
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jidapa Watcharakitti | -- | 2253 | 2022-05-28 05:53:43 | | | |
| 2 | Lindsay Dong | Meta information modification | 2253 | 2022-05-30 03:33:09 | | |
Video Upload Options
Consumer trends towards environmentally friendly products are driving plastics industries to investigate more benign alternatives to petroleum-based polymers. In the case of adhesives, one possibility to achieve sustainable production is to use non-toxic, low-cost starches as biodegradable raw materials for adhesive production. While native starch contains only hydroxyl groups and has limited scope, chemically modified starch shows superior water resistance properties for adhesive applications. Esterified starches, starches with ester substituents, can be feasibly produced and utilized to prepare bio-based adhesives with improved water resistance. Syntheses of esterified starch materials can involve esterification, transesterification, alkylation, acetylation, succinylation, or enzymatic reactions.
1. Introduction
Starch is one of the most abundant polysaccharides in nature, consisting of amylose and amylopectin in various ratios depending on the botanical source. Starch has been used extensively in food, medicine, and agricultural products. Due to its low cost, renewable nature and biodegradable quality, it is attracting the attention of plastic industries as a potential building block for petroleum-free adhesives, especially for paper and wood surfaces. However, the hydrophilicity of native starch makes formulating waterproof adhesives from it challenging as the starch hydroxyl groups easily form hydrogen bonds with water [1]. The introduction of hydrophobic functional groups (e.g., esters) onto native starch chains can improve its water resistance, and this can be carried out using chemical, mechanochemical, or enzymatic methods.
2. Esterified Starch
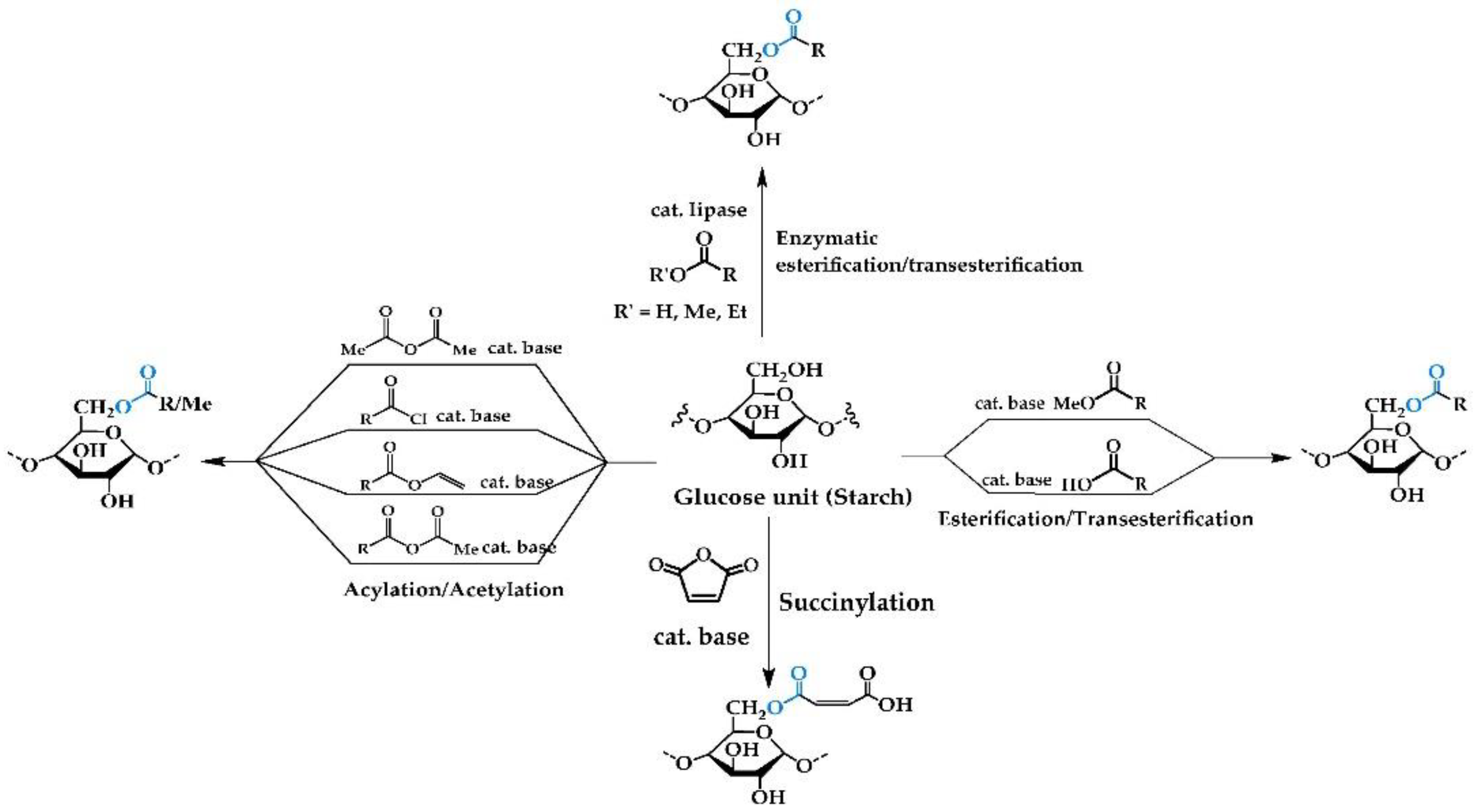
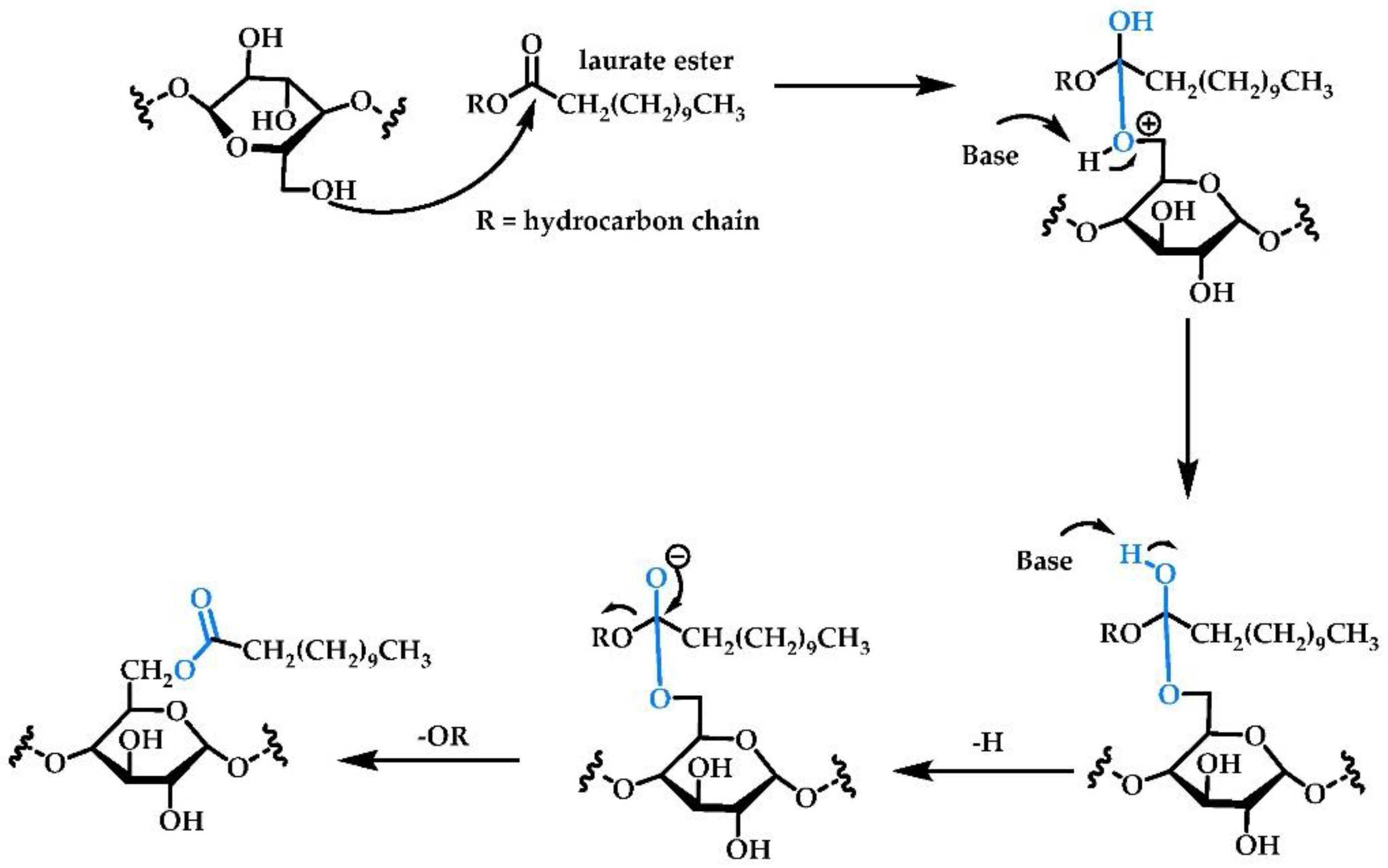
2.1. Chemical Reactions
2.1.1. Esterification/Transesterification
2.1.2. Acylation/Acetylation/Alkylation
2.1.3. Succinylation
2.1.4. Enzymatic Reactions with Free/Immobilized Lipase
2.1.5. Mechanochemical Processes
2.2. Properties of Starches
2.2.1. Degree of Substitution
2.2.2. Morphology of Native and Modified Starches
2.2.3. Viscosity of Starch Pastes
2.2.4. Solubility/Hydrophobicity
2.2.5. Thermal Properties of Starches
2.3. Adhesives Derived from Esterified Starch
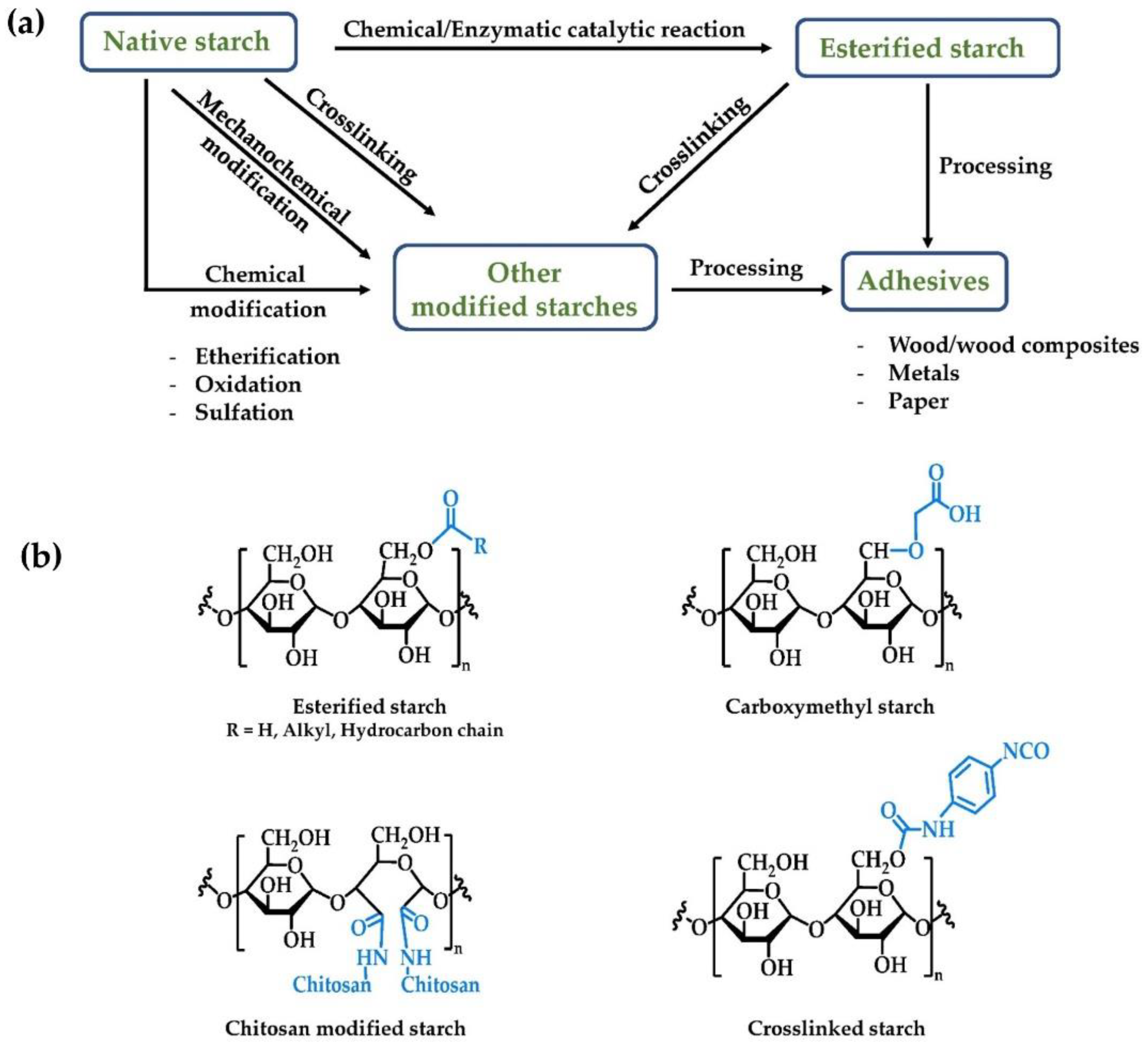
3. Other Modified Starches for Adhesive Applications
| Reaction of Modified Starch | Starch | Properties | Utilization | Ref. |
|---|---|---|---|---|
| Oxidation and modification with chitosan | Corn | Improved dry and wet shear strength of plywood | An adhesive film for plywood | [38] |
| Oxidation using H2O2 and then crosslinking with B-pMDI and citric acid | Corn | Improved physical properties, mechanical properties, and water resistance | Medium density fiberboard | [39] |
| Oxidation using KMnO4, then crosslinking and copolymerization with polyamide and methyl methacrylate | Corn | Improved wet shear strength and water resistance | An adhesive for plywood | [40] |
| Oxidation using KMnO4, polycondensation reaction with urea and addition of nano-TiO2 | Corn | The nano-TiO2 effectively improves dry shear strength and viscosity of the nano-TiO2-U-OSt adhesive. | An adhesive | [41] |
| Oxidation using H2O2 and crosslinking with polyamidoamine-epichlorohydrin (PAH) | Rice | Enhanced thermal stability, hydrophobicity, wet-cohesion, and adhesiveness | An adhesive for wood composites | [42] |
| Etherification with carboxymethyl and use of POCl3 as crosslinking agent | Wheat | The modified starch mixed with PVA improves solid content, heat and water resistance but decrease viscosity. | Adhesive for particleboard | [34] |
| Etherification with epichlorohydrin | Oil palm | Improved mechanical strength (modulus, elasticity, and internal bond), solid content and viscosity | Adhesive for particleboard | [43] |
| Graft copolymerization with glycidyl methacrylate (GMA) and crosslinking with sodium trimetaphosphate (STMP). | Cassava | Improved water resistance and bonding strength | An adhesive for plywood | [44] |
| Graft copolymerization with sodium dodecyl sulfate (SDS) | Micronized (MS) | Improved shear strength and decreased viscosity of micronized starch with increasing SDS contents | Wood adhesive | [45] |
| Graft copolymerization with lignin | Corn | Improved adhesive bond strength and moisture resistance, including extended shelf-life. | An adhesive for paper | [46] |
| Crosslinking with polyphenylene isocyanate (PAPI) with poly vinyl alcohol (PVOH) as a protective colloid | Cassava | Improved water resistance, shear strength, mobility and storage stability of starch adhesive | Wood adhesive | [47] |
| Crosslinking with lignin | Corn | Lignin improved the strength and water resistance of adhesive | An adhesive for cardboard application | [48] |
Etherification can be used to modify the water resistance properties of starch through the introduction of lipophilic functional groups. This is generally accomplished by treating starch with epoxides, such as propylene or ethylene oxides.
Crosslinking, the process of forming nonpolar covalent bonds between the hydroxyl groups of starch, is also a strategy for improving the utility of starches in adhesives. Crosslinked starch exhibits superior mechanical (tensile strength), thermal, and water stability compared to native starch. [49][50][51].
Another convenient method to modify starch is by grafting synthetic monomers or polymers with desirable properties onto the natural starch backbone. In these instances, it is desirable if the crystallinity and biodegradability properties of the starch are unchanged. Grafting typically occurs at the Cl–C2 end groups, and C2–C3 glycol groups on the starch glucose units. Graft copolymers produced from addition of vinyl- or other monomeric acrylates show improved water resistance and shear strength over natural starch [52].
The unique properties of nanoparticles such as their small size, high surface energy, and the ability to functionalize their surfaces render them attractive for the development of high-performance composite materials. Nano-TiO2, nano-SiO2, and montmorillonite (MMT) nanoparticles have been used as crosslinking or grafting agents to improve the characteristics of biopolymer-based adhesives in recent years [53][54]. Significant improvements in water resistance and bonding strength were found when nanoTiO2, [41][55] saline coupling agents [53], vinyl acetate [56], acrylate [32][56] and polyamide [42][57] were used as crosslinking or grafting agents with the starch for adhesive purposes.
Natural polymers derived from biomass (lignin, cellulose, hemicellulose), and chitosan have the potential to be used as bridging agents in adhesive formulations. These may be useful in systems containing inorganic materials and organic components such as starch, and their use can improve the adhesive properties of the formulation [38][42][46]. Blending starch with hydrophobic biopolymers (such as lignin) could be a strategy for improving the water-resistance of the adhesive and altering its mechanical properties.
References
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888.
- Junistia, L.; Sugih, A.K.; Manurung, R.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis of Higher Fatty Acid Starch Esters using Vinyl Laurate and Stearate as Reactants. Starch-Starke 2008, 60, 667–675.
- Oikawa, H.; Ichihara, A.; Sakamura, S. Biosynthetic Study of Betaenone B: Origin of the Oxygen Atoms and Accumulation of Deoxygenated Intermediate using P-450 Inhibitor. J. Chem. Soc. Chem. Commun. 1988, 600–602.
- Biswas, A.; Shogren, R.L.; Selling, G.; Salch, J.; Willett, J.L.; Buchanan, C.M. Rapid and environmentally friendly preparation of starch esters. Carbohydr. Polym. 2008, 74, 137–141.
- Tupa, M.; Maldonado, L.; Vázquez, A.; Foresti, M.L. Simple organocatalytic route for the synthesis of starch esters. Carbohydr. Polym. 2013, 98, 349–357.
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of fatty-acid esters of starch and theirblends with LDPE. J. Appl. Polym. Sci. 1997, 65, 705–721.
- Aburto, J.; Alric, I.; Thiebaud, S.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Panayiotou Synthesis, Characterization, and Biodegradability of Fatty-Acid Esters of Amylose and Starch. J. Appl. Polym. Sci. 1999, 74, 1440–1451.
- Tupa, M.V.; Altuna, L.; Herrera, M.L.; Foresti, M.L. Preparation and Characterization of Modified Starches Obtained in Acetic Anhydride/Tartaric Acid Medium. Starch-Starke 2020, 72, 1900300.
- Amos, R.C.; Mesnager, J.; Kuska, M.; Gauthier, M. Production of Cyclic Anhydride-Modified Starches. Polymers 2021, 13, 1504.
- Kim, H.S.; Choi, H.S.; Kim, B.Y.; Baik, M.Y. Characterization of Acetylated Corn Starch Prepared under Ultrahigh Pressure (UHP). J. Agric. Food Chem. 2010, 58, 3573–3579.
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263.
- Huang, Q.; Fu, X.; He, X.; Luo, F.; Yu, S.; Li, L. The effect of enzymatic pretreatments on subsequent octenyl succinic anhydride modifications of cornstarch. Food Hydrocoll. 2010, 24, 60–65.
- Hu, H.; Liu, W.; Shi, J.; Huang, Z.; Zhang, Y.; Huang, A.; Yang, M.; Qin, X.; Shen, F. Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non-conventional technology. Starch-Starke 2016, 68, 151–159.
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starch-Starke 2012, 64, 188–197.
- Lu, X.; Luo, Z.; Fu, X.; Xiaos, Z. Two-Step Method of Enzymatic Synthesis of Starch Laurate in Ionic Liquids. J. Agric. Food Chem. 2013, 61, 9882–9891.
- Prasertpornsakun, N.; Raita, M.; Laosiripojana, N.; Champreda, V. Biocatalytic synthesis of starch esters by immobilized lipase on magnetic microparticles. Biosci. Biotechnol. BioChem. 2015, 79, 1750–1758.
- Horchani, H.; Chaâbounib, M.; Gargouria, Y.; Sayaria, A. Solvent-free lipase-catalyzed synthesis of long-chain starch esters using microwave heating: Optimization by response surface methodology. Carbohydr. Polym. 2010, 79, 466–474.
- Luna, C.; Luna, D.; Calero, J.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo-Escamilla, C. 7—Biochemical catalytic production of biodiesel. In Handbook of Biofuels Production, 2nd ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 165–199.
- Ilesanmi, O.I.; Adekunle, A.E.; Omolaiye, J.A.; Olorode, E.M.; Ogunkanmi, A.L. Isolation, optimization, and molecular characterization of lipase producing bacteria from contaminated soil. Sci. Afr. 2020, 8, e00279.
- Osbon, Y.; Kumar, M. Biocatalysis and Strategies for Enzyme Improvement. In Biophysical Chemistry: Advance Applications, 1st ed.; Khalid, M.A.A., Ed.; IntechOpen: London, UK, 2020; Volume 7.
- Zhang, Y.; Gan, T.; Hu, H.; Huang, Z.; Huang, A.; Zhu, Y.; Feng, Y.; Yang, M. A Green Technology for the Preparation of High Fatty Acid Starch Esters: Solid-Phase Synthesis of Starch Laurate Assisted by Mechanical Activation with Stirring Ball Mill as Reactor. Ind. Eng. Chem. Res. 2014, 53, 2114–2120.
- Huang, Z.Q.; Xie, X.L.; Chen, Y.; Lu, J.P.; Tong, J.P. Ball-milling treatment effect on physicochemical properties and features for cassava and maize starches. Comptes Rendus Chim. 2008, 11, 73–79.
- Huang, Z.Q.; Lu, J.P.; Li, X.H.; Tong, Z.F. Effect of mechanical activation on physicochemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135.
- Wang, S.Y.; Zhang, C.; Liu, Q.Q.; Wang, Z.J.; Wan, K.X.; Qian, J.Y.; Zhang, L.; Wu, C.; Li, Q. Modification of potato starch by critical melting pretreatment combined with freeze-thawing: Preparation, morphology, structure, and functionality. LWT 2022, 158, 113109.
- Ojogbo, E.; Blanchard, R.; Mekonnen, T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure–Property Correlations. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2611–2622.
- Wang, Z.; Zhu, H.; Huang, J.; Ge, Z.; Guo, J.; Feng, X.; Xu, Q. Improvement of the bonding properties of cassava starch-based wood adhesives by using different types of acrylic ester. Int. J. Biol. Macromol. 2019, 126, 603–611.
- Hui, R.; Qi-he, C.; Liang, F.M.; Qiong, X.; Guo-qing, H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86.
- Muljana, H.; Knoop, S.V.D.; Keijzer, D.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis of fatty acid starch esters in supercritical carbon dioxide. Carbohydr. Polym. 2010, 82, 346–354.
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features, and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130.
- Vamadevan, V.; Bertoft, E.; Seetharaman, K. On the importance of organization of glucan chains on thermal properties of starch. Carbohydr. Polym. 2013, 92, 1653–1659.
- Silaket, P.; Chatakanonda, P.; Tran, T.; Wansuksri, R.; Piyachomkwan, K.; Sriroth, K. Thermal properties of esterified cassava starches and their maltodextrins in various water systems. Starch-Starke 2014, 66, 1022–1032.
- Xu, Q.; Wen, J.; Wang, Z. Preparation and Properties of Cassava Starch-based Wood Adhesives. BioResources 2016, 11, 6756–6767.
- Owodunni, A.A.; Lamaming, J.; Hashim, R.; Abdulwahab Taiwo, O.F.; Hussin, M.H.; Mohamad Kassim, M.H.; Bustami, Y.; Sulaiman, O.; Mohamad Amini, M.H.; Hiziroglu, S. Properties of green particleboard manufactured from coconut fiber using a potato starch-based adhesive. BioResources 2020, 15, 2279–2292.
- Lamaming, J.; Heng, N.B.; Owodunni, A.A.; Lamaming, S.Z.; Khadir, N.K.A.; Hashim, R.; Sulaiman, O.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; et al. Characterization of rubberwood particleboard made using carboxymethyl starch mixed with polyvinyl alcohol as adhesive. Compos. Part B Eng. 2020, 183, 107731.
- Zhang, Z.; Macquarrie, D.; Clark, J.H.; Matharu, A.S. Chemical modification of starch and the application of expanded starch and its esters in hot melt adhesive. RSC Adv. 2014, 4, 41947–41955.
- Wang, Z.; Li, Z.; Gu, Z.; Hong, Y.; Cheng, L. Preparation, characterization, and properties of starch-based wood adhesive. Carbohydr. Polym. 2012, 88, 699–706.
- Huicochea, E.F. Introductory Chapter: Starch Modifications. Applications of Modified Starches; Huicochea, E.F., Villalobos, R.R., Eds.; InTechOpen: London, UK, 2018; p. 78.
- Li, D.; Zhuang, B.; Wang, X.; Wu, Z.; Wei, W.; Aladejana, J.Y.; Hou, X.; Yves, K.G.; Xie, Y.; Liu, J. Chitosan used as a specific coupling agent to modify starch in preparation of adhesive film. J. Clean. Prod. 2020, 277, 123210.
- Lubis, M.A.R.; Park, B.D.; Hong, M.K. Tuning of Adhesion and Disintegration of Oxidized Starch Adhesives for the Recycling of Medium Density Fiberboard. BioResources 2020, 15, 5156–5178.
- Su, M.; Wu, J.; Pan, P.; Wang, H. Preparation, and characterization of a water-resistant polyamide-oxidized starch-methyl methacrylate eco-friendly wood adhesive. Int. J. Biol. Macromol. 2022, 194, 763–769.
- Zhao, X.F.; Peng, L.Q.; Wang, H.L.; Wang, Y.B.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr. Polym. 2018, 181, 1112–1118.
- Yin, H.; Zheng, P.; Zhang, E.; Rao, J.; Lin, Q.; Fan, M.; Zhu, Z.; Zeng, Q.; Chen, N. Improved wet shear strength in eco-friendly starch-cellulosic adhesives for woody composites. Carbohydr. Polym. 2020, 250, 116884.
- Sulaiman, N.S.; Hahim, R.; Amini, M.H.M.; Sulaiman, O.; Hiziroglu, S. Evaluation of the Properties of Particleboard Made Using Oil Palm Starch Modified with Epichlorohydrin. BioResources 2013, 8, 283–301.
- Chen, X.; Sun, C.; Wang, Q.; Tan, H.; Zhang, Y. Preparation of glycidyl methacrylate grafted starch adhesive to apply in high-performance and environment-friendly plywood. Int. J. Biol. Macromol. 2022, 194, 954–961.
- Chen, L.; Xiong, Z.; Li, Q.; Din, Z.U.; Xiong, H. Sodium dodecyl sulfate improves the properties of bio-based wood adhesive derived from micronized starch: Microstructure and rheological behaviors. Int. J. Biol. Macromol. 2019, 140, 1026–1036.
- Wu, Q.; Shao, W.; Xia, N.; Wang, P.; Kong, F. A separable paper adhesive based on the starch―lignin composite. Carbohydr. Polym. 2020, 229, 115488.
- Jiang, Y.; Chen, Q.; Tan, H.; Gu, J.; Zhang, Y. A Low-Cost, Formaldehyde-Free, and High-Performance Starch-based Wood Adhesive. BioResources 2019, 14, 1405–1418.
- Nasiri, A.; Wearing, J.; Dubé, M.A. Using Lignin to Modify Starch-Based Adhesive Performance. ChemEngineering 2020, 4, 3.
- Detduangchan, N.; Sridach, W.; Wittaya, T. Enhancement of the properties of biodegradable rice starch films by using chemical crosslinking agents. Int. Food Res. J. 2014, 21, 1189–1199.
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch-filled linear low-density polyethylene films. Carbohydr. Polym. 2002, 50, 331–337.
- Seligra, P.G.; Jaramillo, C.M.; Fama, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74.
- Praveen, T.K.; Bhushanam, M.V.N.; Prasad, Y.R. Preparation, Evaluation and Characterization of Starch and Grafted Starch. IIJPSR 2021, 12, 4001–4010.
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation, and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37.
- Li, Z.; Wang, J.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y. Effects of montmorillonite addition on the performance of starch-based wood adhesive. Carbohydr. Polym. 2015, 115, 394–400.
- Chen, L.; Xiong, Z.; Xiong, H.; Wang, Z.; Din, Z.U.; Nawaz, A.; Wang, P.; Hu, C. Effects of nano-TiO2 on bonding performance, structure stability and film-forming properties of starch-g-VAc based wood adhesive. Carbohydr. Polym. 2018, 200, 477–486.
- Zia-ud-Din; Chen, L.; Ullah, I.; Wang, P.K.; Javaid, A.B.; Hu, C.; Zhang, M.; Ahamd, I.; Xiong, H.; Wang, Z. Synthesis, and characterization of starch-g-poly (vinyl acetate-co-butyl acrylate) bio-based adhesive for wood application. Int. J. Biol. Macromol. 2018, 114, 1186–1193.
- Zhang, Y.; Chen, M.; Zhang, J.; Li, J.; Shi, S.Q.; Gao, Q. A High-Performance Bio-Adhesive Using Hyperbranched Aminated Soybean Polysaccharide and Bio-Based Epoxide. Adv. Mater. Interfaces 2020, 7, 2000148.





