
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaume Mora | -- | 2249 | 2022-06-04 17:02:00 | | | |
| 2 | Sirius Huang | Meta information modification | 2249 | 2022-06-06 04:07:50 | | |
Video Upload Options
Curing high-risk neuroblastoma (HR-NB) is a challenging endeavor, which involves the optimal application of several therapeutic modalities. High-dose therapy transformed clinical practice, legislation, and public health policy, and it drove a two-decade period of entrepreneurial oncology. However, no ASCT strategies remain for any solid tumor indication in adults. As with most solid malignancies, higher dosing of cytotoxic agents has not resulted in a clear benefit in survival for HR-NB patients, whereas the long-term toxicity has been well defined. Fortunately, novel approaches such as anti-GD2 immunotherapy have demonstrated a significant survival benefit with a much less adverse impact on the patient’s wellbeing.
1. Hematopoietic Stem-Cell Transplant: Historical Review
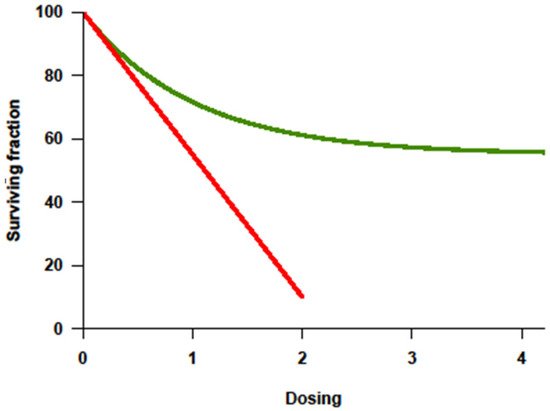
2. The Basic Principle of Transplantation Expanded to Solid Tumors: More Is Not Always Better
3. Anti-GD2 Immunotherapy and ASCT
References
- Jacobson, L.O.; Marks, E.K.; Robson, M.J.; Gaston, E.O.; Zirkle, R.E. Effect of spleen protection on mortality following X-irradiation. J. Lab. Clin. Med. 1949, 34, 1538–1543.
- Lorenz, E.; Uphoff, D.; Reid, T.R.; Shelton, E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J. Natl. Cancer Inst. 1951, 12, 197–201.
- Thomas, E.D.; Lochte, H.L., Jr.; Cannon, J.H.; Sahler, O.D.; Ferrebee, J.W. Supralethal whole body irradiation and isologous marrow transplantation in man. J. Clin. Investig. 1959, 38, 1709–1716.
- Mathé, G.; Amiel, J.L.; Schwarzenberg, L.; Catton, A.; Schneider, M. Adoptive immunotherapy of acute leukemia: Experimental and clinical results. Cancer Res. 1965, 25, 1525–1531.
- McGovern, J.J., Jr.; Russel, P.S.; Atkins, L.; Webster, E.W. Treatment of terminal leukemic relapse by total-body irradiation and intravenous infusion of stored autologous bone marrow obtained during remission. N. Engl. J. Med. 1959, 260, 675–683.
- Santos, G.W.; Sensenbrenner, L.L.; Burke, P.J.; Colvin, M.; Owens Jr, A.H.; Bias, W.B.; Slavin, R.E. Marrow transplantation in man following cyclophosphamide. Transplant. Proc. 1971, 3, 400–404.
- Santos, G.W. Busulfan (Bu) and cyclophosphamide (Cy) or marrow transplantation. Bone Marrow Transplant. 1989, 4, 236–239.
- Frei, E., III; Teicher, B.A.; Holden, S.A.; Cathcart, K.N.; Wang, Y.Y. Preclinical studies and clinical correlation of the effect of alkylating dose. Cancer Res. 1988, 48, 6417–6423.
- Frei, E.J.; Canellos, G.P. Dose: A critical factor in cancer chemotherapy. Am. J. Med. 1980, 69, 585–594.
- Frei, E., III; Antman, K.; Teicher, B.; Eder, P.; Schnipper, L. Bone marrow autotransplantation for solid tumors prospects. J. Clin. Oncol. 1989, 7, 515–526.
- MacNeil, M.; Eisenhauer, E.A. Adults: High-dose chemotherapy: Is it standard management for any common solid tumor? Ann. Oncol. 1999, 10, 1145–1161.
- Livingston, R.B. Dose intensity and high dose therapy. Cancer 1994, 74, 1177–1182.
- Marina, N.M.; Pappo, A.S.; Parham, D.M.; Cain, A.M.; Rao, B.N.; Poquette, C.A.; Pratt, C.B.; Greenwald, C.; Meyer, W.H. Chemotherapy dose-intensification for pediatric patients with Ewing’s family of tumors and desmoplastic small round-cell tumors: A feasibility study at St. Jude Children’s Research Hospital. J. Clin. Oncol. 1999, 17, 180–190.
- Whelan, J.; Khan, A.; Sharma, A.; Rothermundt, C.; Dileo, P.; Michelagnoli, M.; Seddon, B.; Strausss, S. Interval compressed vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide, etoposide in patients with advanced Ewing’s and other Small Round Cell Sarcomas. Clin. Sarcoma Res. 2012, 21, 12.
- Cheung, N.-K.V.; Heller, G. Chemotherapy Dose Intensity Correlates Strongly With Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. 1991, 9, 1050–1058.
- Savarese, D.M.F.; Hsieh, C.-C.; Stewart, F.M. Clinical Impact of Chemotherapy Dose Escalation in Patients With Hematologic Malignancies and Solid Tumors. J. Clin. Oncol. 1997, 15, 2981–2995.
- Rettig, R.A.; Jacobson, P.D.; Farquhar, C.M.; Aubry, W.M. False Hope: Bone Marrow Transplantation for Breast Cancer; Oxford University Press: London, UK, 2007; ISBN 978-0195187762.
- Haveman, L.M.; van Ewijk, R.; van Dalen, E.C.; Breunis, W.B.; Kremer, L.C.; van den Berg, H.; Dirksen, U.; Merks, J.H. High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with primary metastatic Ewing sarcoma. Cochrane Database Syst. Rev. 2021, 9, CD011405.
- Delafoy, M.; Verschuur, A.; Scheleirmacher, G.; Tabone, M.D.; Sudour-Bonnange, H.; Thébaud, E.; Freycon, C.; Notz-Carrère, A.; Boulanger, C.; Pellier, I.; et al. High-dose chemotherapy followed by autologous stem cell rescue in Wilms tumors: French report on toxicity and efficacy. Pediatric Blood Cancer 2022, 69, e29431.
- Peinemann, F.; Enk, H.; Smith, L.A. Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas. Cochrane Database Syst. Rev. 2017, 4, CD008216.
- Peinemann, F.; Kröger, N.; Bartel, C.; Grouven, U.; Pittler, M.; Erttmann, R.; Kulig, M. High-dose chemotherapy followed by autologous stem cell transplantation for metastatic rhabdomyosarcoma--a systematic review. PLoS ONE 2011, 6, e17127.
- Sauerbrey, A.; Bielack, S.; Kempf-Bielack, B.; Zoubek, A.; Paulussen, M.; Zintl, F. High-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) as salvage therapy for relapsed osteosarcoma. Bone Marrow Transplant. 2001, 27, 933–937.
- Häberle, B.; Maxwell, R.; von Schweinitz, D.; Schmid, I. High Dose Chemotherapy with Autologous Stem Cell Transplantation in Hepatoblastoma does not Improve Outcome. Results of the GPOH Study HB99. Klin. Pädiatrie 2019, 231, 283–290.
- Furtwängler, R.; Kager, L.; Melchior, P.; Rübe, C.; Ebinger, M.; Nourkami-Tutdibi, N.; Niggli, F.; Warmann, S.; Hubertus, J.; Amman, G.; et al. High-dose treatment for malignant rhabdoid tumor of the kidney: No evidence for improved survival-The Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH) experience. Pediatric Blood Cancer 2018, 65.
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Pa-tients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735.
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957.
- Kushner, B.H.; Kramer, K.; Cheung, N.-K.V. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte macrophage colony-stimulating factor for neuro-blastoma. J. Clin. Oncol. 2001, 19, 4189–4194.
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334.
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629.
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) þ Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189.
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173.
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309.
- Kushner, B.H.; O’Reilly, R.J.; Mandell, L.R.; Gulati, S.C.; LaQuaglia, M.; Cheung, N.K. Myeloablative combination chemotherapy without total body irradiation for neuroblastoma. J. Clin. Oncol. 1991, 9, 274–279.
- Kushner, B.H.; Gulati, S.C.; O’Reilly, R.J.; Heller, G.; Cheung, N.-K.V. Autografting with bone marrow exposed to multiple courses of very high dose cyclophosphamide in vivo and to 4-hydroperoxy-cyclophosphamide in vitro. Med. Pediatric Oncol. 1990, 18, 454–458.
- Kushner, B.H.; Hadju, S.I.; Gulati, S.C.; Erlandson, R.A.; Exelby, P.R.; Lieberman, P.H. Extracranial primitive neuroectodermal tumors: The Memorial Sloan-Kettering Cancer Center experience. Cancer 1991, 67, 1825–1829.
- Kushner, B.H.; Gulati, S.C.; Kwon, J.H.; O’Reilly, R.J.; Exelby, P.R.; Cheung, N.-K.V. High-dose melphalan with 6-hydroxydopamine-purged autologous bone marrow transplantation for poor-risk neuroblas-toma. Cancer 1991, 68, 242–247.
- Kushner, B.H.; Cheung, N.-K.V.; Kramer, K.; Dunkel, I.J.; Calleja, E.; Boulad, F. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transplant. 2001, 28, 551–556.
- Kushner, B.H.; Kramer, K.; Modak, S.; Kernan, N.A.; Reich, L.M.; Danis, K.; Cheung, N.-K.V. Topotecan, thiotepa, and carboplatin for neuroblastoma: Failure to prevent relapse in the central nervous system. Bone Marrow Transplant. 2006, 37, 271–276.
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Kramer, K.; Modak, S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 2012, 30, 3264–3270.
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Modak, S.; Kramer, K.; Roberts, S.S.; Basu, E.M.; Yataghene, K.; Cheung, N.-K.V. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget 2016, 7, 4155–4166.
- Kushner, B.H.; LaQuaglia, M.P.; Modak, S.; Wolden, S.L.; Basu, E.M.; Roberts, S.S.; Kramer, K.; Yataghene, K.; Cheung, I.Y.; Cheung, N.-K.V. MYCN-amplified stage 2/3 neuroblastoma: Excellent survival in the era of anti-GD2 immunotherapy. Oncotarget 2017, 8, 95293–95302.
- Mora, J.; Castañeda, A.; Flores, M.A.; Santa-María, V.; Garraus, M.; Gorostegui, M.; Simao, M.; Perez-Jaume, S.; Mañe, S. The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunothera-py. Results of Two Consecutive Studies. Front. Pharmacol. 2020, 11, 1699.
- Mora, J.; Castañeda, A.; Gorostegui, M.; Santa-María, V.; Garraus, M.; Muñoz, J.P.; Varo, A.; Perez-Jaume, S.; Mañe, S. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatric Blood Cancer 2021, 68, e29121.




