Curing high-risk neuroblastoma (HR-NB) is a challenging endeavor, which involves the optimal application of several therapeutic modalities. High-dose therapy transformed clinical practice, legislation, and public health policy, and it drove a two-decade period of entrepreneurial oncology. However, no ASCT strategies remain for any solid tumor indication in adults. As with most solid malignancies, higher dosing of cytotoxic agents has not resulted in a clear benefit in survival for HR-NB patients, whereas the long-term toxicity has been well defined. Fortunately, novel approaches such as anti-GD2 immunotherapy have demonstrated a significant survival benefit with a much less adverse impact on the patient’s wellbeing.
1. Hematopoietic Stem-Cell Transplant: Historical Review
The era of hematopoietic stem-cell transplantation began after the first atomic bomb explosion, with the pioneering observations by Leon Jacobson et al. in 1949. Mice were protected from the lethal effects of ionizing radiation on bone marrow (BM) by protecting their spleens with lead
[1]. Subsequently, Lorenz and colleagues showed that surviving lethal radiation could also be achieved by infusions of BM
[2]. The discovery showing that all hematopoietic stem cells arise from pluripotent transplantable stem cells led to the use of BM transplantation as a treatment for hematological malignancies. In the years 1959–1965, three landmark papers were published that described the first experiences with transplantation of BM into leukemia patients
[3][4][5][3,4,5]. In the first study, two patients with acute lymphoblastic leukemia received high-dose total body irradiation (TBI), followed by BM grafts from twin siblings
[3]. This was the first example of patients given supralethal irradiation who showed hematological recovery.
The second half of the 1960s provided a significant evolution of high-dose myeloablative conditioning regimens. These consisted of maximally tolerated doses of TBI and/or chemotherapeutic agents such as cyclophosphamide, which not only served to kill cancer cells, but also suppressed the immune system of the host so that BM grafts would not be rejected
[6]. The 1980s and 1990s showed how successful stem-cell transplantation could be safely applied with refined supportive care and broader application using alternative graft donors and sources. T-cell depletion and immunosuppressive agents were shown to minimize graft-versus-host disease. Drugs such as busulfan were identified as alternatives to TBI
[7]. In 1990, E. Donnall Thomas won a Nobel Prize for his pioneering research on stem-cell transplantation for the treatment of hematopoietic diseases, laying the foundations of modern stem-cell replacement therapy for human diseases including cancer. In the year 2000 alone, approximately 18,000 patients worldwide were treated with myeloablative approaches, a strategy accepted as the standard of care for a range of specific indications.
2. The Basic Principle of Transplantation Expanded to Solid Tumors: More Is Not Always Better
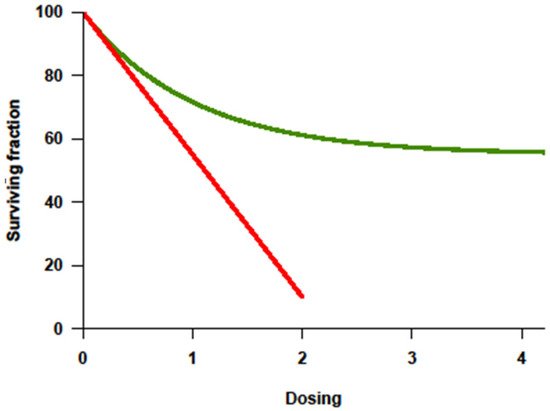
With the improvement of supportive therapy and sophistication in transplant-related techniques, treatment intensity for cancer became highly regarded in the 1990s partly because of the lessons from Hodgkin’s lymphoma. Higher cumulative dosage or higher dose intensity (DI) could be applied since myelotoxicity was no longer limiting in the face of stem-cell rescue. Investigative trials assumed that tumor response correlated positively with the dosage or intensity of drug(s) administered, and that this response would translate into improved overall survival (OS). Among the many preclinical studies, a steep log–linear dose–response curve was consistently documented for some cytotoxic agents (
Figure 1)
[8]. Increased chemotherapy drug dosage correlated with a log-fold increase in cell kill. It was postulated that, if myelotoxicity (the major dose-limiting toxicity of chemotherapeutic agents) could be reversed by stem-cell rescue, cure might be possible by increasing the dose or dose intensity of treatment. The principle supported autologous stem-cell transplant (ASCT) strategies. This linear–log relationship between chemotherapy agent dose and tumor cell killing suggested that, if the drug dose is increased without increasing toxicity to the patient, then a multiple log increase in tumor cell death would be observed
[9][10][9,10]. Few prior clinical studies were available but suggested a positive relationship
[9][10][9,10]. In addition to the limitation of extrapolating in vitro studies to human beings, there are underlying assumptions of this “cure by dosing” hypothesis, i.e., that the dose–response curve in patients is indeed linear, that drug resistance is overcome by increasing treatment intensity, that myelotoxicity is the only dose-limiting concern, and that response will translate into improved survival. Decades later, this hypothesis has been tested in multiple cancer types, and its success has been revealed to be disease-specific and qualified.
Figure 1. Dose–response curve showing log-fold increase in cell killing (
Y-axis = survivor cells) with increase in dosage (
X-axis) as the basis for high-dose therapy strategies. Adapted from original in Frei E III, Canellos GP: Dose: A critical factor in cancer chemotherapy. Am J Med 69:585–594, 1980.
[9].
Chemoresistance, be it intrinsic or acquired, is a key factor impacting response and the ability to achieve a cure. In chemotherapy-sensitive solid cancers, a high initial response rate to chemotherapy is commonly followed by subsequent relapse and resistance to further treatment. Tumors fitting this model usually show a dose–response behavior to drugs given in the conventional dose range. In principle, the drugs causing this effect should be appropriate for further dose escalation, with dose-limiting toxicity (DLT) primarily affecting the hematopoietic organ rescuable with hematopoietic stem cells. Ideally, their nonhematologic effects should be limited, and morbidity and mortality should be minimal. Inherent to this approach is the assumption that resistance can be overcome with the increase in dose, which can be achieved with stem-cell support
[11]. Alkylating agents are the most appropriate agents for dose escalation
[10]. There was evidence from cell lines and animal models of a steep dose–response curve for these compounds
[10], and resistance to alkylating agents had been overcome by a 5–10-fold increase in dose. Their DLT was primarily hematologic, and nonhematologic toxicities at high doses were non-overlapping. In contrast, anthracyclines are not suitable for dose escalation not only because of their cardiac and mucosal toxicities, but also because their cytotoxicity in preclinical studies leveled off. Antimetabolites and vinca alkaloids are also not suitable agents for high-dose therapy (HDT) not only because of their non-marrow toxicity limiting significant escalation, but also because their dose–response curves in vitro and in vivo are not linear
[10]. Dose–response curves for most chemotherapy agents were determined using in vitro and in animal models, with tumor cell killing being directly proportional to both dose and duration of exposure (
Figure 1, red line). Importantly, the steepness of the dose–response curve for most drugs showed a distinct plateau above which more drug does not appear to kill more tumor cells (
Figure 1, green line)
[7]. Furthermore, in humans, it is yet mostly unknown whether a plateau (
Figure 1, green line) is reached when drugs are administered at the maximal tolerated dose (MTD).
The concept of HDT refers to pulsed chemotherapy, which achieves high peak drug concentrations that theoretically increase the overall exposure of tumor cells to supratherapeutic levels of cytotoxic drugs
[12]. The concept of “dose intensity” (DI), however, is not the same as HDT. DI is defined as the amount of drug administered per unit of time (e.g., mg/m
2/week). A dose-intensive regimen may or may not yield high peak concentrations (HDT). Continuous administration of cyclophosphamide might be quite dose-intensive, but it could be associated with lower peak concentrations and less acute toxicity than a similarly dose-intensive, pulsed, high-dose regimen. Different trials have shown the importance of dose intensity in the treatment of small round cell tumors including sarcomas
[13][14][13,14] and neuroblastomas
[15].
Myelosuppression associated with cytotoxic therapy often limited the amount of drug that could be administered. This limitation was overcome to some degree with the use of hematopoietic cytokines such as granulocyte colony-stimulating factor (G-CSF) and techniques to support hematopoiesis such as autologous peripheral blood stem cells and/or BM. Prospective studies in adults with solid tumors showed that dose escalation with cytokine support did not increase cure rates in patients with chemotherapy-responsive solid tumors (testis cancer, small-cell lung cancer (SCLC), advanced ovarian cancer, non-Hodgkin’s lymphoma, or bladder cancer
[16]). The many reasons explaining this lack of benefit are as follows: (1) cytotoxic agents produced toxicity to nonhematopoietic tissues that limited the capacity to escalate doses further; (2) patients received extensive prior treatments that resulted in impairment of the BM function sufficient to blunt the response to cytokines; (3) the use of CSFs did not allow dose escalation beyond twofold higher than conventional doses.
Many clinical trials involving a variety of adult solid tumors were performed in the 1990s testing myeloablative therapy with BM transplant
[11]. For the most common tumors (breast, ovary, and SCLC) compelling evidence to support the use of myeloablative chemotherapy in standard patient management did not emerge. Randomized data comparing standard therapy to HDT was found unconvincing. In breast cancer, eight randomized trials were reported, and the data did not support the use of HDT in breast cancer. In SCLC, insufficient evidence supported the change in practice. Selection bias was found to account for favorable results in uncontrolled studies in these tumors, as many authors pointed out by reviewing outcomes of HDT candidates who received only standard therapy
[11]. HDT was ultimately determined to be ineffective and to have significantly greater toxicity. From its birth in the 1980s to its abandonment in the late 1990s, HDT transformed clinical practice, legislation, and public health policy, as well as drove a long period of entrepreneurial oncology. It also gave rise to one of the most serious cases of research misconduct of the 20th century
[17]. Today, no ASCT strategies remain for any solid tumor indication in adults. For pediatric tumors, except for specific subgroups of lymphomas and germ-cell tumors, no other extracranial solid tumor indication of ASCT has been established as standard management for high-risk cases. ASCT strategies have failed to show benefit for Ewing sarcomas
[18], Wilms tumors
[19], non-rhabdomyosarcoma soft-tissue sarcomas
[20], rhabdomyosarcomas
[21], osteosarcomas
[22], hepatoblastomas
[23], and rhabdoid tumors
[24].
3. Anti-GD2 Immunotherapy and ASCT
Fortunately, chemo-resistant NB is highly responsive to treatments far less toxic than myeloablative therapy, namely, anti-GD2 antibody + granulocyte–macrophage colony-stimulating factor (GM-CSF)
[25][57] ± low-dose chemotherapy
[26][58]. It is noteworthy that two anti-GD2 mAbs—dinutuximab and naxitamab—have received approval from the Food and Drug Administration in the USA.
Immunotherapy targeting GD2 emerged with the aim to eliminate chemotherapy-refractory disease, similar to HDT strategies, in the 1990s and early 2000s. The murine monoclonal antibody (mAb) 3F8 underwent extensive preclinical testing and, when administered with GM-CSF, led to significant responses in patients with refractory NB
[27][59]. Clinical trials of anti-GD2 mAb therapy demonstrated significantly improved EFS and, for the first time, OS when used after major responses to standard induction therapy and ASCT, leading to regulatory approval in the United States and Europe
[28][29][60,61]. The problem is that the two strategies (ASCT and anti-GD2 immunotherapy) targeting minimal residual disease (MRD) have not been formally evaluated independently.
The only randomized controlled trial involved HR-NB patients who received either anti-GD2 mAb dinutuximab-containing immunotherapy or
cis-RA after ASCT (ANBL0032)
[28][60]. The primary objective was an intention-to-treat comparison. At 2 years, the estimated EFS was 66% in the dinutuximab-containing immunotherapy arm and 46% in the standard therapy group. A subsequent report provided longer follow-up data on survival
[30][62]. The survival difference between the treatment groups remained statistically significant with a 5 year EFS of 56.6% for patients randomized to triple (dinutuximab, IL-2, and GM-CSF) immunotherapy versus 46.1% for those randomized to
cis-RA only (
p = 0.042). The 5 year OS was 73.2% versus 56.6% for triple immunotherapy versus
cis-RA only patients, respectively (
p = 0.045). The role of anti-GD2 immunotherapy after HDT and ASCT was clearly shown to be relevant for the survival of patients with HR-NB, and anti-GD2 immunotherapy was then adopted worldwide as the standard of care for all HR-NB patients in remission post ASCT. These results, however, question even further the role of HDT and ASCT in the era of anti-GD2 immunotherapy given that the landmark CCG-3891 study, as described earlier
[31][40], did not show a benefit in OS when tested without anti-GD2 mAb. More recently, a detailed analysis by the SIOPEN of risk factors and the relevance for each of the sequential therapies within the HR-NB multimodal treatment program (chemotherapy, ASCT, and immunotherapy), revealed that the impact of immunotherapy on EFS is significantly influenced by stage and pattern of metastases, but only borderline related to ASCT
[32][63].
Initial evidence of the superiority of anti-GD2 immunotherapy over HDT and ASCT for the management of patients with HR-NB came from the MSKCC group. This group had a great interest in and experience with transplant during the HDT era
[33][34][35][36][37][38][28,34,35,36,37,38] but discontinued it in 2003 when their analyses of sequential studies with or without ASCT and/or mAb 3F8 showed no survival benefit
[39][64]. In 2016, they first reported similar outcomes for HR-NB patients whose consolidative therapy of first CR/VGPR included 3F8/GM-CSF + isotretinoin with or without prior HDT and ASCT
[40][41][65,66]. They were able to accrue a large cohort of more than 100 patients treated without ASCT, a remarkable group of patients given that ASCT has remained in all major studies since the year 2000 and continues today. Independently, another group of researchers were able to recruit a cohort of non-ASCT patients (
n = 54) adding to that from MSKCC, increasing the population of HR-NB patients managed in the current era without ASCT and showing no difference in survival
[42][67]. Recent advances in anti-GD2 immunotherapy, such as more potent mAbs
[43][68], the lesser use of toxic IL-2
[29][61], and the systematic use of GM-CSF, may account for the current lack of survival advantage of ASCT. With all this evidence (or lack of it), discontinuing ASCT for HR-NB would be the logical step forward and consistent with the general consensus among (pediatric and adult) oncologists that this highly toxic treatment from the 20th century should no longer be recommended as long as anti-GD2 immunotherapy is broadly available to all children affected by HR-NB.