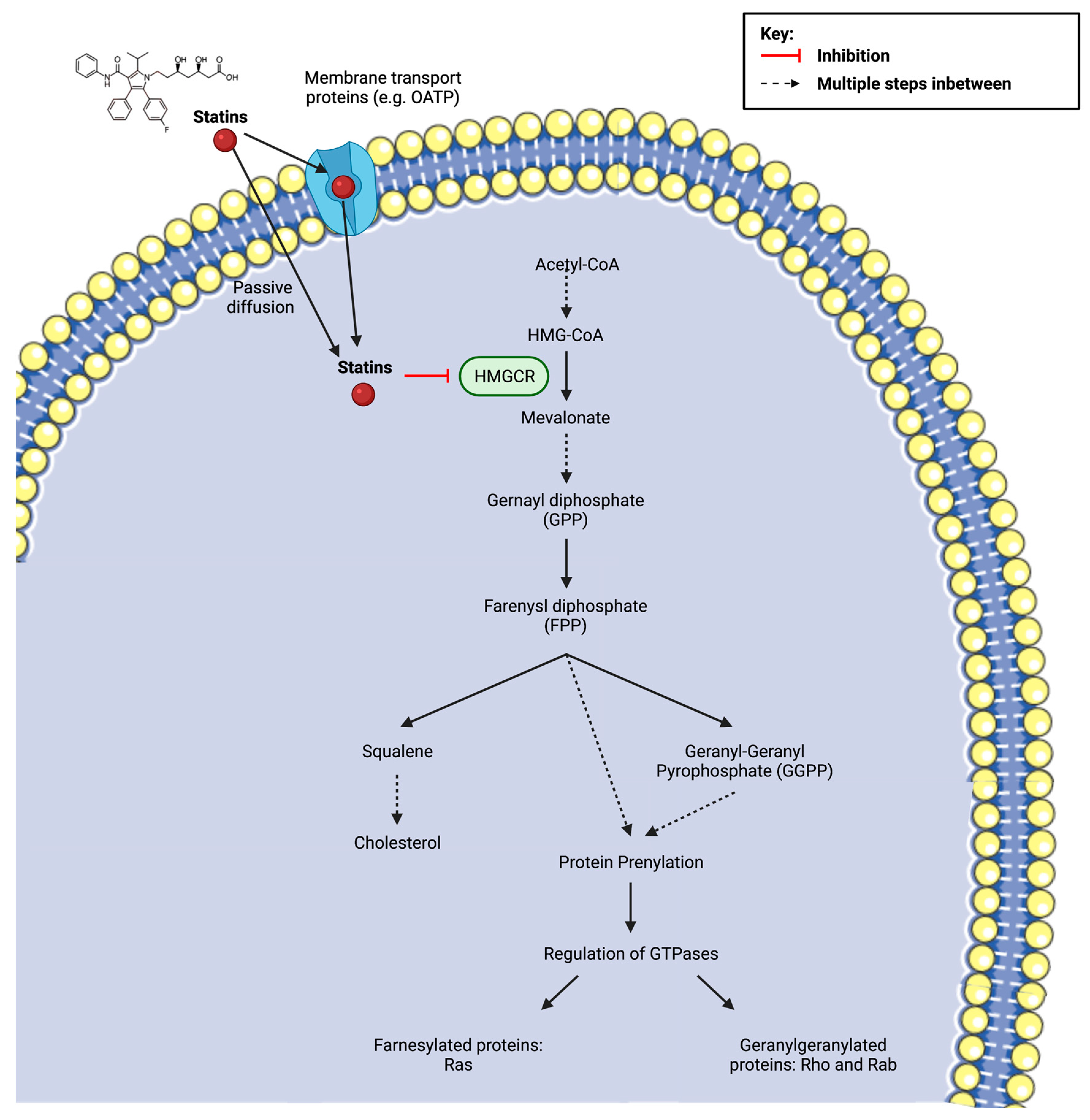
Figure 4), specifically geranylgeranylation, as GGTI-298 (a geranylgeranyltransferase inhibitor), but not FTI-277 (a farnesyl transferase inhibitor) increased LPS-treated CD9 levels to a comparable degree. However, the precise mechanism by which decreased isoprenoid synthesis confers CD9 upregulation is currently unknown.
Figure 4. Statin inhibition of 3-hydroxy-3methylglutaryl coenzyme A (HMG-CoA) reductase (HMGCR) and the subsequent implications on downstream metabolites of the mevalonate pathway, including the synthesis of cholesterol and the isoprenoids farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Protein prenylation, via isoprenoids, is essential for the activation of small guanosine triphosphate (GTP)-binding proteins (Ras, Rho, Rac). The cellular uptake of the drug depends on its solubility. Lipophilic statins are more likely to enter the cell via passive diffusion, whereas hydrophilic statins require protein transporters, such as organic anion transporting polypeptides (OATPs) in hepatocytes. Created with
BioRender.com (accessed on 7 March 2022) and
Smart.Servier.com (accessed on 7 March 2022).
2.1.2. Pro-Inflammatory Modulation of TLR Signalling Pathways
In contrast to the anti-inflammatory properties of statins described above, a growing number of in vitro studies are reporting that statins paradoxically enhance pro-inflammatory signalling in macrophages (
Table 2). LPS-triggered TLR4 activation in macrophages activates both NF-κB and AP-1 transcription factors
[132], which have both been implicated in statin-induced pro-inflammatory responses
[71][96].
In one of the earliest studies
[96] reporting pro-inflammatory effects, it was demonstrated that simvastatin pre-treatment enhanced LPS-induced IL-12p40 (a constituent of the bioactive cytokines IL-12 and IL-23) and TNFα mRNA expression and protein production by a mechanism involving AP-1 and C/EBP transcription factors. Specifically, statin treatment decreased c-FOS binding to the AP-1 promoter region (a negative regulator of the signalling system) whilst simultaneously enhancing JNK-mediated c-Jun phosphorylation, thereby stimulating the transcription of inflammatory genes. In keeping with this, atorvastatin and simvastatin pre-treatment is observed to enhance TLR2/TLR4 ligand-stimulated IL-6 and TNFα production
[60], and various research groups have found statins to induce the activation of the MyD88 pathway transcription factor NF-κB
[70][75] (alongside AP-1). There is evidence that these effects depend on the isoprenoid branch of the mevalonate pathway
[96] and on Rho GTPases
[70][83]. The molecular mechanisms connecting the effects of statins on GTPases and the increased expression of the AP-1 transcription factor remain poorly understood, but it has been suggested that Rho GTPase inactivation by the suppression of prenylation abolishes an inhibitory feedback loop in this pathway, thereby resulting in an enhanced upregulation of cytokine gene expression.
Statins have also been found to enhance pro-inflammatory macrophage responses by increasing NLRP3 inflammasome activation in a p38-dependent manner
[71]. IL-1β is unique compared to most cytokines in that it requires post-translational modification via caspase-1 to reach its mature form, being originally translated as a 33 kDa inactive precursor (pro-IL-1β)
[133]. Caspase-1, in turn, requires NLRP3 inflammasome activation to mediate this process
[134]. Several studies have found that statins promote caspase-1 and NLRP3 activation and have shown that statin-stimulated IL-1β release is dependent on their enhanced activation
[63][67][71]. Statin treatment is proposed to facilitate LPS-induced capase-1 and inflammasome stimulation via its disturbance of isoprenoid biosynthesis, as the effect was reversible with GGPP addition
[67]. Furthermore, the deletion of geranylgeranyltransferase type 1 (GGTase-I; responsible for carrying out GTPase geranylgeranylation) in macrophages mimicked the effects of statins. Later studies by the group suggested that Rac1 mediates the hyperactivity to pro-inflammatory stimuli observed in statin-treated and GGTase-I-deficient macrophages because the deletion of Rac1 abolished the enhanced release of pro-inflammatory cytokines, whereas the deletion of other GTPases (RhoA and Cdc42) did not
[67]. However, how statin-induced hyperactive Rac1 activation may drive the enhancement of LPS-stimulated p38 activation and thus increase pro-inflammatory IL-1β secretion has yet to be explored.
In consideration of the relevance of statins to atherosclerosis management, various research groups have also investigated the effects of statins on macrophage TLR-mediated cytokine responses using endogenous molecules (e.g., LDL and cholesterol crystals), with mixed findings. Lindholm and Nilsson reported that in combination with aggregated LDL (agLDL) loading, statin treatment enhanced secretion of IL-1β and IL-8 but had no effect on TNFα or IL-6 secretion in human primary monocyte-derived macrophages isolated from buffy coats
[98]. Cui et al. also reported statin treatment to strongly enhance mature IL-1β release in murine BMDMs stimulated with a combination of LPS and cholesterol crystals but noted the opposite to be true in THP-1 derived macrophages
[72]. Interestingly, despite the conflicting data between macrophage cell types, these effects were all reported to be isoprenoid dependent
[72][98]. At present, it remains unclear which TLR-pathway signalling elements are affected by statin treatment in ox- and agLDL-stimulated macrophages but, given that (for reasons not completely understood) different TLR4 stimuli induce different cellular responses
[135][136], future studies may find the involvement of signalling components outside of those noted in the LPS experiments.
It has also been suggested that statin-mediated effects on TLR-inflammatory responses may not solely be the result of their action on its signalling pathway but may also result from an increase in membrane CD14 expression
[85]. RAW 264.7 macrophage incubation with lovastatin both alone and in combination with LPS promoted increased CD14 mRNA and protein levels, resulting in greater LPS-induced TNFα secretion. Coincubation of lovastatin-treated macrophages with FPP, GGPP, or water-soluble cholesterol was seen to prevent LPS-induced TNFα levels, suggesting that statin effects on macrophage responses may be regulated at multiple levels.
2.2. Statins Modulate IFN-γR Inflammatory Signalling Pathways
Cytokines are major regulators of macrophage activation, and aberrant secretion is implicated in several disease states, including chronic inflammatory diseases such as atherosclerosis. IFN-γ, particularly, is known to play a role in atherosclerotic development, being highly expressed in lesions
[137] and inducing foam cell formation
[138] in macrophages via increased LDL uptake. IFN-γ exerts its biological activities by binding to a specific cell surface receptor, IFN-γR, which utilises the Jak-STAT pathway in its signal transduction (a recurring theme amongst members of the cytokine receptor superfamily). Through this mechanism, IFN-γ induces the expression of numerous genes that play a role in macrophage inflammatory responses, such as ROS production and communication between macrophages and other immune cells (e.g., T lymphocytes) via chemokine secretion and surface marker expression
[139]. Notably, IFN-γ is also thought to participate in an amplification loop to increase immune system sensitivity, as it has been seen to enhance LPS-induced NF-κB activation and increase TLR expression, whilst in turn, TLR ligands, such as LPS, augment local IFN-γ induction
[139].
2.2.1. Anti-Inflammatory Modulation of IFN-γR Signalling Pathways
In both human and mouse-derived macrophages, a variety of statins have been found to reduce IFN-γ-induced MHC-II expression through the downregulation of the class II transactivator (CIITA), thereby interfering with their ability to prompt T cell activation, indicative of an immunosuppressive impact
[57][58][86][102]. Further examination of this effect provided some insight into the potential molecular basis, with Kwak et al. and Lee et al. finding that statins specifically decrease the expression of CIITA at the transcriptional level, after noting that CIITA mRNA destabilisation did not occur in the presence of simvastatin. The transcription of IFN-γ-inducible CIITA expression is controlled by a large regulatory region containing three independent promoters pI, pIII, and pIV, which, in turn, are controlled by distinct regulatory elements
[140]. As Kwak et al.
[57] had noted that constitutive MHC-II expression, which is controlled by pI and pIII, was not affected by statin treatment it was suggested that pIV may be involved. Lee et al.
[86] therefore focused their investigation on this particular promoter region, discovering that its transcription factors STAT1 and IRF-1 were both downregulated. In addition to this, the team also documented that the addition of GGPP, but not cholesterol, abolished the statin-mediated reduction in IFN-γ-induced MHC-II expression, signifying again that the effect was likely to be dependent on statins’ action as isoprenoid inhibitors. They next tested the effects of two specific inhibitors of Ras superfamily protein prenylation: GGTI-298 and FTI-277. GGTI-298 was found to mimic the inhibitory actions of simvastatin on CIITA expression, but FTI-277 had no effect, indicating the specific involvement of geranylgeranylation. Furthermore, a Rac1-specific inhibitor was also shown to capture this effect, revealing its contribution to IFN-γ-induced STAT1 activation. Another potential factor leading to STAT1 suppression was suggested by Huang et al., who demonstrated that lovastatin and fluvastatin upregulate mRNA expression of the
Socs-
3 gene in macrophages
[78]. SOCS proteins are known to negatively regulate cytokine signalling through their binding to the cytoplasmic domain of recognition receptors
[141]. Regardless of the precise signalling mechanisms involved, the dampening of IFN-γ inflammatory stimulation via STAT1 inhibition has also been found to affect a number of other pro-inflammatory responses, including reduced mRNA expression of chemokines (monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory proteins-1 α and β (MIP-1α/β))
[90][97][103], chemokine receptors (CCRs—
CCR1,
CCR2, and
CCR5)
[97] and cytokines (IL-6), along with reduced NO production
[59].
2.2.2. Pro-Inflammatory Modulation of IFN-γR Signalling Pathways
Interestingly, reports of statins enhancing pro-inflammatory signalling have not cited the involvement of IFN-γR pathways. Indeed, although simvastatin pre-treatment was found to enhance IL-12p40 and TNFα production in murine macrophages stimulated with both IFN-γ and
L.
monocytogenes infection
[102], the researchers highlighted that this was most likely to be the result of TLR-mediated signalling pathways as they found that IFN-γ treatment alone in macrophages had no effect on pro-inflammatory cytokine production. Moreover, in agreement with anti-inflammatory reports, they noted a decreased surface expression of MHC-II. Another study by Linnenberger et al. agreed with this finding that statin treatment had no effect on macrophage stimulation by IFN-γ (despite enhancing LPS-induced expression of TNF, IL-1β, and IL-6)
[75].
2.3. Statins Play Roles in Macrophage Differentiation
Alongside their effects on inflammatory signalling pathways, more recent studies have suggested that statins may directly alter the differentiation of macrophages in vitro. In one study, atorvastatin enhanced an IL-4-induced M2 phenotype via p38 MAPK-dependent PPARγ activation when added at the start of the differentiation process
[64]. However, in other work, macrophages differentiated overnight in the presence of fluvastatin were more reactive to LPS stimulation than those that were not, characterised by a greater secretion of IL-1β and IL-6 and dependent on Rac1-geranylgeranylation
[68]. Taken together, these studies suggest that macrophages differentiated in the presence of statins may be more immune-responsive to various stimuli and therefore can enhance either pro or anti-inflammatory functions depending on the particular stimulating agents they are exposed to.