Consumer trends towards environmentally friendly products are driving plastics industries to investigate more benign alternatives to petroleum-based polymers. In the case of adhesives, one possibility to achieve sustainable production is to use non-toxic, low-cost starches as biodegradable raw materials for adhesive production. While native starch contains only hydroxyl groups and has limited scope, chemically modified starch shows superior water resistance properties for adhesive applications. Esterified starches, starches with ester substituents, can be feasibly produced and utilized to prepare bio-based adhesives with improved water resistance. Syntheses of esterified starch materials can involve esterification, transesterification, alkylation, acetylation, succinylation, or enzymatic reactions.
- biopolymer
- esterified starch
- synthesis
- adhesives
1. Introduction
Starch is one of the most abundant polysaccharides in nature, consisting of amylose and amylopectin in various ratios depending on the botanical source. Starch has been used extensively in food, medicine, and agricultural products. Due to its low cost, renewable nature and biodegradable quality, it is attracting the attention of plastic industries as a potential building block for petroleum-free adhesives, especially for paper and wood surfaces. However, the hydrophilicity of native starch makes formulating waterproof adhesives from it challenging as the starch hydroxyl groups easily form hydrogen bonds with water [1][2]. The introduction of hydrophobic functional groups (e.g., esters) onto native starch chains can improve its water resistance, and this can be carried out using chemical, mechanochemical, or enzymatic methods.
2. Esterified Starch
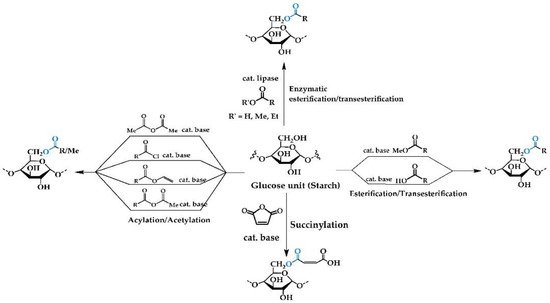
2.1. Chemical Reactions
2.1.1. Esterification/Transesterification
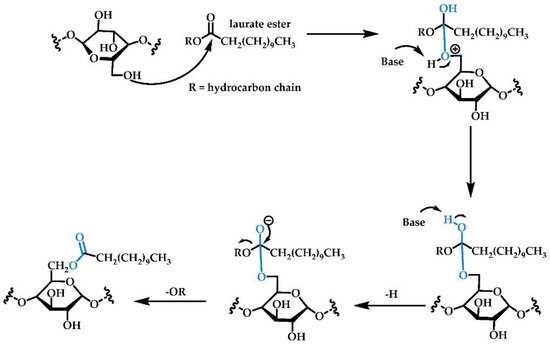
2.1.2. Acylation/Acetylation/Alkylation
2.1.3. Succinylation
Succinylation refers to the appending of side chains to starch by the reaction with succinic anhydride. Succinic anhydride is an electrophile, which undergoes nucleophilic attack by alcohol functionalities on the starch backbone. The esterified starch product has carboxylic acid functionalities at the terminus of each chain, and this allows the product to be incorporated into the adhesive directly or be further modified or functionalized prior to use. While succinylation results in a high degree of substitution [11][12][30,31] the limitation of this reaction is the cost of succinic anhydride. Additionally, the succinylation products have a carboxylic group at the terminal chain that could further promote cross-linking reactions between starch chains [13][32].2.1.4. Enzymatic Reactions with Free/Immobilized Lipase
Esterification of starch with fatty acids can be performed using enzyme catalysis, with lipases playing the key role. The use of enzymes has the advantage of not requiring extreme conditions and expensive starting materials, however the degree of substitution is often low [1][14][15][2,15,33] with immobilized lipase on magnetic microparticles providing a higher degree of substitution in the esterified starch than free lipase [16][34]. Horchani and coworkers also reported lipase immobilized calcium carbonate [17][35] as a catalyst in the esterification reactions between oleic acid and Maize starch. The yields of modified starch and their degree of substitution are varied, significantly depending on reaction conditions (temperature, pH, and lipase enzyme origin) [18][36]. Obtaining lipase from either plant of microorganisms requires multi-step isolation, purification and characterization of lipase to ensure its high purity and enzamic activity [17][19][35,37]. Additional limiations of enzymes such as low thermostability, pH sensitivity, narrow substrate scope, and low reaction yield in non-aqueous solvents [20][38], may result in restrictions in starch modification processes.2.1.5. Mechanochemical Processes
These processes involve reactions taking place without the addition of solvent and utilize mechanical activation, such as ball milling. In the case of starch esterification, ball milling allows for intimate mixing of starch and the esterification agent, giving good yields of product [21][39]. This processing also disrupts the structure and morphology of the starch, and results in changes in physicochemical properties, compared to those of native starch, such as decreased cold-water solubility, transparency, and emulsion stabilization, along with increased gelatinization temperature and viscosity [13][22][23][32,40,41].2.2. Properties of Starches
2.2.1. Degree of Substitution
The degree of substitution (DS) in esterified starch is an important factor dictating the physical properties of the material. It may be determined using 1H-NMR spectroscopy or through an acid–base titration process.2.2.2. Morphology of Native and Modified Starches
The morphology of starch granules (native and modified) can be studied using scanning electron microscopy (SEM), and this technique provides information about the granule shape, and surface features. Starch granules are the vehicle of carbohydrate storage in plant cells, and the morphology of these depends on the biochemistry of the chloroplast/amyloplasts and the physiology of the plant. Normally, native starch granules are elliptical, have a smooth surface, and range from 1 to 100 μm in size [24][46]. Modification can result in the granules becoming irregular in shape with pronounced surface roughness, especially at high degrees of substitution (DS) [24][25][26][46,47,48].2.2.3. Viscosity of Starch Pastes
In addition to the source of starch and conditions used to make the paste, functionalization of the starch can also markedly affect the viscosity of the paste. A study on the paste viscosity of OSA-esterified starch [13][27][32,49] indicated that paste obtained from this material was higher than that of native starch, as a consequence of the bulky OSA functional groups. Acylation of starch gives rise to steric interactions between starch chains, and this influences both hydrophilicity and the degree of hydrogen bonding, and thus viscosity. The viscosities of esterified starches generally decrease as the molecular weight of the ester group increases [28][50].2.2.4. Solubility/Hydrophobicity
Appending larger functional groups onto the starch units results in increased hydrophobicity and lower water solubility. On the other hand, solubility in lower polarity solvents increases [1][2] and this impacts the potential application of these starch derivatives [25][47].2.2.5. Thermal Properties of Starches
The thermal properties of starch are dependent on the amylose content or amylose/amylopectin ratio [29][30][51,52], and are also affected by functionalization. While esterified starches having low degrees of substitution (DS) do not see dramatic changes in onset temperature relative to native starch, starches having higher DS values show a decrease in gelatinization temperature [31][53]. Furthermore, the glass transition temperature (Tg), the temperature at which a glassy state of amorphous materials converts into a rubbery state, is affected by structure. In the case of functionalized starch, steric hindrance between starch chains increases as the functional groups become larger, resulting in higher Tg. On the other hand, the extension of free volume of chain mobility can reduce Tg [31][53].2.3. Adhesives Derived from Esterified Starch
Starch-based adhesives are widely used in the production of plywood [11][32][30,73] and particleboard [33][34][74,75]. Cassava starch esterified using dodecyl succinic anhydride (DDSA) as a reactant [11][30] and crosslinked with polymethylene polyphenyl polyisocyanate (PAPI) results in a formaldehyde-free industrial plywood adhesive with excellent viscosity, bonding strength, and water resistance properties [11][30]. Natural corn starch was converted to high amylose corn starch (HACS) by solvent exchange with ethanol and toluene prior to esterification with propionic anhydride in the presence of catalytic 4-dimethylaminopyridine (DMAP). The DS of these starch propionates was in the range 0.38–2.54, with higher ratios of propionic anhydride which enhanced the adhesion strength relatively to native starch. These modified starches were mixed with glycerol and polyvinyl alcohol and hot-pressed to form hot melt adhesives on Al plates. It was found that these covered a greater surface area and exhibited higher tensile strength than adhesives formed analogously from unmodified starch [35][76]. As mentioned in earlier sections esterified starch from chemical modifications show enhanced hydrophobicity and water resistance, with adhesives formed from this exhibiting improved bonding strength under both dry and wet conditions relative to those from natural starch [36][77]3. Other Modified Starches for Adhesive Applications
The application of native starches in adhesives is limited due to their relatively poor shear strength, low thermal stability, and hydrophilicity [37][84]. Chemical modification is a successful strategy for changing the structure and properties of biopolymers to fit new applications [37][84] with esterification of starches discussed in the previous section being a prime example. In addition to esterification other modifications of starches can include etherification, oxidation, crosslinking, and grafted copolymerization, as shown in Figure 3 and Table 4.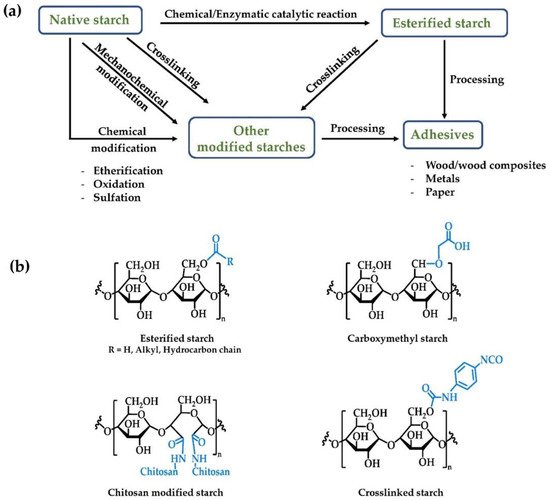
| Reaction of Modified Starch | Starch | Properties | Utilization | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidation and modification with chitosan | Corn | Improved dry and wet shear strength of plywood | An adhesive film for plywood | [38] | [102] | |||||||
| Oxidation using H | 2 | O | 2 | and then crosslinking with B-pMDI and citric acid | Corn | Improved physical properties, mechanical properties, and water resistance | Medium density fiberboard | [39] | [107] | |||
| Oxidation using KMnO | 4 | , then crosslinking and copolymerization with polyamide and methyl methacrylate | Corn | Improved wet shear strength and water resistance | An adhesive for plywood | [40] | [108] | |||||
| Oxidation using KMnO | 4 | , polycondensation reaction with urea and addition of nano-TiO | 2 | Corn | The nano-TiO | 2 | effectively improves dry shear strength and viscosity of the nano-TiO | 2 | -U-OSt adhesive. | An adhesive | [41] | [98] |
| Oxidation using H | 2 | O | 2 | and crosslinking with polyamidoamine-epichlorohydrin (PAH) | Rice | Enhanced thermal stability, hydrophobicity, wet-cohesion, and adhesiveness | An adhesive for wood composites | [42] | [100] | |||
| Etherification with carboxymethyl and use of POCl | 3 | as crosslinking agent | Wheat | The modified starch mixed with PVA improves solid content, heat and water resistance but decrease viscosity. | Adhesive for particleboard | [34] | [75] | |||||
| Etherification with epichlorohydrin | Oil palm | Improved mechanical strength (modulus, elasticity, and internal bond), solid content and viscosity | Adhesive for particleboard | [43] | [109] | |||||||
| Graft copolymerization with glycidyl methacrylate (GMA) and crosslinking with sodium trimetaphosphate (STMP). | Cassava | Improved water resistance and bonding strength | An adhesive for plywood | [44] | [110] | |||||||
| Graft copolymerization with sodium dodecyl sulfate (SDS) | Micronized (MS) | Improved shear strength and decreased viscosity of micronized starch with increasing SDS contents | Wood adhesive | [45] | [111] | |||||||
| Graft copolymerization with lignin | Corn | Improved adhesive bond strength and moisture resistance, including extended shelf-life. | An adhesive for paper | [46] | [101] | |||||||
| Crosslinking with polyphenylene isocyanate (PAPI) with poly vinyl alcohol (PVOH) as a protective colloid | Cassava | Improved water resistance, shear strength, mobility and storage stability of starch adhesive | Wood adhesive | [47] | [112] | |||||||
| Crosslinking with lignin | Corn | Lignin improved the strength and water resistance of adhesive | An adhesive for cardboard application | [48] | [113] |
Etherification can be used to modify the water resistance properties of starch through the introduction of lipophilic functional groups. This is generally accomplished by treating starch with epoxides, such as propylene or ethylene oxides.
Crosslinking, the process of forming nonpolar covalent bonds between the hydroxyl groups of starch, is also a strategy for improving the utility of starches in adhesives. Crosslinked starch exhibits superior mechanical (tensile strength), thermal, and water stability compared to native starch. [49][50][51][88,89,90].
Another convenient method to modify starch is by grafting synthetic monomers or polymers with desirable properties onto the natural starch backbone. In these instances, it is desirable if the crystallinity and biodegradability properties of the starch are unchanged. Grafting typically occurs at the Cl–C2 end groups, and C2–C3 glycol groups on the starch glucose units. Graft copolymers produced from addition of vinyl- or other monomeric acrylates show improved water resistance and shear strength over natural starch [52][92].
The unique properties of nanoparticles such as their small size, high surface energy, and the ability to functionalize their surfaces render them attractive for the development of high-performance composite materials. Nano-TiO2, nano-SiO2, and montmorillonite (MMT) nanoparticles have been used as crosslinking or grafting agents to improve the characteristics of biopolymer-based adhesives in recent years [53][54][95,96]. Significant improvements in water resistance and bonding strength were found when nanoTiO2, [41][55][97,98] saline coupling agents [53][95], vinyl acetate [56][93], acrylate [32][56][73,93] and polyamide [42][57][99,100] were used as crosslinking or grafting agents with the starch for adhesive purposes.
Natural polymers derived from biomass (lignin, cellulose, hemicellulose), and chitosan have the potential to be used as bridging agents in adhesive formulations. These may be useful in systems containing inorganic materials and organic components such as starch, and their use can improve the adhesive properties of the formulation [38][42][46][100,101,102]. Blending starch with hydrophobic biopolymers (such as lignin) could be a strategy for improving the water-resistance of the adhesive and altering its mechanical properties.
