
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga E. Lebedeva | -- | 2327 | 2022-06-07 13:33:01 | | | |
| 2 | Peter Tang | -34 word(s) | 2293 | 2022-06-08 03:44:25 | | |
Video Upload Options
The problem of recycling polymer waste remains the main one in the context of the growth in the use of plastics. Given the non-renewability of fossil fuels, the task of processing plastic waste into liquid fuels seems to be a promising one. Thermocatalytic conversion is one of the methods that allows obtaining liquid products of the required hydrocarbon range. Clays and clay minerals can be distinguished among possible environmental-friendly, cheap and common catalysts.
1. Introduction
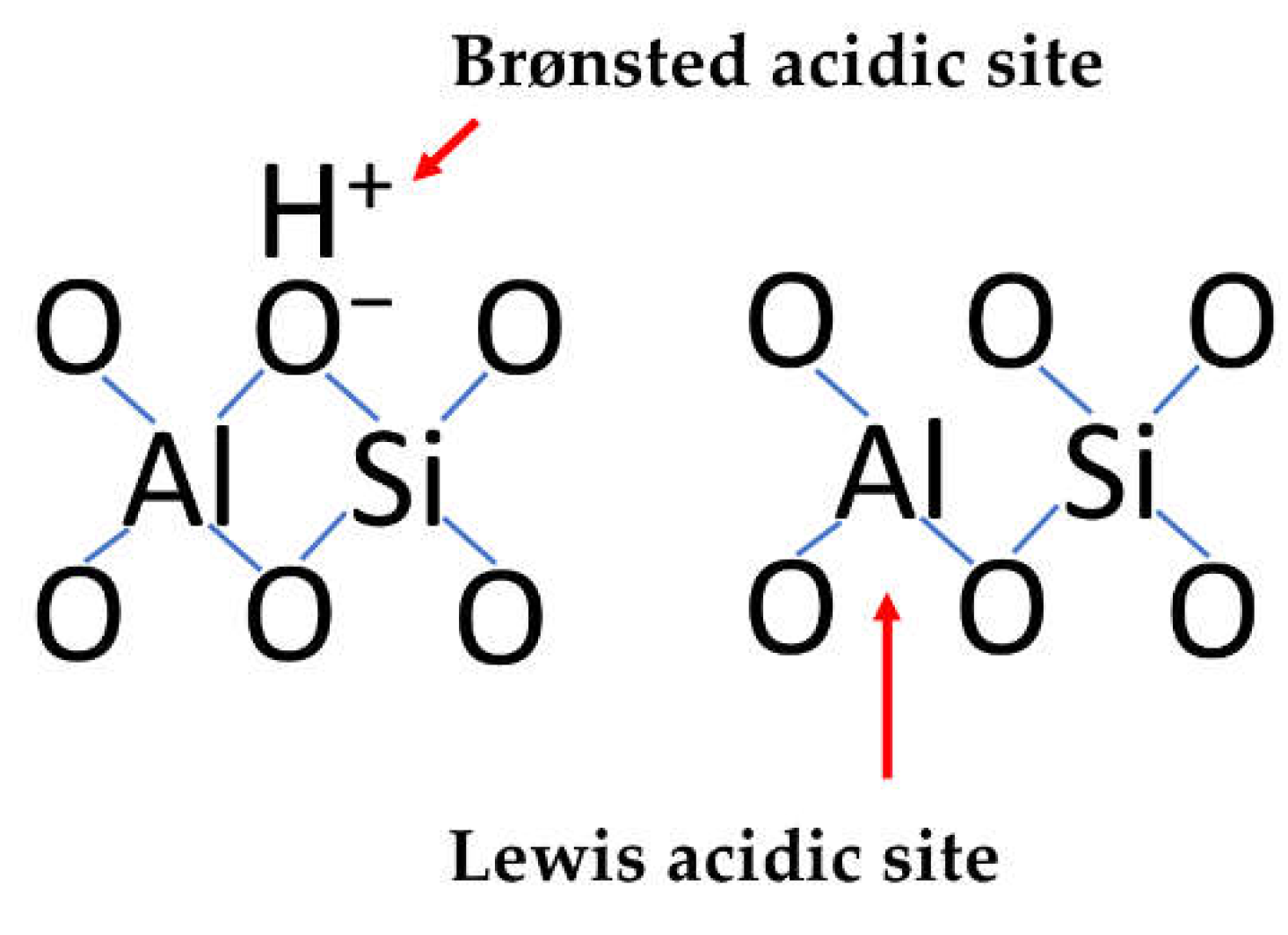
2. Nature of Catalytic Activity of Clays
3. Kaolin Group Catalytic Activity
|
Catalyst |
Plastic |
Temperature, °C |
Highest Liquid Yield, wt% |
Specific Results |
Reference |
|---|---|---|---|---|---|
|
Kaolinite-containing natural clay |
HDPE |
478 |
16 |
Catalyst produced more alkanes than olefins in both gaseous and liquid oil products. |
[3] |
|
Kaolin and its modifications With CH3COOH, HCl, H3PO4, HNO3, and NaOH |
HDPE |
450 |
78.7 |
The liquid fuel consisted of petroleum products range hydrocarbons (C10–C25). |
[4] |
|
Kaolin |
LDPE |
450 |
79.5 |
The oil consists of paraffins and olefins with a predominance of C10–C16 components. |
[5] |
|
Kaolin |
LDPE |
600 |
about 75 |
The first addition of kaolin gives aliphatic compounds and C6–C20 aromatics (90–95%). |
[6] |
|
75% kaolinite with 25% bentonite |
LDPE |
580 |
74.45 |
High yield of paraffins (70.62%). The percentage of aromatics was 5.27%. |
[7] |
|
China clay (kaolinite) |
LDPE |
300 |
84 |
Components with a boiling point of 125–180°C were identified as alkanes, alkenes, and aromatics. |
[8] |
|
Kaolin |
LDPE |
450 |
99.82 |
The highest percentage component is heptane. |
[9] |
|
Al-substituted Keggin tungstoborate/kaolin composite |
LDPE |
295 |
84 |
During the catalytic cracking 70 mol.% of gasoline range hydrocarbons were produced. |
[10] |
|
tungstophosphoric acid/kaolin composite |
LDPE |
335 |
81 |
A high content of benzene-like hydrocarbons (C11–C14). |
[11] |
|
Ahoko kaolin |
PP |
450 |
79.85 |
Liquid products with properties comparable to conventional fuels (gasoline and diesel). |
[12] |
|
Hydrochloric acid/kaolin composite |
PP |
470 |
71.9 |
The condensable hydrocarbons contain dominantly alkanes and alkenes in the range C6–C12. |
[13] |
|
Commercial-grade kaolin clay |
PP |
450 |
89.5 |
Contains olefins, aliphatic, and aromatic hydrocarbons in the oil comparable with liquid fossil fuels. |
[14] |
|
Commercial-grade kaolin clay and kaolin treated with sulfuric acid |
PP |
500 |
92 (acid-treated), 87.5 (neat kaolin) |
The oil from the neat kaolin—C10–C18 products, from the acid-treated kaolin—mainly C9–C13. |
[15] |
|
Kaolin |
PP |
500 |
87.5 |
Fuel properties are identical to the different petroleum fuels. |
[16] |
|
Neat kaolin and kaolin treated with hydrochloric acid |
PP |
400–500 |
71.9 |
The highest yield of liquid hydrocarbons was achieved with kaolin clay treated with 3M HCl. |
[17] |
|
Kaolin |
PP/vaseline (4.0 wt%) |
520 |
52.5 |
The gasoline—32.77%, diesel—13.59%, residue—6.14% |
[18] |
|
CuO/kaolin and neat kaolin |
PS |
450 |
96.37 (neat kaolin), 92.48 (CuO/kaolin) |
The oil contained aromatic hydrocarbons, but from CuO/kaolin—85% C10H8 and ~13% C8H8. |
[19] |
|
Zeolite-Y + metakaolin + aluminum hydroxide + sodium silicate all synthesized from kaolin |
HDPE + LDPE + PP + PS + PET |
350 |
46.7 |
Catalyzed fuel samples consist of 93% gasoline and 7% diesel fraction. |
[20] |
|
Kaolin |
Virgin HDPE, HDPE waste and mixed plastic waste |
425 |
79 |
The catalyst was the most selective in producing diesel, which yielded 63%. |
[21] |
|
Halloysite treated with hydrochloric acid |
PS |
450 |
90.2 |
Aromatic compounds of more than 99%. The main product is styrene (58.82%). |
[22] |
4. Smectite Group Catalytic Activity
|
Catalyst |
Plastic |
Temperature, °C |
Highest Liquid Yield, wt% |
Specific Results |
Reference |
|---|---|---|---|---|---|
|
Bentonite (50 wt%)/spent fluid catalytic cracking catalyst (FCC) |
HDPE |
500 |
100 |
High yields of gasoline C5–C11 (50 wt%) The yield of C12–C20 hydrocarbons—8–10 wt%. |
[24] |
|
Pillared bentonite (PILC) intercalated with Fe or Al |
HDPE and heavy gas oil (HGO) |
500 |
>80 |
The oil from the Fe-PILC-Fe-300 catalyst was more similar to the standard diesel. |
[25] |
|
Bentonite (Gachi clay) |
LDPE |
300 |
77 |
Olefin and paraffin hydrocarbons. |
[26] |
|
South Asian clay classified as bentonite andmontmorillonite impregnated with nickel NPs |
LDPE and post-consumer polybags |
350 |
79.23 (LDPE), 76.01 (poly-bags) |
The final products are in the range of gasoline, kerosene, and diesel. |
[27] |
|
Bentonite thin layer loaded with MnO2 nanoparticles (NPs) |
PP |
750 |
Parameters were designed to get off the liquid |
The complete decomposition of plastics with the formation of gases (methane and hydrogen) and coke. |
[28] |
|
Bentonite treated with 0.5M hydrochloric acid |
PS |
400 |
88.78 |
The obtained liquid contains styrene. Toluene and benzene were the major components. |
[29] |
|
Acid-washed bentonite clay (AWBC), Zn/AWBC, Ni/AWBC, Co/AWBC, Fe/AWBC, Mn/AWBC |
PP, HDPE |
300 for PP and 350 for HDPE |
AWBC (PP 68.77, HDPE 70.19), Ni/AWBC (PP 92.76, HDPE 62.07), Co/AWBC (PP 82.8, HDPE 69.31), Fe/AWBC (PP 82.78, HDPE 71.34), Mn/AWBC (PP 80.4, HDPE 81.07), Zn/AWBC (PP 82.50, HDPE 91) |
Co/AWBC/PP (mainly olefins and naphthenes) and Zn/AWBC/HDPE (mainly paraffins and olefins) were the most effective. |
[30] |
|
H2SO4-activated bentonite (synthesized) |
PP + HDPE |
328 |
79 |
The hydrocarbon oil. |
[31] |
|
A mixture of nature bentonite and zeolite (70:30) |
PP, PET |
400 |
78.42 (PP), 72.38 (PP + PET) |
The number of C3–C10 compounds increased. |
[32] |
|
Pelletized bentonite |
PS, PP, LDPE, HDPE |
500 |
88.5 (PS), 90.5 (PP), 87.6 (LDPE), 88.9 (HDPE) |
PS—95% aromatic hydrocarbons; PP, LDPE, and HDPE—aliphatic hydrocarbons; LDPE, and HDPE—diesel fuel (96% similarity); PS—gasohol 91. |
[33] |
|
Calcium bentonite |
PP, LDPE, HDPE, PP + LDPE + HDPE |
500 |
88.5 (PP), 82 (LDPE), 82.5 (HDPE) 81 (PP + LDPE + HDPE) |
The oil contained only a mixture of hydrocarbons and has matching fuel properties as that of fossil fuel. Mixed plastics—C10-C28. |
[34] |
|
Pillared bentonite (Al-PILC, Fe-PILC, Ti-PILC, Zr-PILC) |
HDPE + PS + PP + PET |
300–500 |
68.2 (Al-PILC), 79.3 (Fe-PILC), 62.8 (Ti-PILC), 62.1 (Zr-PILC) |
80.5% diesel fraction was observed in presence of Fe-PILC. |
[1] |
|
Fe/Al pillared montmorillonite mixed with an acid Commercial bentonite as a binder |
HDPE |
600 |
About 40 |
The catalyst gave high yields of waxes, particularly rich in diesel hydrocarbon range (C11–C21). |
[35] |
|
commercial acid-restructured montmorillonite and Al- and Fe/Al-pillared derivative |
MDPE |
300 |
About 70 |
The clay-based catalysts gave higher yields of liquid products in the C15–C20 range. Clay catalysts produce liquid hydrocarbons in the gasoline and diesel range. |
[36] |
|
Al2O3-pillared montmorillonite (calcium rich) |
LDPE |
430 |
70.2 |
Hydrocarbons from C5 to C13. |
[37] |
|
Montmorillonite (Zenith-N) and a pillared derivative |
LDPE |
427 |
68 (montmorillonite), 75 (pillared derivative) |
Clays showed enhanced liquid formation due to their mild acidity. |
[38] |
|
Al-pillared montmorillonite (Al-PILC), and regenerated samples |
LDPE |
360 |
72 (Al-PILC), 68 (regenerated sample) |
These products were in the boiling point range of motor engine fuels. |
[39] |
|
Montmorillonite (Zenith-N) and a pillared derivative |
LDPE |
360 |
75 (montmorillonite), 76 (pillared derivative) |
These products were in the boiling point range of gasoline. |
[40] |
|
Ionically bonding macrocyclic Zr-Zr complex to montmorillonite |
PP |
300–400 |
- |
A low molecular weight waxy product with paraffin wax characteristics was obtained. |
[41] |
|
Untreated and Al-pillared montmorillonite clay |
PS |
400 |
83.2 (untreated clay), 81.6 (Al-pillared clay) |
Styrene was the major product, and ethylbenzene was the second most abundant one in the liquid product. |
[42] |
|
Four different types of montmorillonites: K5, K10, K20, K30 |
LDPE, PP, and the municipal waste plastics |
begins at 250 for mK5 (LDPE), 210–435 for mK20 (PP) |
Data not presented |
The catalytic degradation products contain a relatively narrow distribution of light hydrocarbons. |
[43] |
|
Organically modified montmorillonite/Co3O4 |
PP + HDPE + PS |
700 |
59.6 |
The catalyst promoted the degradation of mixed plastics into light hydrocarbons and aromatics. |
[44] |
|
cloisite 15 A as a natural montmorillonite modified with a quaternary ammonium salt |
Industrial grade of HDPE, which was a copolymer with 1-hexene (1.5 wt%) as comonomer |
473.7 |
Data not presented |
It was found that the nano clay reduces the temperature at a maximum degradation rate. |
[45] |
|
Commercial acid-restructured saponite and Al- and Fe/Al-pillared derivatives |
MDPE |
300 |
About 70 |
The clay-based catalysts gave higher yields of liquid products in the C15–C20 range. Clay catalysts produce liquid hydrocarbons in the gasoline and diesel range. |
[36] |
|
Saponite, with a small number of impurities, mainly sepiolite and a pillared derivative |
LDPE |
427 |
83 (saponite), 82 (coked pillared derivative) |
Clays showed enhanced liquid formation due to their mild acidity. |
[38] |
|
Al-pillared saponite and regenerated samples |
LDPE |
360 |
72 (pillared saponite), 67 (regenerated sample) |
These products were in the boiling point range of motor engine fuels. |
[39] |
|
Saponite and a pillared derivative |
LDPE |
360 |
68 (saponite), 72 (pillared derivative) |
These products were in the boiling point range of gasoline. |
[40] |
|
Commercial acid-restructured beidellite and Al- and Fe/Al-pillared derivatives |
MDPE |
300 |
About 70 |
The clay-based catalysts gave higher yields of liquid products in the C15–C20 range. The catalysts produce liquid hydrocarbons in the gasoline and diesel range. |
[36] |
5. Other Clay Minerals’ Catalytic Activity
|
Catalyst |
Plastic |
Temperature, °C |
Highest Liquid Yield, wt% |
Specific Results |
Reference |
|---|---|---|---|---|---|
|
Commercial sepiolite |
PE, PP, PS, EVA |
432.65 (PE), 401.65 (PP), 449.75 (PS), 459.85 (EVA) |
Data not presented |
Clay reduces the decomposition temperatures of PE and PP. However, steric effects associated with the PS and EVA substituents nullify this catalytic behavior. |
[46] |
|
Tetraethyl silicate modified vermiculite, Co, and Ni intercalated vermiculite |
PP + PE |
300-480 |
80.6 (organic vermiculite), 73.2 (Co/verm), 70.7 (Ni/verm), 73.9 (Co/Ni/verm) |
The obtained liquid is mainly composed of C9–C12 and C13–C20. |
[47] |
|
Talc (French chalk) |
LDPE |
300 |
91 |
Components with a boiling point of 125–180°C were identified as alkanes, alkenes, and aromatics. |
[8] |
|
Talc (plastic filler) |
PP |
620 |
About 23 |
The liquid product contained a higher aromatic content (57.9%) and a lower n-alkene content (5.8%). |
[48] |
|
Pyrophyllite treated with hydrochloric acid |
PS |
450 |
88.3 |
The catalysts showed selectivity to aromatics over 99%. Styrene (63.40%) is the major product, and ethylbenzene is the second-most abundant one (6.93%). |
[22] |
6. Catalytic Activity of Mixed Natural Clays
|
Catalyst |
Plastic |
Temperature, °C |
Highest Liquid Yield, wt% |
Specific Results |
Reference |
|---|---|---|---|---|---|
|
Acid-activated fire clay (Pradeep Enterprises, Ajmeri Gate, Delhi) |
HDPE |
450 |
41.4 |
The identified compounds were mainly paraffins and olefins with a carbon number range of C6–C18. |
[49] |
|
Indian Fuller’s earth (Multan clay) |
LDPE |
300 |
58.33 |
The obtained liquid contained olefin, paraffin, and aromatic hydrocarbons. Light naphtha—15%, heavy naphtha—35%, middle distillate—60%. |
[50] |
|
Fuller’s earth |
LDPE |
300 |
91 |
Components with a boiling point of 125-180°C were identified as alkanes, alkenes, and aromatics. |
[8] |
|
Natural clay mineral (Indonesia) with LaFeO3 NPs |
PP |
460–480 |
88.8 (5th cycle) |
The liquid fraction: alkanes (44.70%), alkenes (34.84%), cyclo-alkanes (9.87%), cyclo-alkenes (3.07), branched-chain alkanes (2.42%), branched-chain alkenes (0.88%). |
[51] |
|
natural clay with kaolinite, hematite, smectite, quartz |
PS |
410 |
86.68 |
Fuel properties of the liquid fraction obtained showed a good resemblance with gasoline and diesel oil. |
[52] |
|
Red clay (Auburn, Alabama, USA) |
PS and LDPE (co-pyrolysis with a lignin) |
500, 600, 700, 800 |
data not presented |
The carbon yield of a lignin-derived compound, guaiacol, increased during co-pyrolysis of lignin with LDPE, and PS with red clay as a catalyst. |
[53] |
|
Shwedaung clay, Mabisan clay |
HDPE + LDPE + PS + PP + PET |
210-380 |
65.81 (Shwedaung clay), 67.06 (Mabisan clay) |
Fuel can be used internal combustion engine after distillation. Char can be used as solid fuel. |
[54] |
|
Fe-restructured clay (Fe-RC) |
PE + PP + PS + PVC + PET |
450 |
83.73 |
High selectivity for the C9–C12 and C13–C19 oil fractions, which are the major constituents of kerosene and diesel fuel. |
[55] |
|
Romanian natural clays: Vadu Crişului clay and Lugoj clay |
PS + PET + PVC |
420 |
62.18 (Vadu Crişului clay), 54.98 (Lugoj clay) |
The liquid products contained monoaromatic compounds such as styrene, toluene, ethylbenzene, or alpha-methylstyrene. |
[56] |
References
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-Pillared Clays for Efficient Catalytic Pyrolysis of Mixed Plastics. Chem. Eng. J. 2017, 317, 800–809.
- Giese, R.F. Kaolin Group Minerals. In Sedimentology; Springer: Dordrecht, The Netherlands, 1978; pp. 651–655.
- Liu, M.; Zhuo, J.K.; Xiong, S.J.; Yao, Q. Catalytic Degradation of High-Density Polyethylene over a Clay Catalyst Compared with Other Catalysts. Energy Fuels 2014, 28, 6038–6045.
- Kumar, S.; Singh, R.K. Optimization of Process Parameters by Response Surface Methodology (RSM) for Catalytic Pyrolysis of Waste High-Density Polyethylene to Liquid Fuel. J. Environ. Chem. Eng. 2014, 2, 115–122.
- Panda, A.K.; Singh, R.K. Thermo-Catalytic Degradation of Low Density Polyethylene to Liquid Fuel over Kaolin Catalyst. Int. J. Environ. Waste Manag. 2014, 13, 104.
- Luo, W.; Fan, Z.; Wan, J.; Hu, Q.; Dong, H.; Zhang, X.; Zhou, Z. Study on the Reusability of Kaolin as Catalysts for Catalytic Pyrolysis of Low-Density Polyethylene. Fuel 2021, 302, 121164.
- Soliman, A.; Farag, H.A.; Nassef, E.; Amer, A.; ElTaweel, Y. Pyrolysis of Low-Density Polyethylene Waste Plastics Using Mixtures of Catalysts. J. Mater. Cycles Waste Manag. 2020, 22, 1399–1406.
- Khan, K.; Hussain, Z. Comparison of the Catalytic Activity of the Commercially Available Clays for the Conversion of Waste Polyethylene into Fuel Products. J. Chem. Soc. Pakistan 2011, 33, 956–959.
- Erawati, E.; Hamid; Martenda, D. Kinetic Study on the Pyrolysis of Low-Density Polyethylene (LDPE) Waste Using Kaolin as Catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012071.
- Attique, S.; Batool, M.; Yaqub, M.; Goerke, O.; Gregory, D.H.; Shah, A.T. Highly Efficient Catalytic Pyrolysis of Polyethylene Waste to Derive Fuel Products by Novel Polyoxometalate/Kaolin Composites. Waste Manag. Res. 2020, 38, 689–695.
- Attique, S.; Batool, M.; Jalees, M.I.; Shehzad, K.; Farooq, U.; Khan, Z.; Ashraf, F.; Shah, A.T. Highly Efficient Catalytic Degradation of Low-Density Polyethylene Using a Novel Tungstophosphoric Acid/Kaolin Clay Composite Catalyst. Turkish J. Chem. 2018, 42, 684–693.
- Hakeem, I.G.; Aberuagba, F.; Musa, U. Catalytic Pyrolysis of Waste Polypropylene Using Ahoko Kaolin from Nigeria. Appl. Petrochem. Res. 2018, 8, 203–210.
- Uzair, M.A.; Waqas, A.; Khoja, A.H.; Ahmed, N. Experimental Study of Catalytic Degradation of Polypropylene by Acid-Activated Clay and Performance of Ni as a Promoter. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 3618–3624.
- Panda, A.K.; Singh, R. Catalytic Performances of Kaoline and Silica Alumina in the Thermal Degradation of Polypropylene. J. Fuel Chem. Technol. 2011, 39, 198–202.
- Panda, A.K.; Singh, R. Conversion of Waste Polypropylene to Liquid Fuel Using Acid-Activated Kaolin. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 997–1004.
- Panda, A.K.; Singh, R. Experimental Optimization of Process for the Thermo-Catalytic Degradation of Waste Polypropylene to Liquid Fuel. Adv. Energy Eng. 2013, 1, 74–84.
- Uzair, M.A.; Waqas, A.; Afzal, A.; Ansari, S.H.; Anees ur Rehman, M. Application of Acid Treated Kaolin Clay for Conversion of Polymeric Waste Material into Pyrolysis Diesel Fuel. In Proceedings of the 2014 International Conference on Energy Systems and Policies (ICESP), Islamabad, Pakistan, 24–26 November 2014; pp. 1–4.
- Ribeiro, A.M.; Machado Júnior, H.F.; Costa, D.A. Kaolin and Commercial fcc Catalysts in the Cracking of Loads of Polypropylene under Refinary Conditions. Braz. J. Chem. Eng. 2013, 30, 825–834.
- Hadi, B.; Sokoto, A.M.; Garba, M.M.; Muhammad, A.B. Effect of Neat Kaolin and Cuo/Kaolin on the Yield and Composition of Products from Pyrolysis of Polystyrene Waste. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 148–153.
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N.; Obasi, H.C.; Ugbaja, M.I. Study on the Effect of Kankara Zeolite-Y-Based Catalyst on the Chemical Properties of Liquid Fuel from Mixed Waste Plastics (MWPs) Pyrolysis. Polym. Bull. 2021, 78, 377–398.
- Auxilio, A.R.; Choo, W.-L.; Kohli, I.; Chakravartula Srivatsa, S.; Bhattacharya, S. An Experimental Study on Thermo-Catalytic Pyrolysis of Plastic Waste Using a Continuous Pyrolyser. Waste Manag. 2017, 67, 143–154.
- Cho, K.-H.; Jang, B.-S.; Kim, K.-H.; Park, D.-W. Performance of Pyrophyllite and Halloysite Clays in the Catalytic Degradation of Polystyrene. React. Kinet. Catal. Lett. 2006, 88, 43–50.
- Altaner, S.P. Smectite Group. In Sedimentology; Springer: Dordrecht, The Netherlands, 1978; pp. 1120–1124.
- Elordi, G.; Olazar, M.; Castaño, P.; Artetxe, M.; Bilbao, J. Polyethylene Cracking on a Spent FCC Catalyst in a Conical Spouted Bed. Ind. Eng. Chem. Res. 2012, 51, 14008–14017.
- Faillace, J.G.; de Melo, C.F.; de Souza, S.P.L.; da Costa Marques, M.R. Production of Light Hydrocarbons from Pyrolysis of Heavy Gas Oil and High Density Polyethylene Using Pillared Clays as Catalysts. J. Anal. Appl. Pyrolysis 2017, 126, 70–76.
- Hussain, Z.; Khan, K.; Jan, M.; Shah, J. Conversion of Low Density Polyethylene into Fuel Products Using Gachi Clay as Catalyst. J. Chem. Soc. Pakistan 2010, 32, 240–244.
- Qureshi, M.; Nisar, S.; Shah, R.; Salman, H. Studies of Liquid Fuel Formation from Plastic Waste by Catalytic Cracking over Modified Natural Clay and Nickel Nanoparticles. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2020, 63, 79–88.
- hamouda, A.; Abdelrahman, A.; Zaki, A.; Mohamed, H. Studying and Evaluating Catalytic Pyrolysis of Polypropylene. Egypt. J. Chem. 2021, 64, 2593–2605.
- Dewangga, P.B.; Rochmadi; Purnomo, C.W. Pyrolysis of Polystyrene Plastic Waste Using Bentonite Catalyst. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012110.
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Influence of Metal-Oxide-Supported Bentonites on the Pyrolysis Behavior of Polypropylene and High-Density Polyethylene. J. Appl. Polym. Sci. 2015, 132, 41221.
- Narayanan, K.S.; Anand, R.B. Experimental Investigation on Optimisation of Parameters of Thermo-Catalytic Cracking Process for H.D.P.E. & P.P. Mixed Plastic Waste with Synthesized Alumina-Silica Catalysts. Appl. Mech. Mater. 2014, 592–594, 307–311.
- Sembiring, F.; Purnomo, C.W.; Purwono, S. Catalytic Pyrolysis of Waste Plastic Mixture. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012020.
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic Pyrolysis of Plastic Waste for the Production of Liquid Fuels for Engines. RSC Adv. 2019, 9, 5844–5857.
- Panda, A.K. Thermo-Catalytic Degradation of Different Plastics to Drop in Liquid Fuel Using Calcium Bentonite Catalyst. Int. J. Ind. Chem. 2018, 9, 167–176.
- Borsella, E.; Aguado, R.; De Stefanis, A.; Olazar, M. Comparison of Catalytic Performance of an Iron-Alumina Pillared Montmorillonite and HZSM-5 Zeolite on a Spouted Bed Reactor. J. Anal. Appl. Pyrolysis 2018, 130, 320–331.
- De Stefanis, A.; Cafarelli, P.; Gallese, F.; Borsella, E.; Nana, A.; Perez, G. Catalytic Pyrolysis of Polyethylene: A Comparison between Pillared and Restructured Clays. J. Anal. Appl. Pyrolysis 2013, 104, 479–484.
- Olivera, M.; Musso, M.; De León, A.; Volonterio, E.; Amaya, A.; Tancredi, N.; Bussi, J. Catalytic Assessment of Solid Materials for the Pyrolytic Conversion of Low-Density Polyethylene into Fuels. Heliyon 2020, 6, e05080.
- Gobin, K.; Manos, G. Polymer Degradation to Fuels over Microporous Catalysts as a Novel Tertiary Plastic Recycling Method. Polym. Degrad. Stab. 2004, 83, 267–279.
- Manos, G.; Yusof, I.Y.; Gangas, N.H.; Papayannakos, N. Tertiary Recycling of Polyethylene to Hydrocarbon Fuel by Catalytic Cracking over Aluminum Pillared Clays. Energy Fuels 2002, 16, 485–489.
- Manos, G.; Yusof, I.Y.; Papayannakos, N.; Gangas, N.H. Catalytic Cracking of Polyethylene over Clay Catalysts. Comparison with an Ultrastable Y Zeolite. Ind. Eng. Chem. Res. 2001, 40, 2220–2225.
- Lal, S.; Anisia, K.S.; Jhansi, M.; Kishore, L.; Kumar, A. Development of Heterogeneous Catalyst by Ionically Bonding Macrocyclic Zr–Zr Complex to Montmorillonite Clay for Depolymerization of Polypropylene. J. Mol. Catal. A Chem. 2007, 265, 15–24.
- Cho, K.-H.; Cho, D.-R.; Kim, K.-H.; Park, D.-W. Catalytic Degradation of Polystyrene Using Albite and Montmorillonite. Korean J. Chem. Eng. 2007, 24, 223–225.
- Tomaszewska, K.; Kałużna-Czaplińska, J.; Jóźwiak, W. Thermal and Thermo-Catalytic Degradation of Polyolefins as a Simple and Efficient Method of Landfill Clearing. PJCT 2010, 12, 50–57.
- Gong, J.; Liu, J.; Jiang, Z.; Chen, X.; Wen, X.; Mijowska, E.; Tang, T. Converting Mixed Plastics into Mesoporous Hollow Carbon Spheres with Controllable Diameter. Appl. Catal. B Environ. 2014, 152–153, 289–299.
- Kebritchi, A.; Nekoomansh, M.; Mohammadi, F.; Khonakdar, H.A. Effect of Microstructure of High Density Polyethylene on Catalytic Degradation: A Comparison Between Nano Clay and FCC. J. Polym. Environ. 2018, 26, 1540–1549.
- Marcilla, A.; Gómez, A.; Menargues, S.; Ruiz, R. Pyrolysis of Polymers in the Presence of a Commercial Clay. Polym. Degrad. Stab. 2005, 88, 456–460.
- Chen, Z.; Wang, Y.; Sun, Z. Application of Co Ni Intercalated Vermiculite Catalyst in Pyrolysis of Plastics. J. Phys. Conf. Ser. 2021, 1885, 032030.
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic Pyrolysis of Plastic Wastes in a Continuous Microwave Assisted Pyrolysis System for Fuel Production. Chem. Eng. J. 2021, 418, 129412.
- Patil, L.; Varma, A.K.; Singh, G.; Mondal, P. Thermocatalytic Degradation of High Density Polyethylene into Liquid Product. J. Polym. Environ. 2018, 26, 1920–1929.
- Hussain, Z.; Khan, K.; Jan, M.; Shah, J. Conversion of Low Density Polyethylene into Fuel Products Using Indian Fuller’s Earth as Catalyst. J. Chem. Soc. Pak. 2010, 32, 790–793.
- Nguyen, L.T.T.; Poinern, G.E.J.; Le, H.T.; Nguyen, T.A.; Tran, C.M.; Jiang, Z. A LaFeO3 Supported Natural-Clay-Mineral Catalyst for Efficient Pyrolysis of Polypropylene Plastic Material. Asia-Pac. J. Chem. Eng. 2021, 16, e2695.
- Ali, G.; Nisar, J.; Shah, A.; Farooqi, Z.H.; Iqbal, M.; Shah, M.R.; Ahmad, H.B. Production of Liquid Fuel from Polystyrene Waste: Process Optimization and Characterization of Pyrolyzates. Combust. Sci. Technol. 2021, 1–14.
- Patil, V.; Adhikari, S.; Cross, P. Co-pyrolysis of Lignin and Plastics Using Red Clay as Catalyst in a Micro-Pyrolyzer. Bioresour. Technol. 2018, 270, 311–319.
- Kyaw, K.; Hmwe, C. Effect of Various Catalysts on Fuel Oil Pyrolysis Process of Mixed Plastic Wastes. Int. J. Adv. Eng. Technol. 2015, 8, 794.
- Lei, J.; Yuan, G.; Weerachanchai, P.; Lee, S.W.; Li, K.; Wang, J.-Y.; Yang, Y. Investigation on Thermal Dechlorination and Catalytic Pyrolysis in a Continuous Process for Liquid Fuel Recovery from Mixed Plastic Wastes. J. Mater. Cycles Waste Manag. 2018, 20, 137–146.
- Filip, M.; Pop, A.; Perhaiţa, I.; Trusca, R.; Rusu, T. The Effect of Natural Clays Catalysts on Thermal Degradation of a Plastic Waste Mixture. Adv. Eng. Forum 2013, 8–9, 103–114.




