Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irena Baranowska-Bosiacka | -- | 2095 | 2022-05-30 14:22:04 | | | |
| 2 | Jessie Wu | + 4 word(s) | 2099 | 2022-05-31 02:51:00 | | | | |
| 3 | Jessie Wu | + 4 word(s) | 2103 | 2022-05-31 02:55:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Baranowska-Bosiacka, I.; Korbecki, J.; Gąssowska-Dobrowolska, M.; , .; Barczak, K.; Chlubek, M. CXCL1 in Noncancerous Diseases of Bone and Muscle. Encyclopedia. Available online: https://encyclopedia.pub/entry/23561 (accessed on 08 February 2026).
Baranowska-Bosiacka I, Korbecki J, Gąssowska-Dobrowolska M, , Barczak K, Chlubek M. CXCL1 in Noncancerous Diseases of Bone and Muscle. Encyclopedia. Available at: https://encyclopedia.pub/entry/23561. Accessed February 08, 2026.
Baranowska-Bosiacka, Irena, Jan Korbecki, Magdalena Gąssowska-Dobrowolska, , Katarzyna Barczak, Mikołaj Chlubek. "CXCL1 in Noncancerous Diseases of Bone and Muscle" Encyclopedia, https://encyclopedia.pub/entry/23561 (accessed February 08, 2026).
Baranowska-Bosiacka, I., Korbecki, J., Gąssowska-Dobrowolska, M., , ., Barczak, K., & Chlubek, M. (2022, May 30). CXCL1 in Noncancerous Diseases of Bone and Muscle. In Encyclopedia. https://encyclopedia.pub/entry/23561
Baranowska-Bosiacka, Irena, et al. "CXCL1 in Noncancerous Diseases of Bone and Muscle." Encyclopedia. Web. 30 May, 2022.
Copy Citation
CXCL1 is a chemokine crucial in inflammation as a chemoattractant for neutrophils in physiology and in selected major non-cancer diseases.
CXCL1
chemokine
cytokine
1. Cartilage and Bone Tissue
1.1. Bone, Fracture Healing, Osteoporosis
CXC motif chemokine ligand 1 (CXCL1) is involved in the physiology and pathology of bone tissue. Most data are based on studies of KC, a mouse paralog for human CXCL1, and for this reason require confirmation in research on humans.
The expression of ligands for CXCR2, such as KC and MIP-2, has been shown to be elevated in murine osteocytes under shear stress [1][2] and by parathormone (PTH) and parathyroid hormone-related protein (PTHrP) [3]. This increase in KC expression causes osteoclast precursors to migrate [1], and the subsequent activation of CXCR2 on these cells enhances osteoclast maturation [4]. This is followed by either bone remodeling or bone resorption under the influence of factors that stimulate KC expression. Human osteoclast precursors exhibit CXCR2 expression [5] but it is reduced during differentiation of these cells into osteoclasts. It appears that CXCL1 may have the same properties in bone as KC, and so may participate in bone modelling in humans, although this should be confirmed by further studies.
Due to the induction of osteoclast maturation by CXCR2 ligands, CXCL1 levels are positively correlated with osteoporosis in humans [6]. Also, bone marrow adipocytes produce ligands for CXCR2 [4], which leads to the weakened bone structure in mice with advanced age or obesity [4]. If a similar mechanism occurs in humans, then this could account for the frequent bone fractures in older people or those with obesity. CXCL1 is also important in fractures—a condition associated with an increase in KC expression in mice [7]. This chemokine is indirectly important in fracture healing, via neutrophils recruited by this chemokine [7].
The expression of ligands for CXCR2 by bone marrow adipocytes may support the formation of cancer bone metastasis in elderly or obese people [4]. Also, the increased expression of ligands for CXCR2 in osteocytes under shear stress and PTHrP may support bone metastasis of some cancers, including breast cancer [2][8]. Not less important is CXCL1 production by cancer cells in bone metastasis [9], as CXCL1 stimulates cancer cell proliferation [10][11][12], as well as participating in bone remodeling during bone metastasis formation [4][9]. If a tumor cell from the blood stops in bone tissue, it causes bone remodeling by secreting CXCL1 and hence bone metastasis.
1.2. Bone Marrow
Ligands for CXCR2, including CXCL1, are important in the self-renewal capacity of hematopoietic stem cells [13]. Human CD34+CD38- express CXCL1 as well as other ligands for CXCR2 such as CXCL2, CXCL6 and CXCL8/IL-8—chemokines crucial for hematopoietic stem cell maintenance.
CXCL1 is also significant in the regulation of whole body immunity via neutrophil egress from the bone marrow [14][15]. Two axes are responsible for the regulation of neutrophil release from the bone marrow. CXCL12/SDF-1→CXCR4 is responsible for the retention of neutrophils and homing of senescent neutrophils to the bone marrow [14], while CXCR2 ligands are responsible for neutrophil egress from the bone marrow [14][15]. Also, pro-inflammatory factors in the blood, such as LPS, increase CXCL1 expression in endothelial cells in the bone marrow—an effect dependent on β-adrenergic signaling [15] The release of neutrophils under the influence of pro-inflammatory factors in the blood is important in the fight against pathogens. Acute inflammation increases the levels of pro-inflammatory cytokines in the blood leading to the mobilization of neutrophils and subsequent accumulation of these cells at sites of intense inflammatory responses.
Chronic inflammation is associated with elevated levels of ligands for CXCR2, including CXCL1, which cause the expansion of monocytic myeloid-derived suppressor cell (MDSC) in the bone marrow as shown in mice [16]. This is associated with CXCR2 activation on granulocyte and macrophage progenitor cells (GMPs) [17], which reduces the expression of Sin3-associated 18 kDa polypeptide (SAP18). This, in turn, results in the activation of extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3), which increases granulocyte monocyte progenitor (GMP) differentiation into macrophages and dendritic cell progenitor cells (MDP) [17]. Subsequently, in the bone marrow, MDP differentiate into monocytic MDSC, resulting in an increase in the number of these cells. This effect is important in diseases with chronic inflammation. Expansion of monocytic MDSC in the bone marrow results in an increase in the number of these cells in the blood, which leads to an overall weakening of the immune system.
1.3. Rheumatoid Arthritis
Rheumatoid arthritis, estimated to affect less than 1% of the human population, is an autoimmune disease that is characterized by chronic inflammation which results in the destruction of joints [18]. One component of the pathophysiology of rheumatoid arthritis is an increase in CXCL1 expression in rheumatoid arthritis patients in the blood [19] and synovial fluid [20][21]. At the same time, CXCL1 expression in synovial fluid is higher in patients with rheumatoid arthritis than in those with osteoarthritis [19][21][22].
CXCL1 in the synovial fluid comes from fibroblast-like synoviocytes (FLS), chondrocytes and neutrophils (Figure 1). In particular, increased CXCL1 expression occurs in the lining layer [23]. FLS increases the expression of CXCL1 under the influence of pro-inflammatory cytokines such as TNF-α and IL-1β [20][24], whose expression is also increased in rheumatoid arthritis patients [25][26]. That means that chronic inflammation in joints increases the expression of TNF-α and IL-1β, which increases the expression of CXCL1. The synovial fluid in patients with rheumatoid arthritis also show increased levels of IL-17 [27], a cytokine that increases CXCL1 expression, particularly in FLS [28]. In FLS, the expression of CXCL1 is also increased by resistin, an adipokine produced by macrophages located in the synovium in patients with rheumatoid arthritis [29].
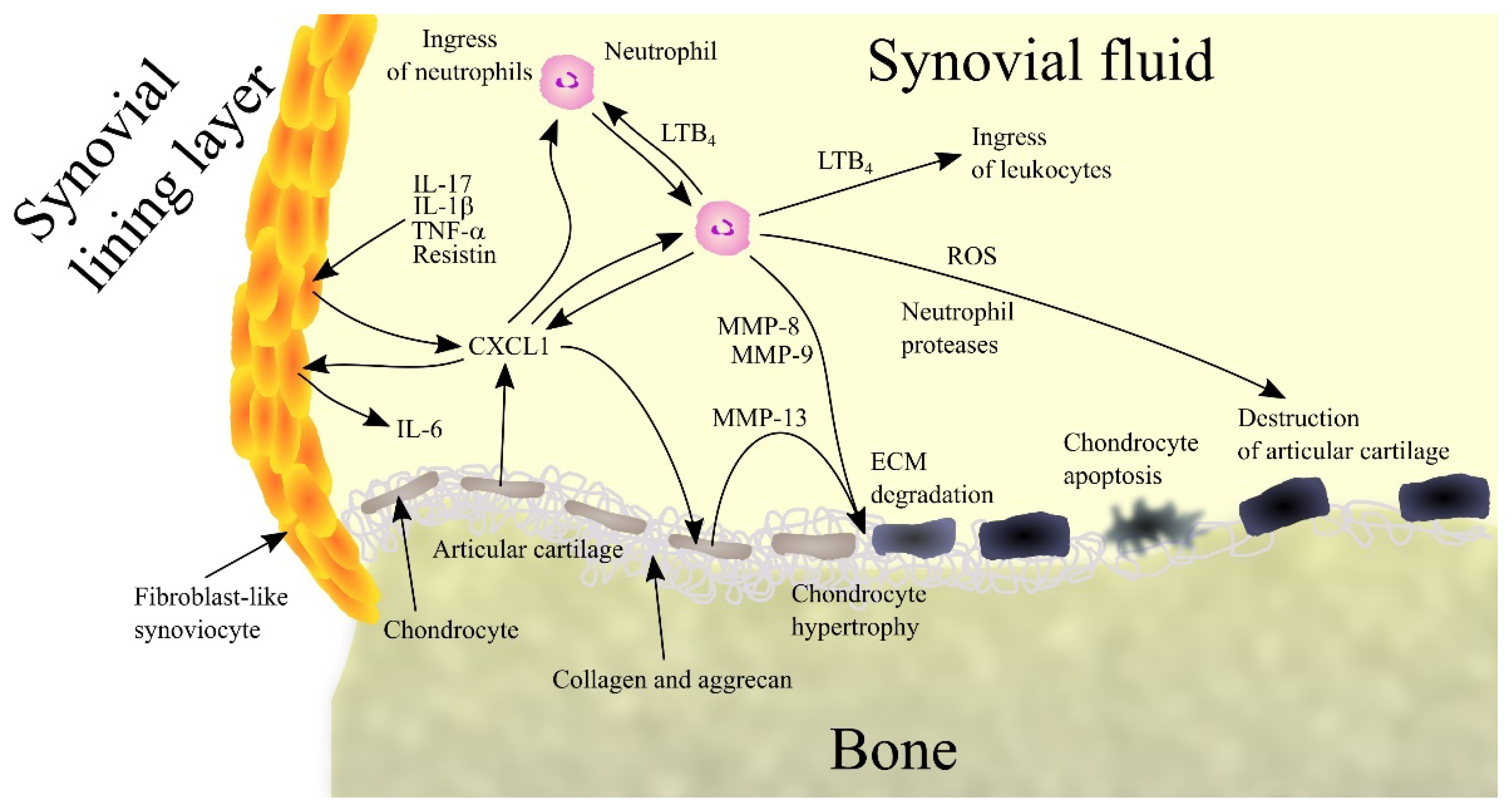
Figure 1. Importance of CXC motif chemokine ligand 1 (CXCL1) and neutrophils in rheumatoid arthritis. Patients with rheumatoid arthritis have elevated levels of interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α), interleukin-17 (IL-17) and resistin in synovial fluid. These factors induce CXCL1 expression in fibroblast-like synoviocytes (FLS), chondrocytes and neutrophils, which leads to an increase in CXCL1 levels in synovial fluid. CXCL1 causes an increase in interleukin-6 (IL-6) expression in FLS. This chemokine causes leukotriene B4 (LTB4) synthesis in neutrophils and thus an ingress of more neutrophils and leukocytes into the joints. CXCL1 also causes an increase in matrix metalloproteinase-13 (MMP-13) expression in chondrocytes, which leads to extracellular matrix (ECM) articular cartilage degradation. Also responsible for this process are matrix metalloproteinase-8 (MMP-8) and matrix metalloproteinase-9 (MMP-9) produced by neutrophils. CXCL1 also causes chondrocyte hypertrophy and apoptosis. Finally, neutrophils secrete reactive oxygen species (ROS) and neutrophil proteases into synovial fluid. All these processes and factors lead to the destruction of articular cartilage and symptoms of rheumatoid arthritis. Abbreviations: CXCL1—CXC motif chemokine ligand 1; ECM—extracellular matrix; IL-1β—interleukin-1β; IL-6—interleukin-6; IL-17—interleukin-17; LTB4—leukotriene B4; MMP—matrix metalloproteinase; ROS - reactive oxygen species; TNF-α - tumor necrosis factor α; Source: own elaboration.
CXCL1 participates in rheumatoid arthritis by acting on various cells in the joints. It causes hypertrophy of chondrocytes [30] resulting in an elevated expression of MMP-13, an enzyme that degrades collagen and aggrecan. This results in degradation of ECM in articular cartilage followed by apoptosis of chondrocytes and degradation of cartilage in the joints.
CXCL1 also acts on FLS. Although it does not cause the proliferation of FLS [31], it does reduce collagen production in these cells, which interferes with the normal function of these cells in the joints. CXCL1 also increases the production of IL-6 in FLS [21], one of the factors causing an increase in IL-6 in synovial fluid in patients with rheumatoid arthritis; such a response does not occur in healthy individuals [21][24]. IL-6 is a cytokine that is involved in rheumatoid arthritis by causing bone resorption and by participating in inflammatory reactions [32].
CXCL1 causes an ingress of neutrophils into the joints [33][34], a process that also appears to require LTB4 [34]. CXCL1 has also been shown to act on neutrophils in the joints by increasing the production and secretion of LTB4 in these cells [34]. This bioactive lipid causes an ingress of leukocytes into joints, where they act destructively on joint tissue and thus contribute to the pathogenesis of rheumatoid arthritis. Neutrophils also produce MMP-8 and MMP-9 which degrade collagen [35]. Neutrophils also produce ROS, which have a destructive effect on joint tissue, and various proteases such as elastase, cathepsin G and proteinase-3, which are involved in joint tissue destruction and inflammatory reactions.
CXCL1 can also increase osteoclast activity, which leads to bone erosion [4][5][6]. However, the importance of CXCL1 in the destruction of bone tissue in the joints of patients with rheumatoid arthritis is yet to be thoroughly investigated.
2. Muscles
CXCL1 may play an important physiological role, particularly in muscle function. However, due to the lack of an appropriate research model, these are assumptions drawn from a mouse model for changes in KC chemokine expression. Therefore, this physiological aspect requires further studies on humans.
2.1. Muscle Physiology
Exercise is associated with an increase in the expression of IL-6 and CXC chemokines that are ligands for CXCR2, such as KC and lipopolysaccharide-induced CXC chemokine (LIX) in the muscle and blood of mice [36][37][38][39][40]. Significantly, the increase in KC expression in the muscle is independent of IL-6 [40]. IL-6 from the muscle travels via the blood to the liver, where the expression of KC increases, which then is responsible for the increase in blood KC levels [38]. KC also acts in an autocrine manner on muscle via CXCR2, which induces an increase in muscle insulin responsiveness, specifically an increase in glucose transporter 4 (GLUT4) recycling [36]. However, the same authors in a later study question the effect of KC and LIX on GLUT4 recycling in muscle [37]. KC also increases fatty acid oxidation [39] and muscle angiogenesis (Figure 2) [39].
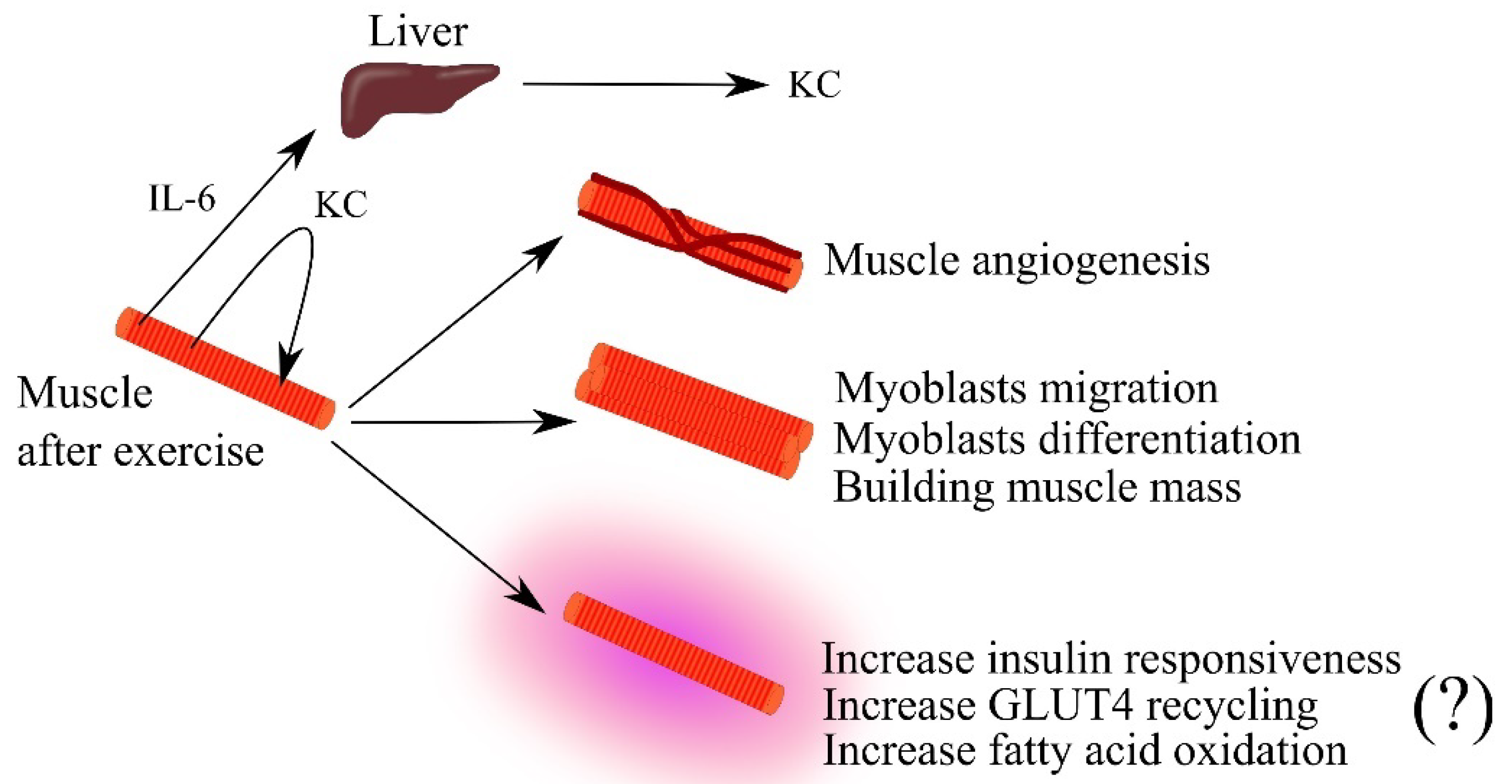
Figure 2. The importance of keratinocyte-derived chemokine (KC) in skeletal muscle physiology. Intense effort induces an increase in the expression of interleukin-6 (IL-6) and KC in muscle. IL-6 travels through the bloodstream to the liver where it increases KC expression. This leads to an increase in blood levels of this chemokine. KC secreted by muscle acts in an autocrine manner, causing muscle angiogenesis, the growth of muscle mass by acting on myoblasts, and increasing muscle efficiency by increasing insulin responsiveness and fatty acid oxidation in the muscle. Abbreviations: (?)—mechanism in question; GLUT4—glucose transporter 4; IL-6—interleukin-6; KC—keratinocyte-derived chemokine; Source: own elaboration.
KC is also considered a myokine as it causes proliferation, self-renewal of satellite cells and myogenesis from satellite cells—stem cells present in muscles that participate in regeneration [41]. KC is also a chemotactic factor for myoblasts and causes myogenic differentiation of these cells [37]. As a consequence of the action of KC, there is an expansion of the muscle and an increase in muscle efficiency. Also of note is the exercise-induced increase in blood levels of KC in mice, and most likely also CXCL1 in humans [38]. KC and CXCL1 cause the mobilization of neutrophils from the bone marrow, whose function is to destroy pathogens [14]. It can be speculated that exercise in the described mechanism may enhance immunity.
The expression of ligands for CXCR2 is also subject to upregulation in muscle regeneration, as shown by studies in cattle [42]. Their role in muscle regeneration is additionally indicated by the fact that their expression is tightly regulated by myostatin [42]. Further research in this area is required to determine the exact mechanism of muscle regeneration.
2.2. Muscle, CXC Motif Chemokine Ligand 1 (CXCL1) and Obesity
CXCL1 participates in muscle disease mechanisms. Saturated fatty acids, particularly palmitate, cause myotube loss [43] which is associated with a decrease in the expression of certain myokines. At the same time, palmitate also increases the expressions of CXCL1 in human muscle and KC in mouse muscle [41]. In mice, KC stimulates proliferation and self-renewal of satellite cells [41] and thus it counteracts the negative effects of palmitate on muscle. A similar mechanism may occur in humans—palmitate may increase the expression of CXCL1 in muscle which then inhibits the adverse effect of this acid. This process is of importance as ~60% of the North American and European populations are overweight [44].
2.3. Tumor-Induced Muscle Wasting
CXCL1 may also participate in tumor-induced muscle atrophy, one of the components of cancer cachexia [45][46]. This section shows the effect that chronic inflammation has on muscle, as in advanced cancer. Patients with breast cancer [47], esophageal squamous cell carcinoma [48], ovarian cancer [49] and renal cell carcinoma [50] have elevated levels of CXCL1 in the blood. In addition, studies in mouse models have shown that factors from tumorigenesis increase KC expression in muscle [45]. KC, produced in muscle as well as secreted from a tumor, impairs myoblast differentiation, leading to muscle atrophy. This effect is also enhanced by other factors from the tumor such as insulin like growth factor binding protein 3 (IGFBP3) and CC motif chemokine ligand 2 (CCL2) [45]. Another mechanism by which KC causes tumor-induced muscle atrophy is the infiltration of skeletal muscle by immune cells, including neutrophils and macrophages [45]. These cells suppress myogenic differentiation, leading to tumor-induced muscle atrophy.
References
- Govey, P.M.; Jacobs, J.M.; Tilton, S.C.; Loiselle, A.E.; Zhang, Y.; Freeman, W.M.; Waters, K.M.; Karin, N.J.; Donahue, H.J. Integrative transcriptomic and proteomic analysis of osteocytic cells exposed to fluid flow reveals novel mechano-sensitive signaling pathways. J. Biomech. 2014, 47, 1838–1845.
- Dwivedi, A.; Kiely, P.A.; Hoey, D.A. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem. Biophys. Res. Commun. 2021, 534, 14–20.
- Onan, D.; Allan, E.H.; Quinn, J.M.; Gooi, J.H.; Pompolo, S.; Sims, N.A.; Gillespie, M.T.; Martin, T.J. The chemokine Cxcl1 is a novel target gene of parathyroid hormone (PTH)/PTH-related protein in committed osteoblasts. Endocrinology 2009, 150, 2244–2253.
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin. Exp. Metastasis 2015, 32, 353–368.
- Grassi, F.; Piacentini, A.; Cristino, S.; Toneguzzi, S.; Cavallo, C.; Facchini, A.; Lisignoli, G. Human osteoclasts express different CXC chemokines depending on cell culture substrate: Molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem. Cell Biol. 2003, 120, 391–400.
- Hu, Y.; Wang, L.; Zhao, Z.; Lu, W.; Fan, J.; Gao, B.; Luo, Z.; Jie, Q.; Shi, X.; Yang, L. Cytokines CCL2 and CXCL1 may be potential novel predictors of early bone loss. Mol. Med. Rep. 2020, 22, 4716–4724.
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cells Mater. 2016, 32, 152–162.
- Washam, C.L.; Byrum, S.D.; Leitzel, K.; Ali, S.M.; Tackett, A.J.; Gaddy, D.; Sundermann, S.E.; Lipton, A.; Suva, L.J. Identification of PTHrP(12-48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol. Biomark. Prev. 2013, 22, 972–983.
- Lee, Y.C.; Gajdosik, M.S.; Josic, D.; Clifton, J.G.; Logothetis, C.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell. Proteom. 2015, 14, 471–483.
- Li, A.; Varney, M.L.; Singh, R.K. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin. Exp. Metastasis 2004, 21, 571–579.
- Bolitho, C.; Hahn, M.A.; Baxter, R.C.; Marsh, D.J. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr. Relat. Cancer 2010, 17, 929–940.
- Bhat, K.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. GROα overexpression drives cell migration and invasion in triple negative breast cancer cells. Oncol. Rep. 2017, 38, 21–30.
- Sinclair, A.; Park, L.; Shah, M.; Drotar, M.; Calaminus, S.; Hopcroft, L.E.; Kinstrie, R.; Guitart, A.V.; Dunn, K.; Abraham, S.A.; et al. CXCR2 and CXCL4 regulate survival and self-renewal of hematopoietic stem/progenitor cells. Blood 2016, 128, 371–383.
- Martin, C.; Burdon, P.C.; Bridger, G.; Gutierrez-Ramos, J.C.; Williams, T.J.; Rankin, S.M. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 2003, 19, 583–593.
- Ao, T.; Kikuta, J.; Sudo, T.; Uchida, Y.; Kobayashi, K.; Ishii, M. Local sympathetic neurons promote neutrophil egress from the bone marrow at the onset of acute inflammation. Int. Immunol. 2020, 32, 727–736.
- Shi, H.; Han, X.; Sun, Y.; Shang, C.; Wei, M.; Ba, X.; Zeng, X. Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 2018, 109, 3826–3839.
- Han, X.; Shi, H.; Sun, Y.; Shang, C.; Luan, T.; Wang, D.; Ba, X.; Zeng, X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019, 10, 598.
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038.
- Skrzypkowska, M.; Stasiak, M.; Sakowska, J.; Chmiel, J.; Maciejewska, A.; Buciński, A.; Słomiński, B.; Trzonkowski, P.; Łuczkiewicz, P. Cytokines and chemokines multiplex analysis in patients with low disease activity rheumatoid arthritis. Rheumatol. Int. 2022, 42, 609–619.
- Hogan, M.; Sherry, B.; Ritchlin, C.; Fabre, M.; Winchester, R.; Cerami, A.; Bucala, R. Differential expression of the small inducible cytokines GRO alpha and GRO beta by synovial fibroblasts in chronic arthritis: Possible role in growth regulation. Cytokine 1994, 6, 61–69.
- Hou, S.M.; Chen, P.C.; Lin, C.M.; Fang, M.L.; Chi, M.C.; Liu, J.F. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 2020, 22, 251.
- Borzi, R.M.; Mazzetti, I.; Macor, S.; Silvestri, T.; Bassi, A.; Cattini, L.; Facchini, A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: Constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999, 455, 238–242.
- König, A.; Krenn, V.; Toksoy, A.; Gerhard, N.; Gillitzer, R. Mig, GRO alpha and RANTES messenger RNA expression in lining layer, infiltrates and different leucocyte populations of synovial tissue from patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Virchows Arch. 2000, 436, 449–458.
- Bertazzolo, N.; Punzi, L.; Stefani, M.P.; Cesaro, G.; Pianon, M.; Finco, B.; Todesco, S. Interrelationships between interleukin (IL)-1, IL-6 and IL-8 in synovial fluid of various arthropathies. Agents Actions 1994, 41, 90–92.
- Hussein, M.R.; Fathi, N.A.; El-Din, A.M.; Hassan, H.I.; Abdullah, F.; Al-Hakeem, E.; Backer, E.A. Alterations of the CD4+, CD8+ T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: Preliminary observations. Pathol. Oncol. Res. 2008, 14, 321–328.
- Zheng, Y.; Sun, L.; Jiang, T.; Zhang, D.; He, D.; Nie, H. TNFα promotes Th17 cell differentiation through IL-6 and IL-1β produced by monocytes in rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 385352.
- Roşu, A.; Mărgăritescu, C.; Stepan, A.; Muşetescu, A.; Ene, M. IL-17 patterns in synovium, serum and synovial fluid from treatment-naïve, early rheumatoid arthritis patients. Rom. J. Morphol. Embryol. 2012, 53, 73–80.
- Kehlen, A.; Thiele, K.; Riemann, D.; Langner, J. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin. Exp. Immunol. 2002, 127, 539–546.
- Sato, H.; Muraoka, S.; Kusunoki, N.; Masuoka, S.; Yamada, S.; Ogasawara, H.; Imai, T.; Akasaka, Y.; Tochigi, N.; Takahashi, H.; et al. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 263.
- Olivotto, E.; Vitellozzi, R.; Fernandez, P.; Falcieri, E.; Battistelli, M.; Burattini, S.; Facchini, A.; Flamigni, F.; Santi, S.; Facchini, A.; et al. Chondrocyte hypertrophy and apoptosis induced by GROalpha require three-dimensional interaction with the extracellular matrix and a co-receptor role of chondroitin sulfate and are associated with the mitochondrial splicing variant of cathepsin B. J. Cell. Physiol. 2007, 210, 417–427.
- Unemori, E.N.; Amento, E.P.; Bauer, E.A.; Horuk, R. Melanoma growth-stimulatory activity/GRO decreases collagen expression by human fibroblasts. Regulation by C-X-C but not C-C cytokines. J. Biol. Chem. 1993, 268, 1338–1342.
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol. Ther. 2020, 7, 473–516.
- Coelho, F.M.; Pinho, V.; Amaral, F.A.; Sachs, D.; Costa, V.V.; Rodrigues, D.H.; Vieira, A.T.; Silva, T.A.; Souza, D.G.; Bertini, R.; et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheumatol. 2008, 58, 2329–2337.
- Grespan, R.; Fukada, S.Y.; Lemos, H.P.; Vieira, S.M.; Napimoga, M.H.; Teixeira, M.M.; Fraser, A.R.; Liew, F.Y.; McInnes, I.B.; Cunha, F.Q. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheumatol. 2008, 58, 2030–2040.
- O’Neil, L.J.; Kaplan, M.J. Neutrophils in Rheumatoid Arthritis: Breaking Immune Tolerance and Fueling Disease. Trends Mol. Med. 2019, 25, 215–227.
- Nedachi, T.; Fujita, H.; Kanzaki, M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1191–E1204.
- Nedachi, T.; Hatakeyama, H.; Kono, T.; Sato, M.; Kanzaki, M. Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E866–E878.
- Pedersen, L.; Pilegaard, H.; Hansen, J.; Brandt, C.; Adser, H.; Hidalgo, J.; Olesen, J.; Pedersen, B.K.; Hojman, P. Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J. Physiol. 2011, 589, 1409–1420.
- Pedersen, L.; Olsen, C.H.; Pedersen, B.K.; Hojman, P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E831–E840.
- Farmawati, A.; Kitajima, Y.; Nedachi, T.; Sato, M.; Kanzaki, M.; Nagatomi, R. Characterization of contraction-induced IL-6 up-regulation using contractile C2C12 myotubes. Endocr. J. 2013, 60, 137–147.
- Masuda, S.; Tanaka, M.; Inoue, T.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; Satoh-Asahara, N. Chemokine (C-X-C motif) ligand 1 is a myokine induced by palmitate and is required for myogenesis in mouse satellite cells. Acta Physiol. 2018, 222, e12975.
- Iwasaki, S.; Miyake, M.; Hayashi, S.; Watanabe, H.; Nagasawa, Y.; Terada, S.; Watanabe, K.; Ohwada, S.; Kitazawa, H.; Rose, M.T.; et al. Effect of myostatin on chemokine expression in regenerating skeletal muscle cells. Cells Tissues Organs 2013, 198, 66–74.
- Yang, M.; Wei, D.; Mo, C.; Zhang, J.; Wang, X.; Han, X.; Wang, Z.; Xiao, H. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 2013, 12, 104.
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10.
- Hogan, K.A.; Cho, D.S.; Arneson, P.C.; Samani, A.; Palines, P.; Yang, Y.; Doles, J.D. Tumor-derived cytokines impair myogenesis and alter the skeletal muscle immune microenvironment. Cytokine 2018, 107, 9–17.
- Callaway, C.S.; Delitto, A.E.; Patel, R.; Nosacka, R.L.; D’Lugos, A.C.; Delitto, D.; Deyhle, M.R.; Trevino, J.G.; Judge, S.M.; Judge, A.R. IL-8 Released from Human Pancreatic Cancer and Tumor-Associated Stromal Cells Signals through a CXCR2-ERK1/2 Axis to Induce Muscle Atrophy. Cancers 2019, 11, 1863.
- Divella, R.; Daniele, A.; Savino, E.; Palma, F.; Bellizzi, A.; Giotta, F.; Simone, G.; Lioce, M.; Quaranta, M.; Paradiso, A.; et al. Circulating levels of transforming growth factor-βeta (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013, 33, 1491–1497.
- Zhang, H.; Yue, J.; Jiang, Z.; Zhou, R.; Xie, R.; Xu, Y.; Wu, S. CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017, 8, e2790.
- Wang, Q.; Li, D.; Zhang, W.; Tang, B.; Li, Q.Q.; Li, L. Evaluation of proteomics-identified CCL18 and CXCL1 as circulating tumor markers for differential diagnosis between ovarian carcinomas and benign pelvic masses. Int. J. Biol. Markers 2011, 26, 262–273.
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J. Immunol. 2005, 175, 5351–5357.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
31 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




